UV-Surface Treatment of Fungal Resistant Polyether Polyurethane Film-Induced Growth of Entomopathogenic Fungi
Abstract
:1. Introduction
2. Results
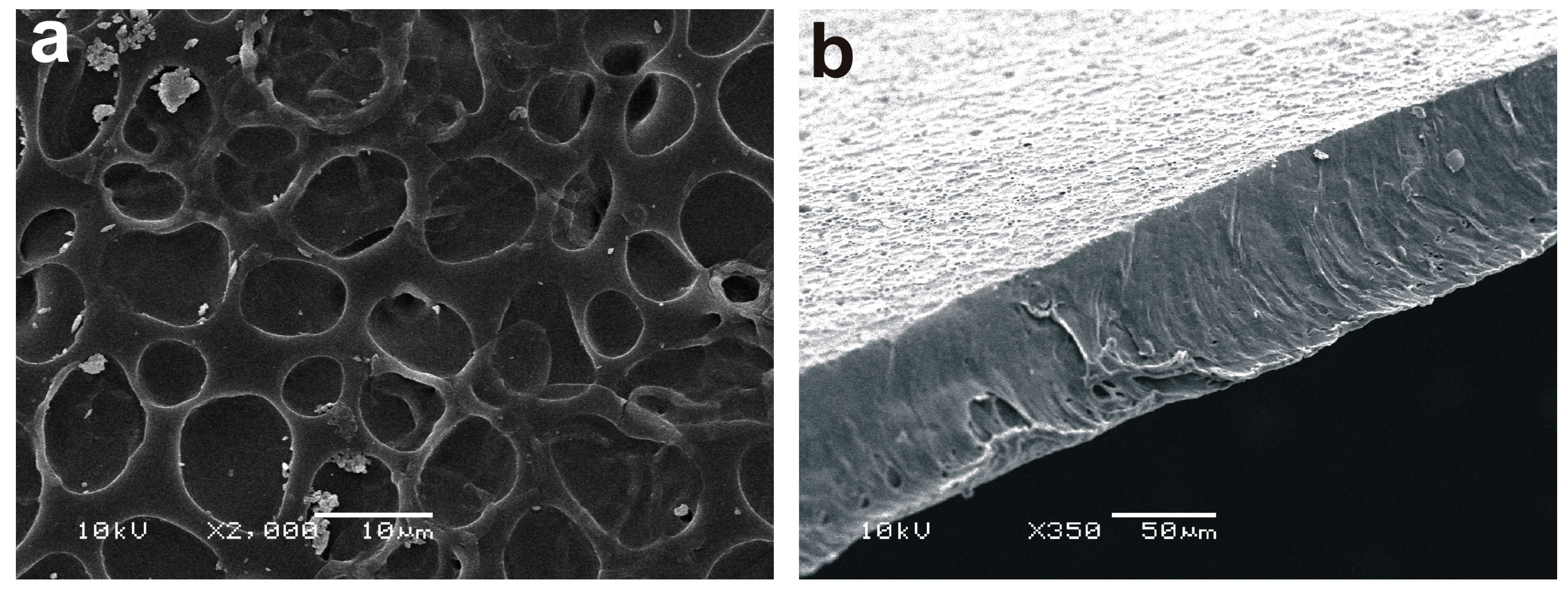
2.1. Surface Characterization of the Polyether Polyurethane Films
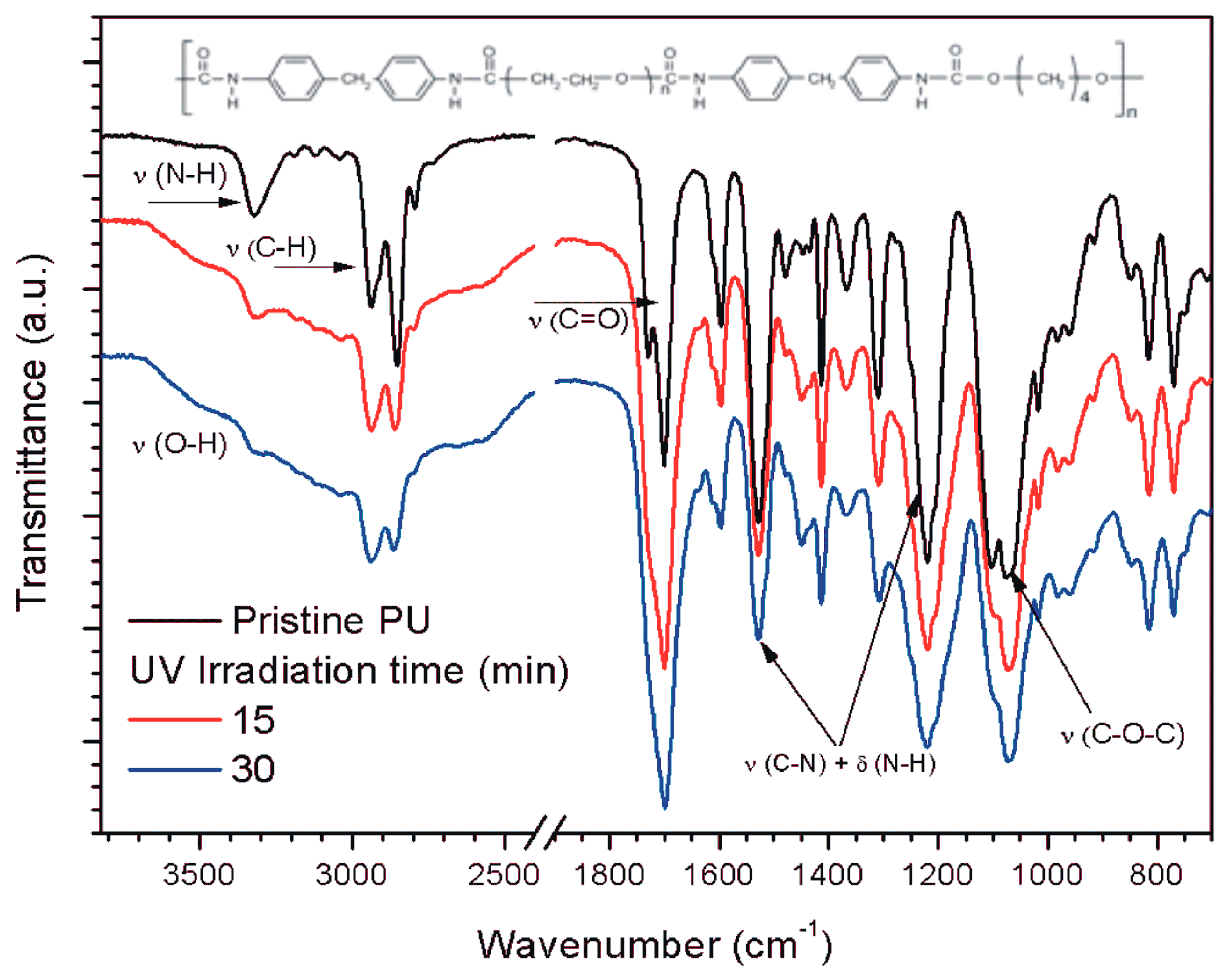
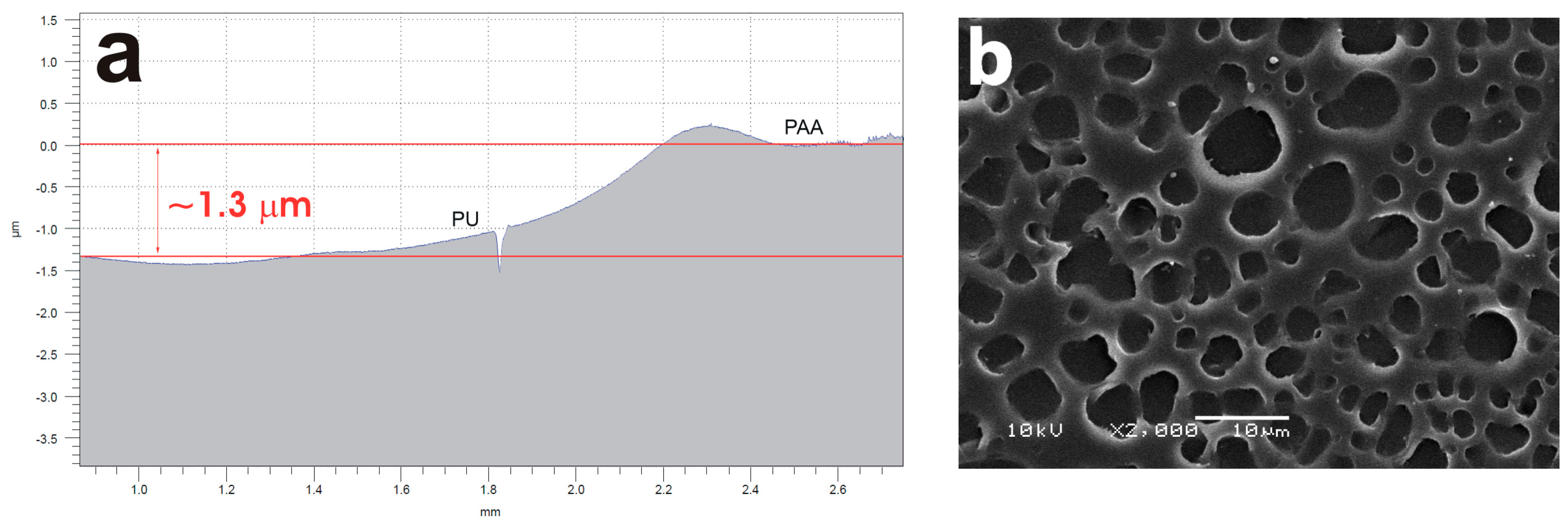
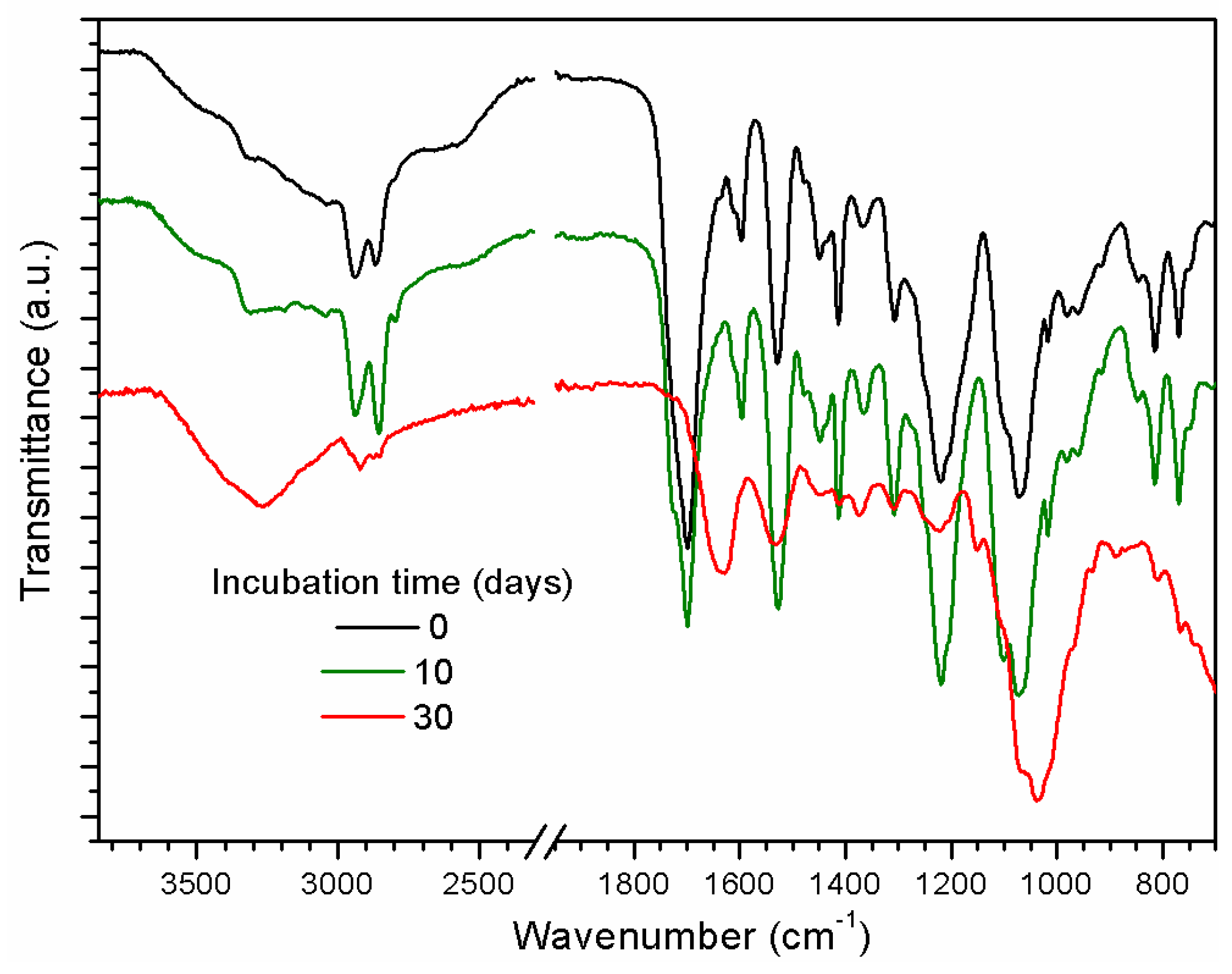
2.2. Surface Modification of the Polyether PU Films by Ultraviolet-Assited Photochemistry
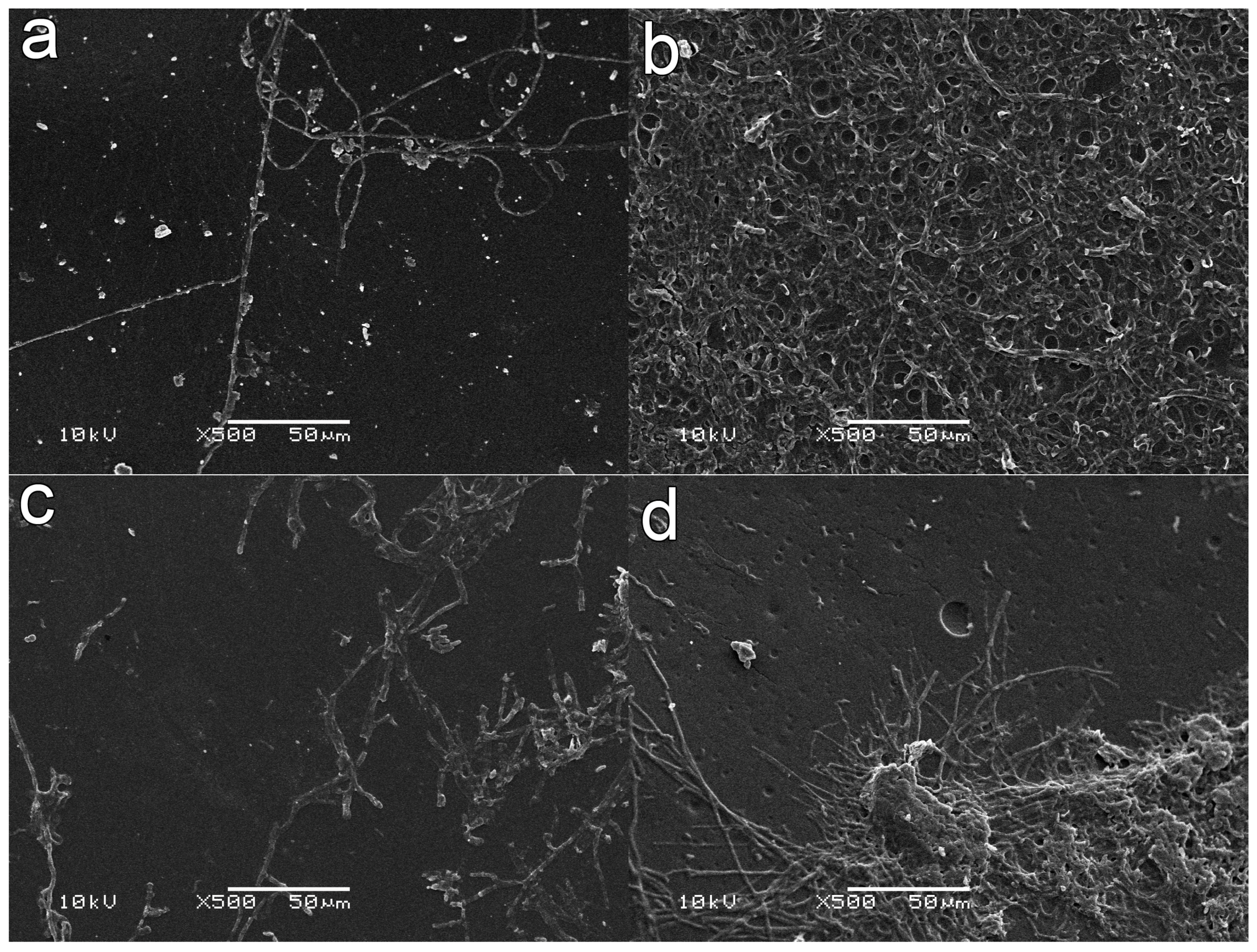
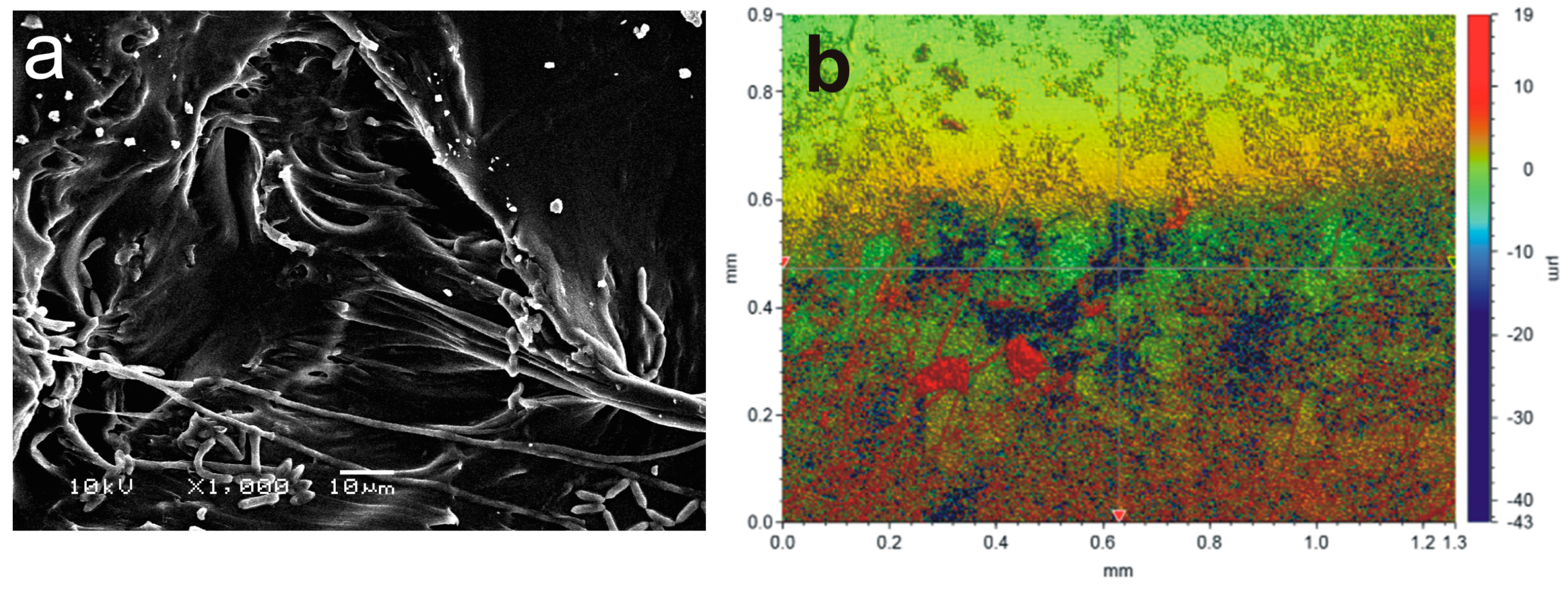
2.3. Induced Growth of Entomopathogenic Fungi
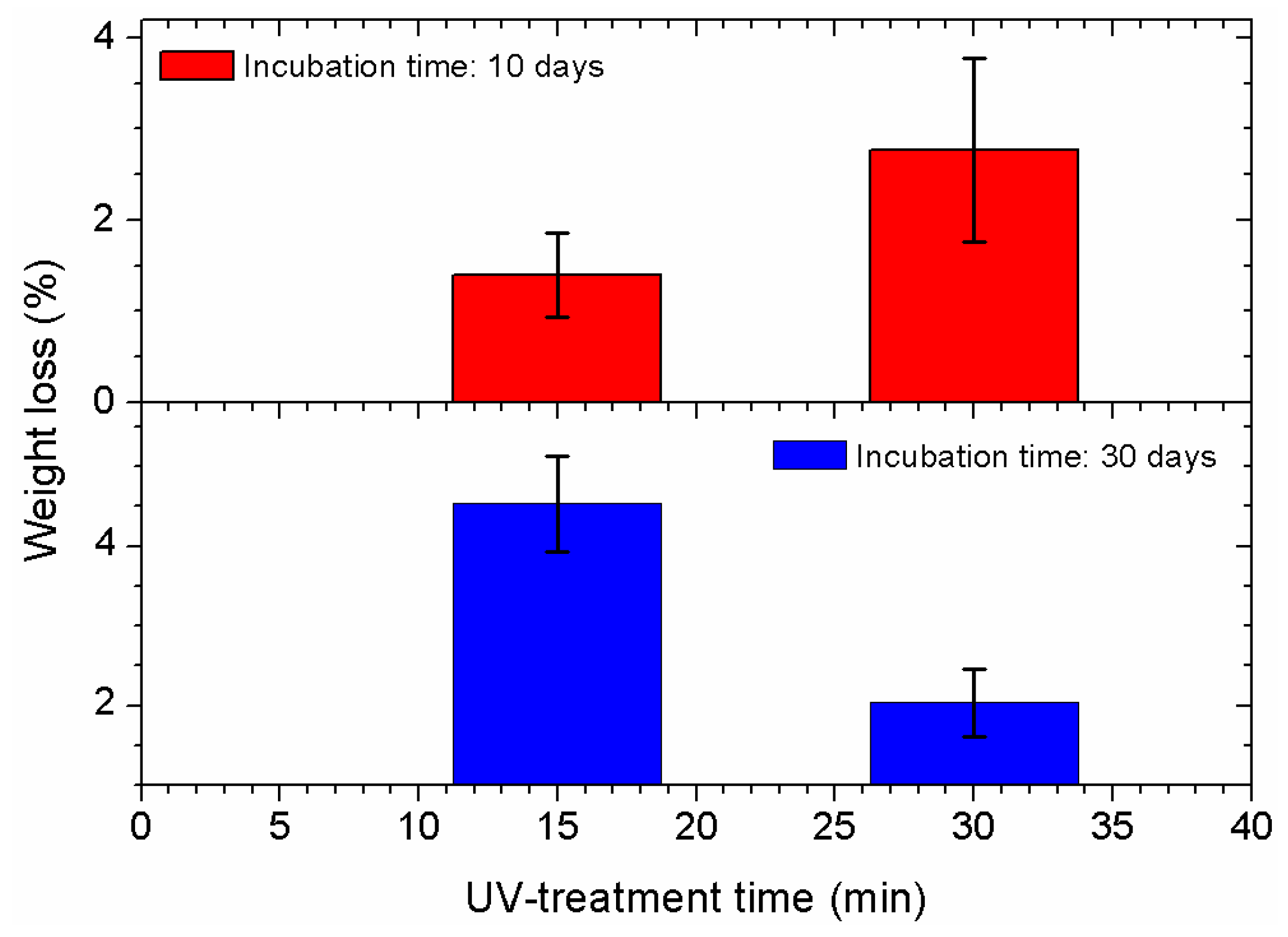
2.4. Weight Change Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Film Preparation and UV-Surface Modification
4.3. Surface Characterization
4.4. Weight Change Measurements
4.5. Fungi Maintenance
4.6. Fungi Inoculationand Growth Behavior Evaluation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar]
- Coltro, L.; Gasparino, B.F.; Queiroz, G.D.C. Plastic materials recycling: The importance of the correct identification. Polímeros 2008, 18, 119–125. [Google Scholar]
- Jayasekara, R.; Harding, I.; Bowater, I.; Lonergan, G. Biodegradability of a selected range of polymers and polymer blends and standard methods for assessment of biodegradation. J. Polym. Environ. 2005, 13, 231–251. [Google Scholar]
- Oehlmann, J.R.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B 2009, 364, 2047–2062. [Google Scholar]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [PubMed]
- Loredo-Trevino, A.; Gutiérrez-Sánchez, G.; Rodríguez-Herrera, R.; Aguilar, C.N. Microbial enzymes involved in polyurethane biodegradation: A review. J. Polym. Environ. 2012, 20, 258–265. [Google Scholar]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [PubMed]
- Zafar, U.; Houlden, A.; Robson, G.D. Fungal communities associated with the biodegradation of polyester polyurethane buried under compost at different temperatures. Appl. Environ. Microbiol. 2013, 79, 7313–7324. [Google Scholar]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar]
- Cosgrove, L.; McGeechan, P.L.; Handley, P.S.; Robson, G.D. Effect of biostimulationand bioaugmentation on degradation of polyurethane buried in soil. Appl. Environ. Microbiol. 2010, 76, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, U.; Nzeram, P.; Langarica-Fuentes, A.; Houlden, A.; Heyworth, A.; Saiani, A.; Robson, G.D. Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour. Technol. 2014, 158, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, L.; McGeechan, P.L.; Robson, G.D.; Handley, P.S. Fungal communities associated with degradation of polyester polyurethane in soil. Appl. Environ. Microbiol. 2007, 73, 5817–5824. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef]
- Griffin, G.J.L. Synthetic polymers and the living environment. Pure Appl. Chem. 1980, 52, 399–407. [Google Scholar] [CrossRef]
- Kessler, F.; Steffens, D.; Lando, G.A.; Pranke, P.; Weibel, D.E. Wettability and cell spreading enhancement in poly(sulfone) and polyurethane surfaces by UV-assisted treatment for tissue engineering purposes. Tissue Eng. Regen. Med. 2014, 11, 23–31. [Google Scholar] [CrossRef]
- Kessler, F.; Marconatto, L.; Rodrigues, R.d.S.B.; Lando, G.A.; Schank, A.; Vainstein, M.H.; Weibel, D.E. Biodegradation improvement of poly(3-hydroxy-butyrate) films by entomopathogenic fungi and UV-assisted surface functionalization. J. Photochem. Photobiol. B 2014, 130, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.; Kûhn, S.; Radtke, C.; Weibel, D.E. Controlling the surface wettability of poly(sulfone) films by UV-assisted treatment: Benefits in relation to plasma treatment. Polym. Int. 2013, 62, 310–318. [Google Scholar] [CrossRef]
- Kumar, A.P.; Pandey, J.K.; Kumar, B.; Singh, R.P. Photo-bio-degradability of agro waste and ethylene-propylene copolymers composites under abiotic and biotic environments. J. Polym. Environ. 2006, 14, 203–212. [Google Scholar] [CrossRef]
- Labuzek, S.; Nowak, B.; Pajak, J. Biodegradation of aged composition of polyethylene with synthetic polyester. Polimery 2006, 33, 27–32. [Google Scholar]
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Savelyev, Y.; Markovskaya, L.; Savelyeva, O.; Akhranovich, E.; Parkhomenko, N.; Travinskaya, T. Degradable polyurethane foams based on disaccharides. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gupta, B.; Plummer, C.; Bisson, I.; Frey, P.; Hilborn, J. Plasma-induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) films: Characterization and human smooth muscle cell growth on grafted films. Biomaterials 2002, 23, 863–871. [Google Scholar] [CrossRef]
- Lee, S.-D.; Hsiue, G.-H.; Chang, P.C.-T.; Kao, C.-Y. Plasma-induced grafted polymerization of acrylic acid and subsequent grafting of collagen onto polymer film as biomaterials. Biomaterials 1996, 17, 1599–1608. [Google Scholar] [CrossRef]
- Bai, M.; Wilske, B.; Buegger, F.; Esperschütz, J.r.; Bach, M.; Frede, H.-G.; Breuer, L. Relevance of nonfunctional linear polyacrylic acid for the biodegradation of superabsorbent polymer in soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.O.; Hitchcock, A.P.; Cornelius, R.M.; Brash, J.L.; Scholl, A.; Doran, A. Using X-PEEM to study biomaterials: Protein and peptide adsorption to a polystyrene-poly(methyl methacrylate)-b-polyacrylic acid blend. J. Electron Spectrosc. Relat. Phenom. 2013, 185, 406–416. [Google Scholar] [CrossRef]
- Wu, C.-S. Characterization and biodegradability of polyester bioplastic-based green renewable composites from agricultural residues. Polym. Degrad. Stab. 2012, 97, 64–71. [Google Scholar] [CrossRef]
- Sartori, S.; Rechichi, A.; Vozzi, G.; D’Acunto, M.; Heine, E.; Giusti, P.; Ciardelli, G. Surface modification of a synthetic polyurethane by plasma glow discharge: Preparation and characterization of bioactive monolayers. React. Funct. Polym. 2008, 68, 809–821. [Google Scholar] [CrossRef]
- Chen, J.P.; Chiang, Y.P. Surface modification of non-woven fabric by DC pulsed plasma treatment and graft polymerization with acrylic acid. J. Membr. Sci. 2006, 270, 212–220. [Google Scholar] [CrossRef]
- Alves, P.; Coelho, J.F.J.; Haack, J.; Rota, A.; Bruinink, A.; Gil, M.H. Surface modification and characterization of thermoplastic polyurethane. Eur. Polym. J. 2009, 45, 1412–1419. [Google Scholar] [CrossRef]
- Chiono, V.; Mozetic, P.; Boffito, M.; Sartori, S.; Gioffredi, E.; Silvestri, A.; Rainer, A.; Giannitelli, S.M.; Trombetta, M.; Nurzynska, D.; et al. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Weibel, D.E.; Michels, A.F.; Horowitz, F.; Cavalheiro, R.S.; Mota, G.V.S. Ultraviolet-induced surface modification of Polyurethane films in the presence of oxygen or acrylic acid vapours. Thin Solid Films 2009, 517, 5489–5495. [Google Scholar] [CrossRef]
- Feng, J.; Wen, G.; Huang, W.; Kang, E.-T.; Neoh, K.G. Influence of oxygen plasma treatment on poly(ether sulphone) films. Polym. Degrad. Stab. 2006, 91, 12–20. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lin, C.-K.; Lin, C.-R.; Lin, C.-H. Polymerization-like grafting of thermoplastic polyurethane by microwave plasma treatment. Surf. Coat. Technol. 2006, 200, 3380–3384. [Google Scholar] [CrossRef]
- Dhayal, M.; Bradley, J.W. Time-resolved electric probe measurements in the pulsed-plasma polymerisation of acrylic acid. Surf. Coat. Technol. 2005, 194, 167–174. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Shaw, H.Y.; Jeng, Y.S.; Cheng, L.P. Formation of multilayer poly(acrylic acid)/poly(vinylidene fluoride) composite membranes for pervaporation. J. Appl. Polym. Sci. 2004, 93, 2266–2274. [Google Scholar] [CrossRef]
- Weibel, D.E.; Vilani, C.; Habert, A.C.; Achete, C.A. Surface modification of polyurethane membranes using low-powered gas plasmas. Influence on the pervaporation processes. J. Membr. Sci. 2007, 293, 124–132. [Google Scholar] [CrossRef]
- Vilani, C.; Weibel, D.E.; Zamora, R.R. M.; Habert, A.C.; Achete, C.A. Study of the influence of the acrylic acid plasma parameters on silicon and polyurethane substrates using XPS and AFM. Appl. Surf. Sci. 2007, 254, 131–134. [Google Scholar] [CrossRef]
- Weibel, D.E.; Vilani, C.; Habert, A.C.; Achete, C.A. Surface modification of polyurethane membranes using RF-plasma treatment with polymerizable and non-polymerizable gases. Surf. Coat. Technol. 2006, 201, 4190–4194. [Google Scholar] [CrossRef]
- Ward, L.J.; Schofield, W.C.E.; Badyal, J.P.S.; Goodwin, A.J.; Merlin, P.J. Atmospheric pressure plasma deposition of structurally well-defined polyacrylic acid films. Chem. Mater. 2003, 15, 1466–1469. [Google Scholar] [CrossRef]
- Lando, G.A.; Weibel, D.E. Universidade Federal de Rio Grande do Sul,Porto Alegre, R.S. PU films irradiated with UV in a presence of a controlled flow of oxygen gas. 2016.
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and properties of a polyester polyurethane-degrading enzyme from comamonasacidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [PubMed]
- Tan, F.T.; Cooper, D.G.; Maric, M.; Nicell, J.A. Biodegradation of a synthetic co-polyester by aerobic mesophilic microorganisms. Polym. Degrad. Stab. 2008, 93, 1479–1485. [Google Scholar]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Kay, M.J.; McCabe, R.W.; Morton, L.H.G. Chemical and physical changes occurring in polyester polyurethane during biodegradation. Int. Biodeterior. Biodegrad. 1993, 31, 209–225. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Sasek, V.; Vitasek, J.; Chromcova, D.; Prokopova, I.; Brozek, J.; Nahlik, J. Biodegradation of synthetic polymers by composting and fungal treatment. Folia Mocrobiol. 2006, 51, 425–430. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Erlandsson, B.; Hakkarainen, M.; Karlsson, S. Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegraded polyethylene. J. Environ. Chem. Eng. 1998, 6, 187–195. [Google Scholar]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.J.; Morton, L.H.G.; Prince, E.L. Bacterial degradation of polyester polyurethane. Int. Biodeterior. 1991, 27, 205–222. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Karlsson, S. Aspects of biodeterioration of inert and degradable polymers. Int. Biodeterior. Biodegrad. 1993, 31, 161–170. [Google Scholar] [CrossRef]
- Ghazali, R.; Mei, L.C.; Shaari, N.Z. K.; Yusof, M.; Ahmad, S. Preliminary study on microbial degradation of flexible polyurethane foams—Physico-mechanical and weight changes during fungal deterioration. J. Oil Palm Res. 2005, 17, 103–109. [Google Scholar]
- Sadi, R.K.; Fechine, G.J.M.; Demarquette, N.R. Photodegradation of poly(3-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2318–2327. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef] [PubMed]
- Cove, D.J. The induction and repression of nitrate reductase in the fungus Aspergillusnidulans. Biochim. Biophys. Acta 1966, 113, 51–56. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lando, G.A.; Marconatto, L.; Kessler, F.; Lopes, W.; Schrank, A.; Vainstein, M.H.; Weibel, D.E. UV-Surface Treatment of Fungal Resistant Polyether Polyurethane Film-Induced Growth of Entomopathogenic Fungi. Int. J. Mol. Sci. 2017, 18, 1536. https://doi.org/10.3390/ijms18071536
Lando GA, Marconatto L, Kessler F, Lopes W, Schrank A, Vainstein MH, Weibel DE. UV-Surface Treatment of Fungal Resistant Polyether Polyurethane Film-Induced Growth of Entomopathogenic Fungi. International Journal of Molecular Sciences. 2017; 18(7):1536. https://doi.org/10.3390/ijms18071536
Chicago/Turabian StyleLando, Gabriela Albara, Letícia Marconatto, Felipe Kessler, William Lopes, Augusto Schrank, Marilene Henning Vainstein, and Daniel Eduardo Weibel. 2017. "UV-Surface Treatment of Fungal Resistant Polyether Polyurethane Film-Induced Growth of Entomopathogenic Fungi" International Journal of Molecular Sciences 18, no. 7: 1536. https://doi.org/10.3390/ijms18071536






