Ubiquitination in Periodontal Disease: A Review
Abstract
:1. Introduction
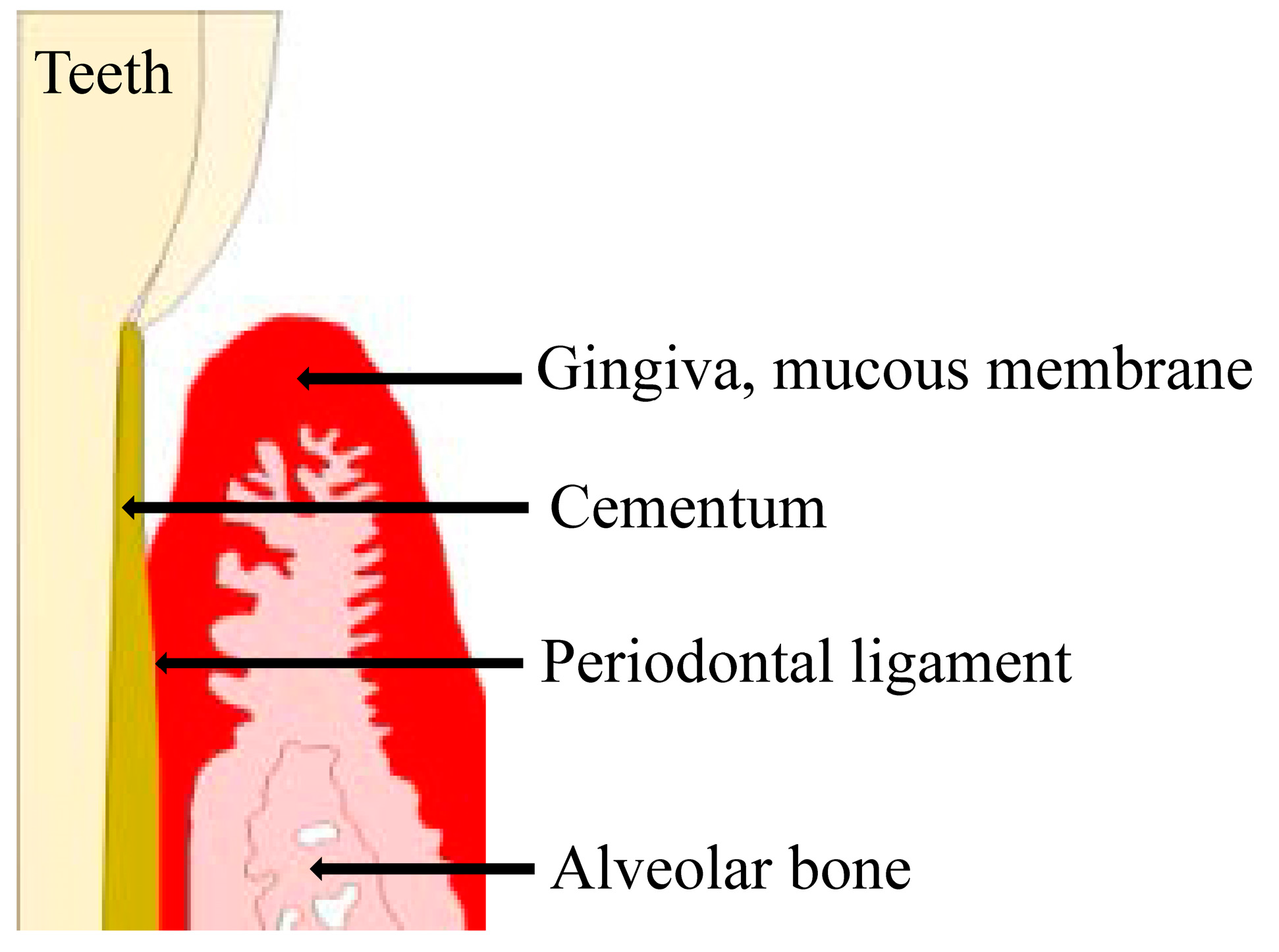
2. Periodontal Disease
3. Bacterial Pathogens of Periodontal Disease
4. Ubiquitination in Periodontal Disease
5. Future Directions for Periodontal Research
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Listgarten, M.A. Pathogenesis of periodontitis. J. Clin. Periodontol. 1986, 13, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Listgarten, M.A. The role of dental plaque in gingivitis and periodontitis. J. Clin. Periodontol. 1988, 15, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Jin, Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Wozney, J.M. Biological mediators for periodontal regeneration. Periodontol. 2000 1999, 19, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.E.; Listgarten, M.A. The gingival tissues: The architecture of periodontal protection. Periodontol. 2000 1997, 13, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M.; Scannapieco, F.A.; Wactawski-Wende, J.; Grossi, S.G.; Genco, R.J. Supragingival plaque may modify the effects of subgingival bacteria on attachment loss. J. Periodontol. 2006, 77, 808–813. [Google Scholar] [CrossRef] [PubMed]
- O’Brien-Simpson, N.M.; Pathirana, R.D.; Paolini, R.A.; Chen, Y.Y.; Veith, P.D.; Tam, V.; Ally, N.; Pike, R.N.; Reynolds, E.C. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J. Immunol. 2005, 175, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T. Periodontal bacteremia and various vascular diseases. J. Periodontal Res. 2009, 44, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, J.M.; Sylvestersen, K.B.; Bekker-Jensen, S.; Szklarczyk, D.; Poulsen, J.W.; Horn, H.; Jensen, L.J.; Mailand, N.; Nielsen, M.L. Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol. Cell Proteom. 2011, 10, M110.003590. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.J.; Jiang, X.; Birmingham, C.L.; So, N.S.; Brumell, J.H. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr. Biol. 2004, 14, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Haas, A.L.; Wilkinson, K.D. Role of ubiquitin conformations in the specificity of protein degradation: Iodinated derivatives with altered conformations and activities. Arch. Biochem. Biophys. 1986, 250, 400–409. [Google Scholar] [CrossRef]
- Kieffer, A.E.; Goumon, Y.; Ruh, O.; Chasserot-Golaz, S.; Nullans, G.; Gasnier, C.; Aunis, D.; Metz-Boutigue, M.H. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. FASEB J. 2003, 17, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Ponpuak, M.; Davis, A.S.; Roberts, E.A.; Delgado, M.A.; Dinkins, C.; Zhao, Z.; Virgin, H.W., 4th; Kyei, G.B.; Johansen, T.; Vergne, I.; et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 2010, 32, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.N. Modification-specific proteomics: Characterization of post-translational modifications by mass spectrometry. Curr. Opin. Chem. Biol. 2004, 8, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Löe, H. Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar]
- Holmstrup, P. Non-plaque-induced gingival lesions. Ann. Periodontol. 1999, 4, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2000 2004, 34, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol. 2000 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Megson, E.; Fitzsimmons, T.; Dharmapatni, K.; Bartold, P.M. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J. Clin. Periodontol. 2010, 9, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Dawson, D.R., 3rd; Morford, L.A.; Peyyala, R.; Miller, C.S.; Gonzaléz, O.A. Periodontal disease immunology: ‘Double indemnity’ in protecting the host. Periodontol. 2000 2013, 62, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Clin. Periodontol. 2013, 84, S135–S152. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, M.P.; Seymour, G.J. Periodontal disease and systemic illness: Will the evidence ever be enough? Periodontol. 2000 2013, 62, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.; Izard, J.; Sasaki, H.; Sambri, V.; Prati, C.; Müller, R.; Stashenko, P. Treponema denticola in disseminating endodontic infections. J. Dent. Res. 2006, 85, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Smith, A.J. The microbiology of the acute dental abscess. J. Med. Microbiol. 2009, 58, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontol. 2000 2010, 54, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.G.; Seers, C.A.; Tan, K.H.; Reynolds, E.C. Virulence Factors of the Oral Spirochete Treponema denticola. J. Dent. Res. 2011, 90, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Eley, B.M.; Cox, S.W. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: Characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol. 2000 2003, 31, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.M.; Shu, L.; Fu, S.M.; Liu, B.; Xu, X.L.; Wu, J.Z. P. intermedia upregulates MMP-1 and MMP-8 expression in human periodontal ligament cells. FEMS Microbiol. Lett. 2009, 299, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mättö, J.; Saarela, M.; Troil-Lindén, B.V.; Alaluusua, S.; Jousimies-Somer, H.; Asikainen, S. Similarity of salivary and subgingival Prevotella intermedia and Prevotella nigrescens isolates by arbitrarily primed polymerase chain reaction. Oral Microbiol. Immunol. 1996, 11, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, T.J.; Bosch-Tijhof, C.J.; Petit, M.D.; Van der Velden, U. Intra-familial transmission and distribution of Prevotella intermedia and Prevotella nigrescens. J. Periodontal Res. 1997, 32, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Porphyromonas gingivalis-host interactions: Open war or intelligent guerilla tactics? Microbes. Infect. 2009, 11, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kinder, S.A.; Holt, S.C. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J. Bacteriol. 1993, 175, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989, 57, 3204–3209. [Google Scholar] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect. Immun. 1989, 57, 3194–3203. [Google Scholar] [PubMed]
- Yiping, W.H. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbial. 2015, 23, 141–147. [Google Scholar]
- Strauss, J.; Kaplan, G.G.; Beck, P.L.; Rioux, K.; Panaccione, R.; DeVinney, R.; Lynch, T.; Allen-Vercoe, E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm. Bowel Dis. 2011, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- O’Brien-Simpson, N.M.; Paolini, R.A.; Hoffmann, B.; Slakeski, N.; Dashper, S.G.; Reynolds, E.C. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect. Immun. 2001, 69, 7527–7534. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Yilmaz, O. In or out: The invasiveness of oral bacteria. Periodontol. 2000 2002, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.L.; Cui, Y.Q.; Gao, R.; Li, Y.; Fu, Z.C.; Zhang, B.; Guan, C.C. Study of TNF-α, IL-1β and LPS levels in the gingival crevicular fluid of a rat model of diabetes mellitus and periodontitis. Dis. Markers 2013, 34, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Kaneko, T.; Ukai, T.; Yokoyama, M.; Ayon Haro, E.R.; Yoshinaga, Y.; Yoshimura, A.; Hara, Y. Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J. Periodontal Res. 2012, 47, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Ayon Haro, E.R.; Ukai, T.; Yokoyama, M.; Kishimoto, T.; Yoshinaga, Y.; Hara, Y. Locally administered interferon-γ accelerates lipopolysaccharide-induced osteoclastogenesis independent of immunohistological RANKL upregulation. J. Periodontal Res. 2011, 46, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Julie, A. Prokaryotic Ubiqutin-like protein modification. Annu. Rev. Microbiol. 2014, 68, 155–175. [Google Scholar]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and Toll-like receptor (TLR) signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Panek, J.S.; Amar, S. Convergent Synthesis of Novel Muramyl Dipeptide Analogues: Inhibition of Porphyromonas gingivalis-Induced Pro-inflammatory Effects by High Doses of Muramyl Dipeptide. J. Med. Chem. 2016, 59, 6878–6890. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Forsberg, K.; Bischof, F. The role of the ubiquitin-editing enzyme A20 in diseases of the central nervous system and other pathological processes. Front. Mol. Neurosci. 2015, 15. [Google Scholar] [CrossRef]
- Hong, J.Y.; Bae, W.J.; Yi, J.K.; Kim, G.T.; Kim, E.C. Anti-inflammatory and anti-osteoclastogenic effects of zinc finger protein A20 overexpression in human periodontal ligament cells. J. Periodontal Res. 2016, 51, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Umemura, H.; Sogawa, K.; Kawashima, Y.; Kado, S.; Sawai, S.; Nishimura, M.; Kodera, Y.; Matsushita, K.; et al. Proteomic analysis of gingival crevicular fluid for discovery of novel periodontal disease markers. Proteomics 2012, 12, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Wakabayashi, M.; Ishige, T.; Beppu, M.; Nishimura, M.; Matsushita, K.; Nomura, F. Detection of Ubiquitinated Dermcidin in Gingival Crevicular Fluid in Periodontal Disease. Int. J. Pept. Res. Ther. 2016, 22, 249–253. [Google Scholar] [CrossRef]
- Ghosh, A.; Joo, N.E.; Chen, T.C.; Kapila, Y.L. Proapoptotic fibronectin fragment induces the degradation of ubiquitinated p53 via proteasomes in periodontal ligament cells. J. Periodontal Res. 2010, 45, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Chen, G.; Zhao, Y.; Zhao, Y.F.; Liu, B. Increased expression of autophagy-related proteins in keratocystic odontogenic tumours: Its possible association with growth potential. Br. J. Oral Maxillofac. Surg. 2014, 52, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zeidán-Chuliá, F.; Gürsoy, M.; Neves de Oliveira, B.H.; Özdemir, V.; Könönen, E.; Gürsoy, U.K. A Systems Biology Approach to Reveal Putative Host-Derived Biomarkers of Periodontitis by Network Topology Characterization of MMP-REDOX/NO and Apoptosis Integrated Pathways. Front. Cell. Infect. Microbiol. 2016, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, H.F.; Ang, E.S.; Yip, K.; Woloszyn, M.; Zheng, M.H.; Tan, R.X. NF-κB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009, 20, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Jiang, B.; Feng, T.; Xue, J.; Yang, J.; Chen, Z.; Liu, J.; Wei, R.; Zhao, S.; Wang, X.; et al. Expression profiling of white sponge nevus by RNA sequencing revealed pathological pathways. Orphanet J. Rare Dis. 2015, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. The proteasome: Structure, function, and role in the cell. Cancer Treat. Rev. 2003, 29 (Suppl. S1), 3–9. [Google Scholar] [CrossRef]
- Jiang, L.; Song, J.; Hu, X.; Zhang, H.; Huang, E.; Zhang, Y.; Deng, F.; Wu, X. The Proteasome Inhibitor Bortezomib Inhibits Inflammatory Response of Periodontal Ligament Cells and Ameliorates Experimental Periodontitis in Rats. J. Periodontol. 2016, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, J.; Miyauchi, S.; Xie, C.J.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. Effects of the proteasome inhibitor, bortezomib, on cytodifferentiation and mineralization of periodontal ligament cells. J. Periodontal Res. 2015, 50, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Dale, B.A. Actvation of protective response in oral epipithelial cells by Fusobacterium nucleatum and human β-defensin-2. J. Med. Microbial. 2007, 56, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.J. Regulation of NF-κB by ubiqutination. Curr. Opin. Immunol. 2013, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Hong, I.S.; Oh, S.; Cho, S.D.; Lee, K.E. Direct effect of streptozotocin on periodontal ligament cells through myeloid cell leukemia-1. J. Periodontal Res. 2015, 50, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Herrmann, J.; Gossl, M.; Mannheim, D.; Sattler, K.; Meyer, F.B.; Lerman, L.O.; Lerman, A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Maldonado, M.A. The Ubiquitin-Proteasome System and Its Role in Inflammatory and Autoimmune Diseases. Cell. Mol. Immunol. 2006, 3, 255–261. [Google Scholar] [PubMed]
- Stewart, H.L.; Alfred, L.G.; William, E.M. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease Statesome inhibitors at the gene and protein expression profile levels. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar]
- Doris, P.; Domagoj, V.; Ivan, D. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar]
| Authors | Research Object (Bacteria, Cell, Fluid, or Tissues) | Methodolgy | Essence of a Discourse or Summary of Results | Reference |
|---|---|---|---|---|
| Maekawa, et al., 2014 | Porphyromonas gingivalis, Human neutrophils | Innate pattern recognition of P.g is predominantly mediated by TLR2, which synergizes with C5aR during periodontal inflammation. The authors examined Pg interactions with both C5aR and TLR2 using knockout mice and specific inhibitors. | Porphyromonas gingivalis (P.g)-induced co-activation of toll-like receptor 2 (TLR2) and the C5a receptor (C5aR) in neutorophils; the resulting crosstalk leads to ubiqutination and proteasomal degradation of MyD88, thereby inhibiting a host-protective antimicrobial response. This activity requires the C5aR/TLR2- dependent release of transforming growth factor-β1, which mediates ubiquitin-proteasome degradation of MyD88 via the E3 ubiqutin ligase Smad ubiquitin regulatory factor 1. MyD88 is unlikely to contribute to immune evasion mediated by the P.g-induced C5a-TLR2 crosstalk; however, it contributes to P.g infection clearance. | [52] |
| Cai et al., 2016 | Porphyromonas gingivalis | P.g.-exposed macrophages were treated with 10 μg/mL MDP (MDP-low) up-regulated TNF-α by 29%, while 100 μg/mL or higher (MDP-high) significantly decreased the level of TNF-α (16–38%). | API activates the ubiquitin-editing enzyme A20 and restricts ubiquitination of nucleotide-binding oligomerization domain-containing protein 2, consequently inhibiting TNF-α secretion in response to P. gingivalis infection. | [53] |
| Hong, et al., 2016 | Gingival tissues, Human periodontal ligament cell | The concentration of prostaglandin E2 was measured by a radioimmunoassay. Reverse transcription-polymerase chain reactions and Western blot analyses were used to measure the mRNA and protein levels, respectively. Osteoclastic differentiation was assessed in mouse bone marrow-derived macrophages using conditioned medium from LPS- and nicotine-treated hPDLCs. | The ubiquitin-edting protein A20 was upregulated in the gingival tissues and neutrophils of patients with periodontal disease and in LPS-exposed human periodontal ligament cells. | [56] |
| Tsuchida, et al., 2016 | Gingival crevicular fluid | The authors explored the considerable variation in the molecular weights of protein bands using on-membrane digestion and liquid chromatography-tandem mass spectrometry (LC–MS/MS) analyses. In immunoprecipitation experiments, ubiquitin DCD was detected by Western blotting and by immunoprecipitation. | In immunoprecipitation experiments, ubiquitinated antimicrobial peptide dermcidin (DCD) in GCF was detected using Western blotting and immunoprecipitation with antibodies against DCD and mono-/poly-ubiquitinated proteins. | [58] |
| Ghosh, et al., 2010 | Periodontal ligament cells | The authors used immunofluorescence, transfection assays, Western blotting, and ELISAs to show that p53 is degraded by a proteasome pathway in response to a proapoptotic disease-associated fibronectin fragment. | Investigated whether fibronectin fragments induce ubiqutination of p53 and its degradation by the proteasome. Inhibiting either the proteolytic function of the proteasome or suppressing ubiquitin at the protein level prevented degradation of p53 and subsequent apoptosis of primary periodontal ligament cells. | [59] |
| Li, et al., 2012 | Keratocytic odontogenic tumors | They detected the expression of some key autophagy-related proteins in the clinical samples of keratocystic odontogenic tumors (KCOT) and radicular cysts and compared them via real-time quantitative polymerase chain reaction (qPCR) and immunohistochemical analysis, respectively. The correlation between the tested autophagy-related proteins with cell antiapoptotic (Bcl-2) or proliferative (Ki-67) markers in KCOT was explored using a Spearman’s rank correlation, followed by a cluster analysis. | Evaluated the activation status of autophagy in keratocystic odontogenic tumors (KCOT) and detected and compared the expression patterns of some key autophagy-related proteins in clinical samples of KCOT and radicular cysts. Implicated the activation of autophagy in KCOT and showed a possible association with growth potential. | [60] |
| Zeidán-Chuliá, et al., 2016 | Periodontitis-associated bacteria, Human oral neutrophils | Using systems biologytools, the authors aimed to: (1) identify an integrated interactome between matrix metallo proteinase (MMP)-REDOX/nitric oxide (NO) and apoptosis pathways upstream of periodontal inflammation; and (2) characterize the attendant topological network properties to uncover putative biomarkers to be tested in the saliva of patients with periodontitis. | Found Ubiqutin C (UBC), Jun proto-oncogene (JUN), and matrix metalloproteinase-14 (MMP-14) as the most central hub- and non-hub-bottlenecks among the 211 genes/proteins of the whole interactome. Described that UBC, JUN, and MMP-14 are likely an optimal candidate group of host-derived biomarkers, in combination with oral pathogenic bacteria-derived proteins, for detecting periodontitis in its early phase. | [61] |
| Cai, et al., 2015 | Oral epidermis tissues and epithelial cells from the White sponge nevus patients | Sequence analysis of samples from a WSN Chinese family revealed a mutation (332 T > C) in the KRT13 gene that resulted in the amino acid change Leu111Pro. The pathological pathway behind the WSN expression profile was investigated using RNA sequencing (RNA-seq). | Investigated the pathogenesis of white sponge nevus (WSN), a rare periodontal hereditary disease, by expression profiling and found that the ribosome structure was damaged and the translation rate was limited in WSN patients, while ubiquitin-mediated proteolysis was enhanced. This study concluded that the abnormal degradation of keratin 13 protein in WSN patients may be associated with keratin 7 protein and an abnormal ubiquitination process. | [63] |
| Jiang, et al., 2016 | Periodontal ligament cells and ameliorates experimental periodontitis in rats | hPDLCs were treated with lipopolysaccharide (LPS) and pretreated with bortezomib (BTZ). mRNA and protein levels of tumor necrosis factor (TNF)-alpha, interleukin (IL)-1β, IL-6, and IL-8 were determined. The anti-inflammatory mechanism of BTZ was studied. Furthermore, experimental rat periodontitis was induced with ligature and LPS injection, and simultaneously and locally treated with BTZ (three injections/week). Four weeks after treatment, microcomputed tomography, immunohistochemistry, and histopathologic analyses were performed. | Bortezomib (BTZ) was the first proteasome inhibitor for clinical treatment of malignancies. The anti-cancer activity of BTZ is accompanied by an anti-inflammatory effect. Jiang et al., (2016) reported that in an LPS- and ligature-induced periodontal disease rat model, BTZ suppressed the expression of TNF-α, IL-1β, IL-6, and IL-8, reduced the ratio of receptor activation of RANKL/osteoprotegerin, and prevented alveolar bone resorption, suggesting that the anti-inflammatory activity of BTZ has a promising therapeutic effect against periodontal inflammatory responses in periodontal disease. | [65] |
| Kitagaki, et al., 2015 | Periodontal ligament cells | A mouse PDL clone cell line, MPDL22, was cultured in mineralization medium in the presence or absence of bortezomib. The expression of calcification-related genes and calcified-nodule formation was evaluated by real-time PCR and Alizarin Red staining, respectively. | Investigated whether BTZ can induce differentiation of PDL cells into hard tissue-forming cells and found that BTZ enhanced the expression of bone morphogenetic protein-2, which induces cytodifferentiation and mineralization of PDL cells. BTZ induced cytodifferentiation of PDL cells by enhancing the accumulation of B-catenin within the cytosol and nucleus, suggesting that BTZ may be efficacious for use in periodontal regeneration therapy. | [66] |
| Yin, et al., 2007 | Fusobacterium nucleatum, Oral epipithelial cells | Human b-defensin-2 (hBD2) is expressed in normal oral tissue leading to the hypothesis that oral epithelial cells are in an activated state with respect to innate immune responses under normal in vivo conditions. To test this hypothesis, global gene expression was evaluated in GECs in response to stimulation by an F. nucleatum cell wall (FnCW) preparation and hBD2 peptide. FnCW treatment altered 829 genes, while hBD2 altered 209 genes (P,0.005, ANOVA). | F. nucleatum and its cell wall extracts induce the expression of human beta-defensin-2 (hBD2), an antimicrobial and immunomodulatory peptide, in cultured primary human gingival epithelial cells in vitro. F. nucleatum cell wall extracts upregulated the expression of multiple protease inhibitors and suppressed NF-κB function and the ubiquitin/proteasome system. Both F. nucleatum cell wall extracts and hBD2 upregulated genes that may enhance the gingival epithelial barrier. | [67] |
| Shin et al., 2015 | Periodontal ligament cells | To assess the apoptotic effects of STZ on periodontal ligament cells (PDLs), they were treated with or without different concentrations of STZ. Qualitative estimation of apoptotic cell death was obtained via a live/dead assay. The expression levels of apoptosis-related proteins were evaluated by a Western blot analysis. | Streptozotocin (STZ, 2-deoxy-2-3(3-(methyl-3-nitrosoureid)-d-glucopyranose) treatment dramatically reduces Mcl-1 (which induces myeloid leukemia cell differentiation protein) expression in a proteasome-dependent manner, thereby suppressing the growth of PDL cells through the Bax/Bak apoptotic signaling pathway. STZ may play an important role in inducing PDL cell apoptosis as a potential direct inducer of periodontitis in an STZ-induced diabetic animal. | [69] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Ubiquitination in Periodontal Disease: A Review. Int. J. Mol. Sci. 2017, 18, 1476. https://doi.org/10.3390/ijms18071476
Tsuchida S, Satoh M, Takiwaki M, Nomura F. Ubiquitination in Periodontal Disease: A Review. International Journal of Molecular Sciences. 2017; 18(7):1476. https://doi.org/10.3390/ijms18071476
Chicago/Turabian StyleTsuchida, Sachio, Mamoru Satoh, Masaki Takiwaki, and Fumio Nomura. 2017. "Ubiquitination in Periodontal Disease: A Review" International Journal of Molecular Sciences 18, no. 7: 1476. https://doi.org/10.3390/ijms18071476





