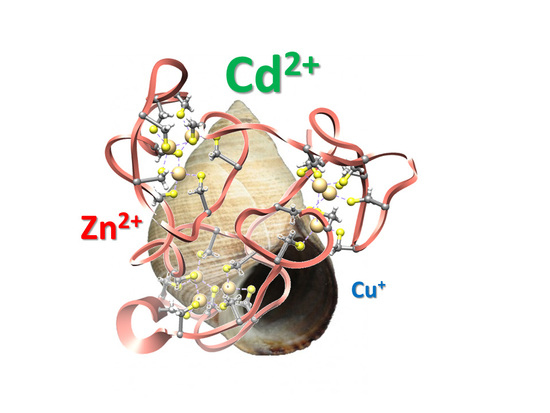
Analysis of Metal-Binding Features of the Wild Type and Two Domain-Truncated Mutant Variants of Littorina littorea Metallothionein Reveals Its Cd-Specific Character
Abstract
:1. Introduction
2. Results and Discussion
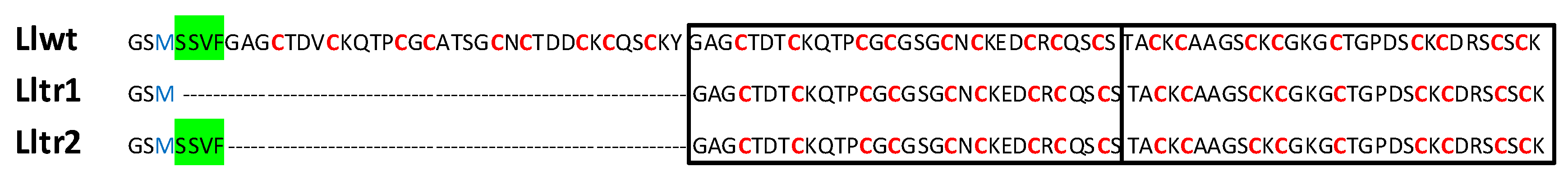
2.1. Characterization of the Metallothionein (MT) System of Littorina littorea
2.2. The LlwtMT, Lltr1MT and Lltr2MT Recombinant Polypeptides
2.3. Zn(II) and Cd(II) Binding Capabilities of LlwtMT, Lltr1MT, and Lltr2MT
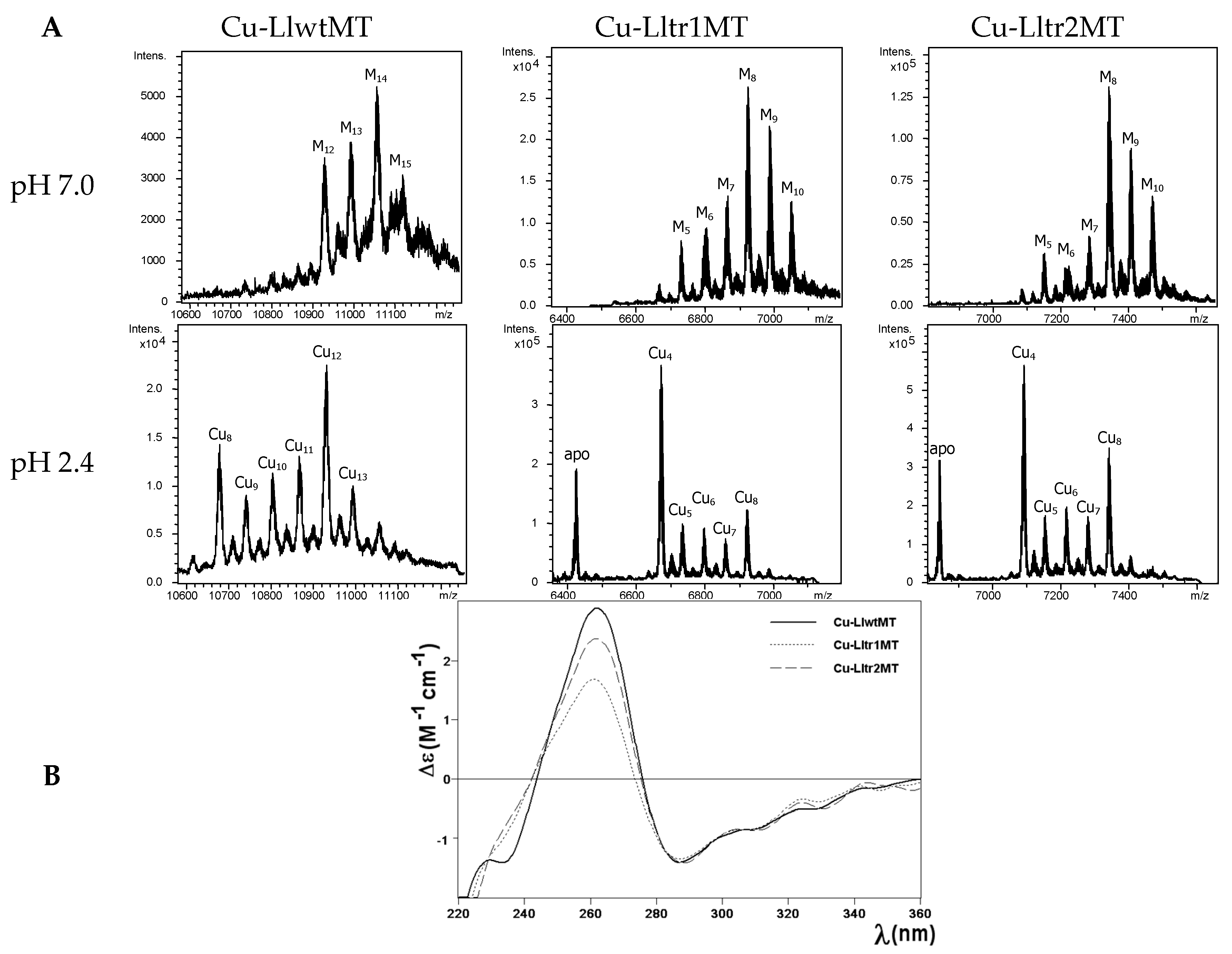
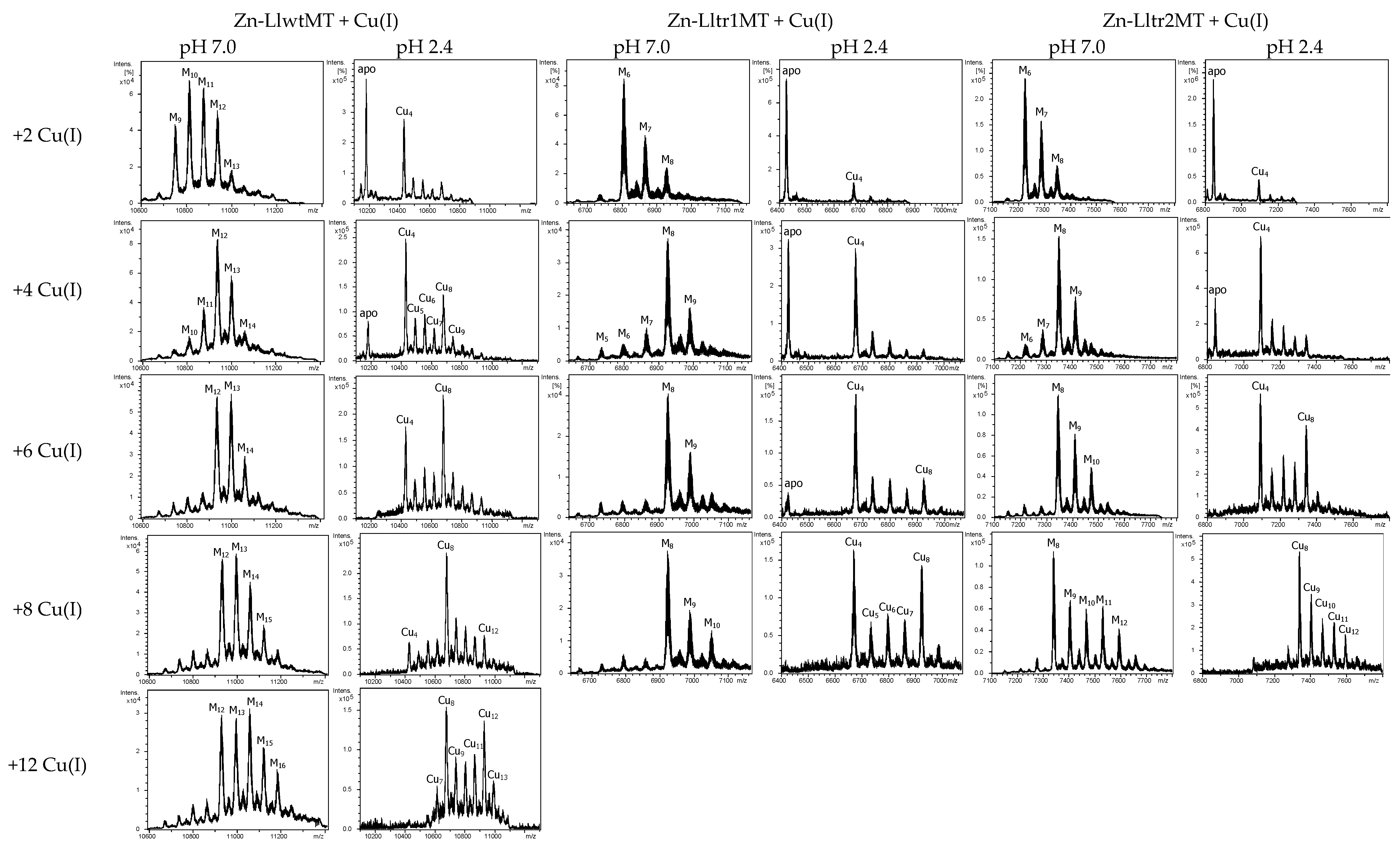
2.4. Cu(I) Binding Capabilities of LlwtMT, Lltr1MT, and Lltr2MT
3. Materials and Methods
3.1. Confirmation of the MT System of Littorina littorea
3.2. Construction and Cloning of the cDNAs Encoding the LlwtMT, Lltr1MT, and Lltr2MT Proteins
3.3. Synthesis and Purification of the Recombinant Zn-, Cd-, and Cu-Complexes of LlwtMT and the Lltr1MT and Lltr2MT Mutants
3.4. Zn(II)/Cd(II) and Zn(II)/Cu(I) Replacement Reactions in the Zn(II)-LlMT Proteins
3.5. Spectroscopic Analyses (ICP-AES, UV-Vis and CD) of the Metal Complexes Formed by the Llmt Proteins
3.6. Electrospray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF MS) of the Metal Complexes Obtained from the LlMT Proteins
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Binz, P.A.; Kägi, J.H.R. Metallothionein: Molecular evolution and classification. In Metallothionein IV; Klassen, C.D., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 7–13. [Google Scholar]
- Iturbe-Espinoza, P.; Gil-Moreno, S.; Lin, W.; Calatayud, S.; Palacios, O.; Capdevila, M.; Atrian, S. The fungus Tremella mesenterica encodes the longest metallothionein currently known: Gene, protein and metal binding characterization. PLoS ONE 2016, 4, e0148651. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Bofill, R.; Palacios, O.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Blindauer, C. Metallothioneins. In RSC Metallobiology: Binding, Transport and Storage of Metal Ions in Biological Cells; Maret, W., Wedd, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; Volume 2, pp. 594–653. [Google Scholar]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Berger, B.; Hunziker, P.E.; Kägi, J.H.R. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 388, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H.R.; Kojima, Y. Chemistry and biochemistry of metallothionein. In Metallothionein II; Kägi, J.H.R., Kojima, Y., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1987; pp. 25–61. [Google Scholar]
- Valls, M.; Bofill, R.; González-Duarte, R.; González-Duarte, P.; Capdevila, M.; Atrian, S. A new insight into metallothionein classification and evolution. The in vivo and in vitro metal binding features of Homarus americanus recombinant MT. J. Biol. Chem. 2001, 276, 32835–32843. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Capdevila, M.; Atrian, S. Independent metal-binding features of recombinant metallothioneins convergently draw a step gradation between Zn- and Cu-thioneins. Metallomics 2009, 1, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lieb, B.; Altenheim, B.; Markl, J. The sequence of a gastropod hemocyanin (HtH1 from Haliotis tuberculata). J. Biol. Chem. 2000, 275, 5675–5681. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Niederstaetter, H.; Thaler, R.; Hödl, E.; Parson, W.; Rossmanith, W.; Dallinger, R. Localisation and quantification of Cd- and Cu-specific metallothionein isoform mRNA in cells and organs of the terrestrial gastropod Helix pomatia. Toxicol. Appl. Pharmacol. 2003, 190, 25–36. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hünziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) is regulated by one single cell type: Differently responsive metal pools in rhogocytes. Am. J. Physiol. 2005, 189, R1185–R1195. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pérez-Rafael, S.; Pagani, A.; Dallinger, R.; Atrian, S.; Capdevila, M. Cognate and noncognate metal ion coordination in metal-specific metallothioneins: The Helix pomatia system as a model. J. Biol. Inorg. Chem. 2014, 19, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moreno, S.; Jiménez-Martí, E.; Palacios, O.; Zerbe, O.; Dallinger, R.; Capdevila, M.; Atrian, S. Does variation of the inter-domain linker sequence modulate the metal binding behaviour of Helix pomatia Cd-metallothionein? Int. J. Mol. Sci. 2016, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hispard, F.; Schuler, D.; de Vaufleury, A.; Scheifler, R.; Badot, P.M.; Dallinger, R. Metal distribution and metallothionein induction after cadmium exposure in the terrestrial snail Helix aspersa (Gastropoda, Pulmonata). Environ. Toxicol. Chem. 2008, 27, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Höckner, M.; Stefanon, K.; de Vaufleury, A.; Monteiro, F.; Pérez-Rafael, S.; Palacios, Ò.; Capdevila, M.; Atrian, S.; Dallinger, R. Physiological relevance and contribution to metal balance of specific and non-specific metallothionein isoforms in the garden snail, Cantareus aspersus. Biometals 2011, 24, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rafael, S.; Monteiro, F.; Dallinger, R.; Atrian, S.; Palacios, Ò.; Capdevila, M. Cantareus aspersus metallothionein metal binding abilities: The unspecific CaCd/CuMT isoform provides hints about the metal preference determinants in metallothioneins. Biochim. Biophys. Acta 2014, 1844, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Beil, A.; Jurt, S.; Niederwanger, M.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R.; Zerbe, O. Structural adaptation of a protein to increased metal stress: NMR structure of a marine snail metallothionein with an additional domain. Angew. Chem. Int. Ed. 2017, 56, 4617–4622. [Google Scholar] [CrossRef] [PubMed]
- English, T.E.; Storey, K.B. Freezing and anoxia stresses induce expression of metallothionein in the foot muscle and hepatopancreas of the marine gastropod Littorina littorea. J. Exp. Biol. 2003, 206, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Villarreal, L.; Capdevila, M.; Atrian, S. The Saccharomyces cerevisiae Crs5 metallothionein metal-binding abilities and its role in the response to zinc overload. Mol. Microbiol. 2007, 63, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; Domenech, J.; Bofill, R.; You, C.; Mackay, E.A.; Kägi, J.H.R.; Capdevila, M.; Atrian, S. The metal-binding features of the recombinant mussel Mytilus edulis MT-10-IV metallothionein. J. Biol. Inorg. Chem. 2008, 13, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Espart, A.; Espín, J.; Ding, C.; Thiele, D.J.; Atrian, S.; Capdevila, M. Full characterization of the Cu-, Zn- and Cd-binding properties of CnMT1 and CnMT2, two metallothioneins of the pathogenic fungus Cryptococcus neoformans acting as virulence factors. Metallomics 2014, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Cols, N.; Romero-Isart, N.; Gonzalez-Duarte, R.; Atrian, S.; Gonzalez-Duarte, P. Recombinant synthesis of mouse Zn3-(and Zn4-(metallothionein 1 domains and characterization of their cadmium(II) binding capacity. Cell. Mol. Life Sci. 1997, 53, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Palacios, O.; Capdevila, M.; Cols, N.; Gonzalez-Duarte, R.; Atrian, S.; Gonzalez-Duarte, P. A new insight into the Ag+ and Cu+ binding sites in metallothionein β domain. J. Inorg. Biochem. 1999, 73, 57–64. [Google Scholar] [CrossRef]
- Bongers, J.; Walton, C.D.; Richardson, D.E.; Bell, J.U. Micromolar protein concentrations and metalloprotein stoichiometries obtained by inductively coupled plasma. Atomic emission spectrometric determination of sulfur. Anal. Chem. 1988, 60, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Domenech, J.; Pagani, A.; Tio, L.; Villarreal, L.; Atrian, S. Zn- and Cd-metallothionein recombinant species from the most diverse phyla may contain sulfide (S2-) ligands. Angew. Chem. Int. Ed. Engl. 2005, 44, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Zaia, J.; Hathout, Y.; Fenselau, C. Retention of thiol protons in two classes of protein zinc ion coordination centers. J. Am. Chem. Soc. 1996, 118, 12242–12243. [Google Scholar] [CrossRef]

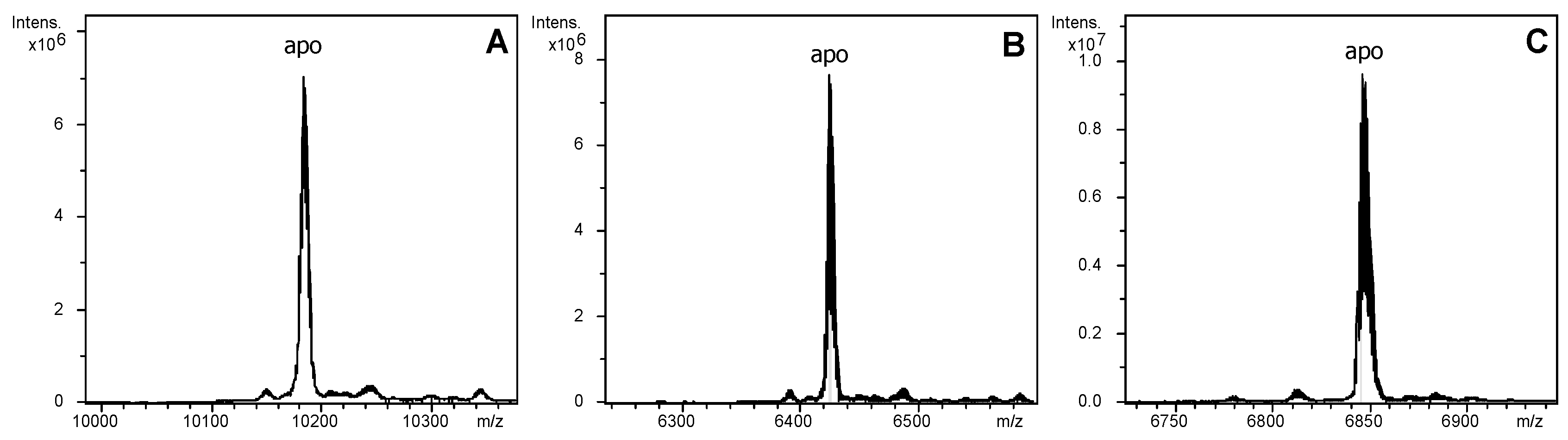
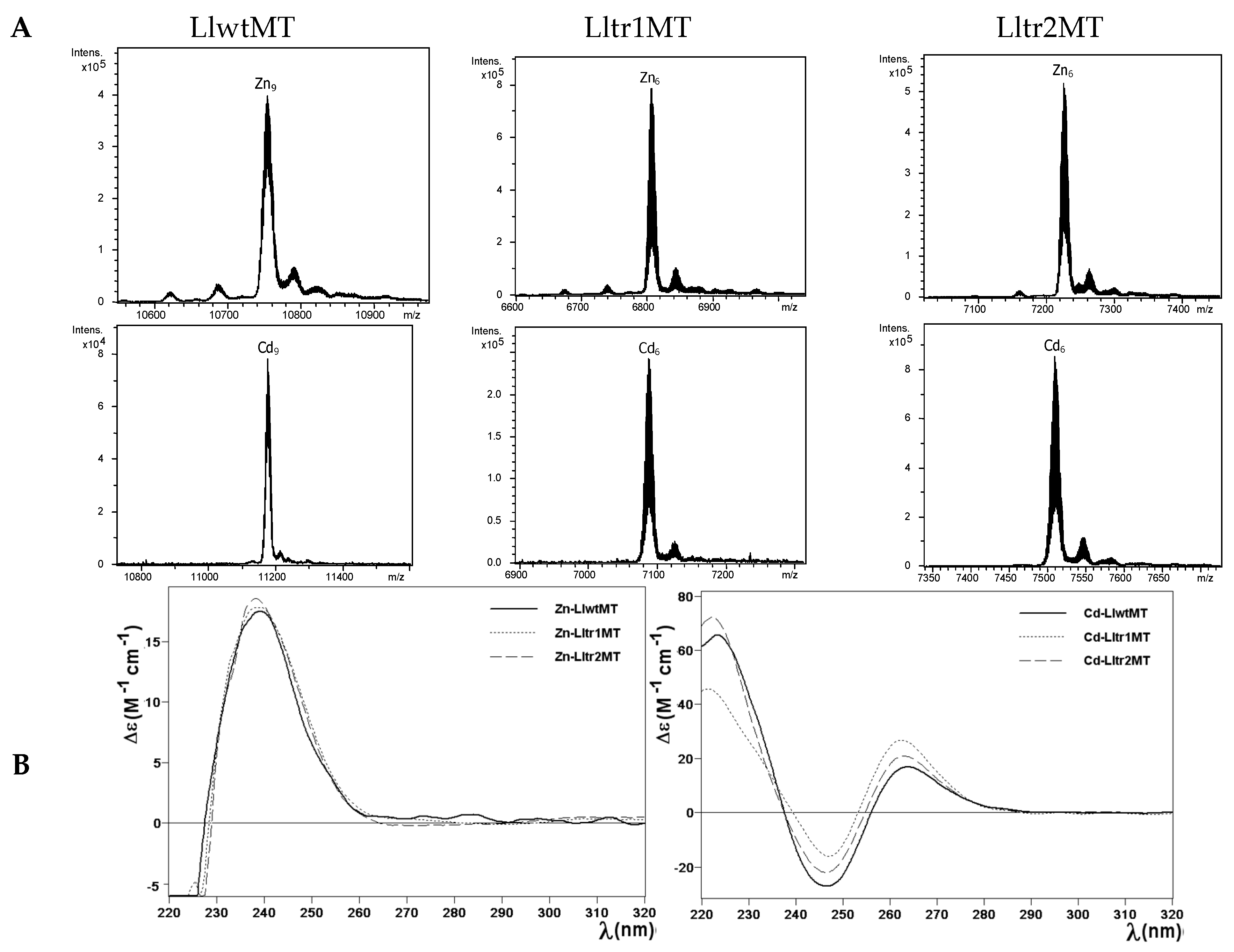


| MT | ICP-AES a | Neutral ESI-MS b | Experimental MM c | Theoretical MM d |
|---|---|---|---|---|
| LlwtMT | 9.5 | Zn9-MT | 10,753 | 10,754.1 |
| Lltr1MT | 5.9 | Zn6-MT | 6807 | 6806.7 |
| Lltr2MT | 6.1 | Zn6-MT | 7226 | 7227.2 |
| LlwtMT | 8.7 | Cd9-MT | 11,177 | 11,177.3 |
| Lltr1MT | 6.3 | Cd6-MT | 7089 | 7088.8 |
| Lltr2MT | 6.5 | Cd6-MT | 7509 | 7509.3 |
| MT | ICP-AES a | Neutral ESI-MS b | Exp MM c | Theor MM d | Acidic ESI-MS b | Exp MM c | Theor MM d |
|---|---|---|---|---|---|---|---|
| LlwtMT | 4.0 Zn 13.3 Cu | M14-MT M13-MT M12-MT M15-MT | 11,058 10,995 10,934 11,122 | 11,059.3 10,996.7 10,934.2 11,121.8 | Cu12-MT | 10,934 | 10,934.2 |
| Cu8-MT | 10,682 | 10,684.0 | |||||
| Cu11-MT | 10,869 | 10,871.6 | |||||
| Cu10-MT | 10,808 | 10,809.1 | |||||
| Cu13-MT | 10,995 | 10,996.7 | |||||
| Cu9-MT | 10,747 | 10,746.5 | |||||
| Cu14-MT | 11,061 | 11,059.3 | |||||
| Lltr1MT | 3.5 Zn 5.8 Cu | M8-MT M9-MT M10-MT M7-MT M6-MT | 6925 6988 7050 6862 6796 | 6926.8 6989.3 7051.9 6864.2 6801.7 | Cu4-MT | 6675 | 6676.6 |
| apo-MT | 6425 | 6426.4 | |||||
| Cu8-MT | 6925 | 6926.8 | |||||
| Cu5-MT | 6738 | 6739.1 | |||||
| Cu6-MT | 6801 | 6801.7 | |||||
| Cu7-MT | 6865 | 6864.2 | |||||
| Lltr2MT | 3.2 Zn 6.5 Cu | M8-MT M9-MT M10-MT M7-MT M6-MT | 7344 7407 7471 7285 7224 | 7347.2 7409.8 7472.3 7284.7 7222.1 | Cu4-MT | 7095 | 7097.0 |
| apo-MT | 6845 | 6846.8 | |||||
| Cu8-MT | 7344 | 7347.2 | |||||
| Cu5-MT | 7161 | 7159.6 | |||||
| Cu6-MT | 7220 | 7222.1 | |||||
| Cu7-MT | 7286 | 7284.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, Ò.; Jiménez-Martí, E.; Niederwanger, M.; Gil-Moreno, S.; Zerbe, O.; Atrian, S.; Dallinger, R.; Capdevila, M. Analysis of Metal-Binding Features of the Wild Type and Two Domain-Truncated Mutant Variants of Littorina littorea Metallothionein Reveals Its Cd-Specific Character. Int. J. Mol. Sci. 2017, 18, 1452. https://doi.org/10.3390/ijms18071452
Palacios Ò, Jiménez-Martí E, Niederwanger M, Gil-Moreno S, Zerbe O, Atrian S, Dallinger R, Capdevila M. Analysis of Metal-Binding Features of the Wild Type and Two Domain-Truncated Mutant Variants of Littorina littorea Metallothionein Reveals Its Cd-Specific Character. International Journal of Molecular Sciences. 2017; 18(7):1452. https://doi.org/10.3390/ijms18071452
Chicago/Turabian StylePalacios, Òscar, Elena Jiménez-Martí, Michael Niederwanger, Selene Gil-Moreno, Oliver Zerbe, Sílvia Atrian, Reinhard Dallinger, and Mercè Capdevila. 2017. "Analysis of Metal-Binding Features of the Wild Type and Two Domain-Truncated Mutant Variants of Littorina littorea Metallothionein Reveals Its Cd-Specific Character" International Journal of Molecular Sciences 18, no. 7: 1452. https://doi.org/10.3390/ijms18071452