Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents
Abstract
:1. Mesenchymal Stem/Stromal Cells in Cell and Immune Therapies
2. MSCs Exert Therapeutic Functions in a Paracrine Manner
3. MSCs Exert Their Therapeutic Effects via Microvesicles and Exosomes
4. Extracellular Vesicles
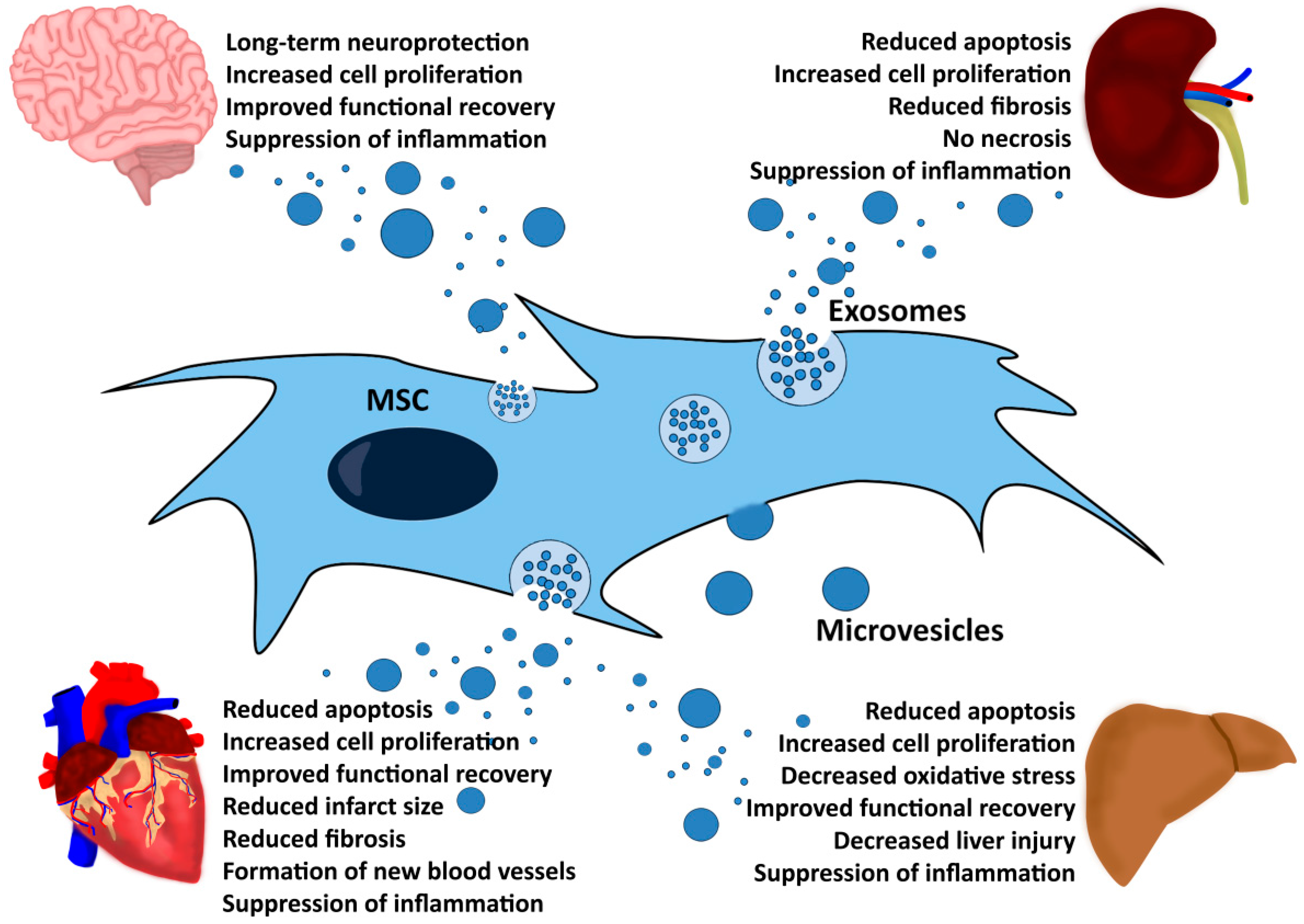
5. MSC-EVs Exert Therapeutic Functions in Different Disease Models
6. MSC-EV Production Strategies
7. Application of MSC-EVs in Animal Models
8. MSC-EVs in the Clinics
9. MSC-EVs as a Novel Therapeutic Agent
10. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luria, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92. [Google Scholar] [PubMed]
- Pittenger, F.M.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringden, O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Ringden, O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2005, 11, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress t-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Heldring, N.; Mager, I.; Wood, M.J.; le Blanc, K.; Andaloussi, S.E. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hmscs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein Tsg-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The Msc: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; el Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Vasculotropic, C.W. Paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Renal Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Borges, F.T.; Simoes, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–3611. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Sarukhan, A.; Dander, E.; Castor, M.; Cibella, J.; Soldani, C.; Trovato, A.E.; Ploia, C.; Luca, G.; Calvitti, M.; et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 2013, 27, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; el Oakley, R.M.; et al. Exosome secreted by msc reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As We wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Giebel, B.; Wodarz, A. Tumor suppressors: Control of signaling by endocytosis. Curr. Biol. 2006, 16, R91–R92. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Ludwig, A.K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. Evpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. Berl. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; da Silva, A.C.; del Portillo, H.; el Andaloussi, S.; et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Iacono, M.L.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mrna. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mrna and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrol. Carlton 2012, 17, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Gu, D.; Xing, X.; Cheng, Z.; Gong, D.; Zhang, G.; Zhu, Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am. J. Transl. Res. 2016, 8, 4289–4299. [Google Scholar] [PubMed]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human wharton’s jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Yip, H.K.; Shao, P.L.; Wu, S.C.; Chen, K.H.; Chen, Y.T.; Yang, C.C.; Sun, C.K.; Kao, G.S.; Chen, S.Y.; et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int. J. Cardiol. 2016, 216, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Lemke, A.; Lange, C. Extracellular vesicles from msc modulate the immune response to renal allografts in a mhc disparate rat Model. Stem Cells Int. 2015, 2015, 486141. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl. Med. 2016, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. Mirna-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Cao, W.; Ma, J.; Sun, L.; Qian, H.; Zhu, W.; Xu, W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015, 2015, 761643. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from mscs ameliorate retinal laser injury partially by inhibition of Mcp-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from gata-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic micrornas for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiang, B.; Wang, X.; Xiang, C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. Hucmsc exosome-derived gpx1 is required for the recovery of hepatic oxidant injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+ CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous Administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and admsc-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. Microrna cluster miR-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. miR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microrna 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017, 26, 243–257. [Google Scholar] [PubMed]
- Hu, B.; Chen, S.; Zou, M.; He, Z.; Shao, S.; Liu, B. Effect of extracellular vesicles on neural functional recovery and immunologic suppression after rat cerebral apoplexy. Cell. Physiol. Biochem. 2016, 40, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Laso-Garcia, F.; Frutos, M.D.G.; Rodriguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci. Rep. 2017, 7, 44433. [Google Scholar] [CrossRef] [PubMed]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.K.; Duhan, V.; Radtke, S.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhang, Z.; Zhang, S.; Yang, H.; Zhang, X.; Pan, J.; Weng, L.; Sha, D.; Zhu, M.; Hu, X.; et al. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain. Res. 2015, 1594, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cell secretes immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, R.; Sun, X.; Duan, Y.; Han, Y.; Zhao, Y.; Qian, H.; Zhu, W.; Xu, W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 2016, 18, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/AKT pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Rager, T.M.; Olson, J.K.; Zhou, Y.; Wang, Y.; Besner, G.E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 2016, 51, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. Hucmsc-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal stem cell microvesicles for treatment of escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, H.; Qin, D.; Yang, L.; Huang, W.; Essandoh, K.; Wang, Y.; Caldwell, C.C.; Peng, T.; Zingarelli, B.; et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci. Rep. 2015, 5, 13721. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.X.; Fan, H.; Tang, Q.; Shou, Z.X.; Zuo, D.M.; Zou, Z.; Xu, M.; Chen, Q.Y.; Peng, Y.; et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS ONE 2015, 10, e0140551. [Google Scholar] [CrossRef] [PubMed]
| Ref. | EV Harvesting Conditioning | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease | MSC Origin | Tissue Source | MSC Isolation | Supplement | Media | Time | EV Isolation | Pre-Processing | Filter | Final Purification Step | Characterization | Tested EV-Marker | |||||||||
| NTA/DLS | Protein | TEM | FLOW | Other | CD9 | CD63 | CD81 | TSG101 | Other | ||||||||||||
| [41] | AKI | Human | UC | unfractionated | 10% | serum free | 48 h | UF + sucrose + UC | 1000× g, 2000× g, 10,000× g | 100 kDa, 0.22 µm | 100,000× g, 60 min | ||||||||||
| [20] | AKI | Human | BM | Ficoll | 10% FBS | 0.5% BSA | o.n. | UC | 2000× g | 100,000× g, 2 × 60 min | |||||||||||
| [40] | AKI | Human | BM | commercial | serum free | 0.5% BSA | o.n. | UC | 10,000× g | 100,000× g, 60 min | |||||||||||
| [17] | AKI | Rat | BM | unfractionated | 20% FCS | EV depletion | 48 h | UC | 800× g, 2000× g, | 0.1 µm | 100,000× g, 2 × 60 min | ||||||||||
| [42] | I/R AKI | Human | UC | unfractionated | 10% FBS | 0.5% BSA | o.n. | UC | 2000× g | 100,000× g, 60 min | |||||||||||
| [43] | I/R AKI | Human | WJ | unfractionated | 10% FBS | 0.5% BSA | o.n. | UC | 2000× g | 100,000× g, 60 min | |||||||||||
| [79] | I/R AKI | Human | UC | Ficoll | 10% FBS | serum free | 24 h/ 48 h | UC | 2000× g | 100,000× g, 60–120 min | |||||||||||
| [45] | I/R AKI | Human | BC | unfractionated | n.d. | serum free | o.n. | UC + Optiprep | 3000× g, | 100,000× g, 120 min 350.000× g, 60 min 100.000× g, 60 min | |||||||||||
| [44] | I/R AKI | Rat | AT | unfractionated | 10% FBS | EV depletion | 96 h | UC | 4500 rpm | 0.22 µm | 120,000× g, 90 min | ||||||||||
| [47] | I/R renal injury | Mouse | BM | commercial | 10% FBS | EV depletion | 48 h | UC | 0.22 µm | n.d. | |||||||||||
| [39] | Renal Injury | Mouse | BM | unfractionated | 10% FBS | n.d. | n.d. | UC | 2000× g, | 100,000× g, 2 × 60 min | |||||||||||
| [52] | Retinal Injury | Human | UC | unfractionated | 10% FBS | serum free | n.d. | UC | 200× g, 2000× g, 10,000× g | 110,000× g, 120 min | |||||||||||
| [46] | Renal Allograft | Rat | BM | unfractionated | 20% FBS | EV depletion | 16 h | UC | 2000× g, 12,000× g | 100,000× g, 70 min | |||||||||||
| [76] | CKD | Human | UC | n.d. | serum free | 0.5% HSA | o.n. | UC | 2000× g | 100,000× g, 60 min | |||||||||||
| [29] | AMI | Human | BM | Ficoll | 10% FCS | EV depletion | n.d. | UC | 1500× g | 100,000× g, 60 min | |||||||||||
| [48] | AMI | Human | UC | unfractionated | 10% FBS | EV depletion, | 48 h | UC+ sucrose | 300× g, 2000× g, 10,000× g, | 100 kDa | 100,000× g, 120 min | ||||||||||
| [49] | AMI | Rat | BM | unfractionated | 10% FBS | EV depletion | 48 h | Precipitation (kit) | 2000× g, | 10,000× g, 60 min | |||||||||||
| [50] | AMI | Rat | BM | n.d. | n.d. | EV depletion | 48 h | Exoquick | 1500× g, 30 min | ||||||||||||
| [51] | AMI | Human | UC | unfractionated | 10% FBS | EV depletion | 48 h | UF + sucrose + UC | 300× g, 2000× g, 10,000× g, | 100 kDa, | 100,000× g, 120 min | ||||||||||
| [54] | AMI | Rat | BM | unfractionated | 15% FBS | EV depletion | 48 h | Exoquick | 3000× g | 100 kDa | 1500× g, 30 min | ||||||||||
| [21] | AMI | Human | ESC | sorting of CD105+ | 10% FCS | n.d. | n.d. | TFF + filter steps | 10, 1000, 500, 300 kDa | 100 kDa | |||||||||||
| [53] | AMI | Mouse | BM | unfractionated | serum free | serum free | n.d. | Exoquick | 3000× g | 0.3 µm | 1500× g, 30 min | ||||||||||
| [80] | AMI | Human | UC | unfractionated | 10% FBS | EV depletion | 48 h | UF + sucrose + UC | 300× g, 2000× g, 10,000× g, | 100 kDa | 100,000× g, 120 min | ||||||||||
| [81] | I/R injury | Human | ESC | sorting of CD105 + | n.d. | serum free | 72 h | TFF + HPLC | 500× g | 0.22 µm, 100 kDa | Chromatography | ||||||||||
| [58] | ALiI | Human | ESC | sorting of CD105+ | 10% FCS | serum free | 72 h | TFF+HPLC | 100 kDa | Chromatography | |||||||||||
| [59] | ALiI | Human | UC | unfractionated | 10% FCS | EV depletion | 48 h | UC + Sucrose +UF | 2.000× g, | 100 kDa, 0.22 µm | 100,000× g, 60 min | ||||||||||
| [55] | Hepatic failure | Human | MB | Ficoll | 20% FBS | 20 % FBS | 24 h | Exoquick | 2000× g, | 0.22 µm, 30 kDa | 1500× g, 30 min | ||||||||||
| [56] | Hepatic failure | Mouse Human | BM | commercial unfractionated | FBS | EV depletion | 48 h | UC | 300× g, 2000× g, 10,000× g, | 100,000× g, 70 min | |||||||||||
| [60] | Liver fibrosis | Human | UC | unfractionated | 10% FBS | EV depletion | 24 h | UF + Sucrose + UC | 1000× g, 2000× g, 10,000× g | 100,000× g, 60 min | |||||||||||
| [57] | I/R injury | Human | iPSC | iPS derived | 10% FBS | serum free | 48 h | UF | 300× g, 2000× g, 4000× g, | 0.22 µm | Amicon Ultra 15 | ||||||||||
| [66] | Stroke | Rat | BM | unfractionated | 20% FBS | EV depletion | 24 h | UC | 10,000× g | 0.22 µm | 100,000× g, 180 min | ||||||||||
| [67] | Stroke | Rat | BM | unfractionated | 20% FBS | EV depletion | 24 h | UC + Sucrose | 100,000× g | 0.22 µm | 100.000× g, 180 min | ||||||||||
| [69] | Stroke | Rat | BM | unfractionated | 20% FBS | EV depletion | 24 h | UC | 3000× g, 10,000× g | 0.22 µm | 100,000× g, 120 min | ||||||||||
| [65] | Stroke | Rat | BM | unfractionated | 20% FCS | EV depletion | 24 h | UC | 3000× g, 10,000× g | 0.22 µm | 100,000× g, 120 min | ||||||||||
| [63] | Stroke | Human | BM | Ficoll | 5% PL | 5% PL | 48 h | PEG + UC | 0.22 µm | 110,000× g, 2 h | |||||||||||
| [64] | Stroke | mini-pigs | AT | unfractionated | 10% FBS | EV depletion | 96 h | UC | 4500 rpm | 0.22 µm | 120,000× g, 90 min | ||||||||||
| [62] | TBI | Human | BM | Ficoll | 20% FBS | EV depletion | 48 h | Exoquick | 1500× g, 30 min | ||||||||||||
| [61] | TBI | Human | BM | unfractionated | 17% FBS | serum free | 6-48 h | UC | 2565× g | 100,000× g, 60–720 min | |||||||||||
| [68] | TBI | Rat | BM | unfractionated | 20% FBS | EV depletion | 48 h | Exoquick | 1500× g, 30 min | ||||||||||||
| [73] | Brain injury | Human | BM | Ficoll | 10% PL | 10% PL | 48 h | PEG + UC | 10,000× g | 0.22 µm | 110,000× g, 2 h | ||||||||||
| [72] | Brain injury | Human | BM | unfractionated | 10% PL | 10% PL | 48 h | PEG | 10,000× g | 0.22 µm | 1500× g, 30 min | ||||||||||
| [70] | Cerebral apoplexy | Human | BM | Ficoll | 5% PL | culture media | 48 h | PEG | 0.22 µm | n.d. | |||||||||||
| [71] | SCI | Rat | AT | digestion | n.d. | EV depletion | 24 h | Kit (miRCURY) | 3200× g, 30 min | ||||||||||||
| [75] | GvHD | Human | BM | unfractionated | 5% PL | culture media | 48 h | PEG + UC | 0.22 µm | 100,000× g, 120 min | |||||||||||
| [82] | GvHD | Human | UC | unfractionated | serum free | serum free | 48 h | UC | 2000× g | 100,000× g, 2 × 120 min | |||||||||||
| [83] | Enterocolitis | Mouse | BM | unfractionated | 10% FBS | serum free | 48 h | Kit (P100 Pure Exo) | |||||||||||||
| [84] | Diabetes | Rat | BM | unfractionated | 15% FBS | EV depletion | 24 h | Precipitation (Kit) | 10,000× g, 60 min | ||||||||||||
| [85] | Radiation damage | Human | BM | commercial | 15% FBS | EV depletion | 7 days | UC | 300× g, 2000× g, 10,000× g, | 100,000× g, 60 min | |||||||||||
| [86] | Wound healing | Human | UC | unfractionated | serum free | serum free | 48 h | UC + sucrose | 1000× g, 2000× g, 10,000× g | 100 kDa, 0.22 µm | 100,000× g, 60 min | ||||||||||
| [87] | Wound healing | Human | UC | unfractionated | 10% FBS | n.d. | 24 h | UC | 10,000× g | 0.22 µm | 100,000× g, 180 min | ||||||||||
| [88] | ALuI | Human | BM | n.d. | n.d. | 0.5% HSA | 48 h | UC | 3000× g | 100,000× g, 60 min | |||||||||||
| [89] | ALuI | Human | BM | commercial | 10% FCS | 0.5% HSA | 48 h | UC | 10,000× g | 100,000× g, 60 min | |||||||||||
| [90] | Airway inflammation | Human | BM | n.d. | 20% FBS | serum free | 48 h | UC | 3000× g | 100,000× g, 2 × 60 min | |||||||||||
| [78] | Graft rejection | Human | ESC | differentiation | serum free | serum free | 72 h | TFF + HPLC | 100 kDa | Chromatography | |||||||||||
| [91] | Sepsis | Mouse | BM | unfractionated | 15% FBS | EV depletion | 24 h | UC | 3000× g, 13,000× g | 0.22 µm | 36,000 rpm, 180 min | ||||||||||
| [92] | Colitis | Rat | BM | unfractionated | 10% FBS | serum free | 48 h | UC | 2000× g | 100,000× g, 2 × 60 min | |||||||||||
| Organ | Reference | Disease | Animal | Xenogenic Application | Functional Testing In Vitro | Application | EV Dose | No. of Injections | Factors | Immunomodulatory Effects | Described Effects After MSC-EV Application | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Strain | Gender | |||||||||||
| Kidney | [41] | AKI | Rat | SD | f | y | Renal capsule | 200 µg | 1 | Bcl-2, Bax | Reduced apoptosis Increased cell proliferation | ||
| [20] | AKI | Mouse | SCID | m | y | EV uptake | i.v. | 15 µg | 1 | mRNA dependent | RNA shuttled in MV associated with immune regulation | Morphological recovery Reduced apoptosis Increased cell proliferation | |
| [40] | AKI | Mouse | SCID | m | y | Apoptosis assay | i.v. | 100 µg 100 µg + 50 µg | 1 or 5 | RNA dependent, ACTB, POLR2E SUMO-1 | Improved survival Single injection: increased renal function, morphology and survival (although negative impact in the long-term) Multiple injections: decreased mortality (no impact in the long-term) | ||
| [17] | AKI | Rat | Wistar | f | n | i.v. | 100 µg/mL | 1 | mRNA dependent | Infiltrated lymphocytes T-B cell count higher, NK reduced TNFα transcripts reduced | Increased cell proliferation No necrosis | ||
| [42] | I/R AKI | Rat | n.d. | m | y | i.v. | 100 µg | 1 | RNA dependent, VEGF, HIF-1α | Reduced fibrosis Increased vessel density Reduced apoptosis Increased cell proliferation | |||
| [43] | I/R AKI | Rat | SD | m | y | i.v. | 100 µg | 1 | miR16, miR15b, miR15a | Reduced infiltration of macrophages (CD68 +) | Reduced apoptosis Increased cell proliferation | ||
| [79] | I/R AKI | Rat | SD | m | y | T-reg induction T-cell proliferation | i.a. | n.d. | 1 | Apolipoprotein, galectins CD73, CD90 | No necrosis No tubular dilation No cast formation | ||
| [45] | I/R AKI | Mouse | SCID | m | y | i.v. | 1 × 105 CE | 1 | RNA dependent | Increased tubular cell proliferation | |||
| [44] | I/R AKI | Rat | SD | m | n | i.v. | 100 µg | 1 | NFĸB, IL-1ß, MIF, PAI-1, COX-2 re | Reduced inflammatory reaction reduced TNFα | Reduced oxidative stress Reduced mitochondrial damage | ||
| [47] | I/R renal injury | Mouse | Balb/C | n.d. | n | Renal capsule | 200 µg | 1 | CCR-2 | Inhibition effect on recruitment of Monocytes and Macrophages | CCR2 enriched in Evs → binding to extracellular CCL-2 | ||
| [39] | Renal injury | Mouse | C57BL/6 | n.d. | n | i.v. | 30 µg | 3 | Lymphocyte infiltration | Improved renal function Decreased injury Prevented fibrosis | |||
| [46] | Renal allograft | Rat | Lewis | m | n | n.d. | n.d. | 1 | Infiltrated lymphocytes T- and B-cell count higher, NK cells reduced TNFα transcripts reduced | no difference in kidney function | |||
| Heart | [29] | AMI | Rat | Wistar | m | y | Border zone heart | 20 µL | 4 | Formation of new blood vessels Reduced infarct size | |||
| [48] | AMI | Rat | SD | n.d. | y | Apoptosis assay | i.v. | 400 µg | 1 | AKT overexpression, PDGF-D | Formation of new blood vessels Improved cardiac function | ||
| [49] | AMI | Rat | SD | m | n | Infarct border | 20 µg | 1 | miR29, miR24 upregulated miR34, miR130, miR378 downregulated | Reduced inflammation | No fibrosis Improved cardiac function Increased cell proliferation and migration | ||
| [50] | AMI | Rat | SD | m | n | T-cell proliferation Tube formation EV uptake | Infarct border | 80 µg | 1 | Decreased proliferation of inflammatory cells | Formation of new blood vessels Improved cardiac function | ||
| [51] | AMI | Rat | SD | m | y | Tube formation EV uptake | i.v. | 400 µg | 1 | Bcl2 | Improved cardiac function Reduced fibrosis Increased cardiomyocyte proliferation | ||
| [54] | AMI | Rat | SD | f | n | Intramyocardial | 4 × 106 CE | 1 | miR22, miR19, PTEN | Improved cardiac function Reduced infarct size Reduced apoptosis | |||
| [21] | AMI | Mouse | n.d. | n.d. | y | i.v. | 0.4 µg | 1 | Independent of immune cells | Reduced infarct size | |||
| [53] | AMI | Mouse | C57BL/6 | n.d. | n | Infarct border | 1 µg | 1 | miR122 | Reduced apoptosis Reduced fibrosis Improved cardiac function | |||
| Liver | [58] | Acute liver injury | Mouse | C57BL/6 | m | y | i.s. | 0.4 µg | 1 | HGF, HGFR protein, IL6ST/gp130, TNFRSF1A/TNFR1, CXCL2/MIP-2 protein, iNOS, NO, COX2, MIP-2 | Decreased apoptosis Decreased liver injury Induced hepatocyte proliferation | ||
| [59] | Acute liver injury | Mouse | BALB/c | n.d. | y | i.v. or oral | 8/16/32 mg/kg BW | 1 | GPX1, Bcl2, ROS, MDA | Reduced serum levels of pro-inflammatory cytokines | Rescued liver failure Increased viability Decreased oxidative stress | ||
| [55] | Hepatic failure | Mouse | C57BL/6 | m | y | EV uptake Apoptosis assay | i.v. | 1 µg/µL | 1 | Caspase-3, TNF-α, IL-6, IL-1ß | Inhibitory immunomodulation of activated MNCs decreased NK-cells | Reduced apoptosis Improved liver function | |
| [56] | Hepatic failure | Mouse | C57BL/6 | m | Y * | i.p./i.v. | 2 ×108 to 2 × 1010 EVs | 1 | Y-RNA-1, MIP2, IL-6, IL-1 alpha, MIP-3 beta, IP-10, MCP-1, MCP-3 | No apoptosis Reduced hepatic injury Improved survival | |||
| [57] | Hepatic I/R injury | Rat | SD | m | y | i.v. | 600 µg | 1 | TNF-α, IL-6, HMGB-1 | Reduced inflammatory markers Reduced infiltration of inflammatory cells | Reduced necrosis/ apoptosis Decreased liver injury Decreased oxidative stress Induced hepatocyte proliferation | ||
| Brain | [66] | Stroke | Rat | Wistar | m | n | i.v. | 100 µg | 1 | Improved neurological function Neurovascular remodeling | |||
| [69] | Stroke | Rat | Wistar | m | n | n.d. | 100 µg | 1 | miR-133 | Improved functional recovery | |||
| [65] | Stroke | Rat | Wistar | m | n | i.v. | 100 µg | 1 | miR17-92 Cluster PTEN | Improved neurological function Increased neural remodeling | |||
| [63] | Stroke | Mouse | C57BL/6 | m | y | i.v. | 2 × 106 CE | 3 | Reduced T-call activation B-cell, NK-cell, T-cell lymphopenia | Long-term neuroprotection Increased angioneogenesis | |||
| [64] | Stroke | Rat | n.d. | m | y | i.v. | 100 µg | 1 | MMP-9, IL-1ß, TNFα, RANTES, PAI-1, NF-KB, iNOS, NOX-1, NOX-2, c-casp3, c-PARP p-SMAD3, TGF-ß, SMAD1/5, BMP-2 | Reduced infiltration of CD11+ and CD68+cells | Decreased oxidative stress Increased angiogenesis | ||
| [62] | TBI | Rat | Wistar | m | y | i.v. | 100 µg | 1 | Reduced neuroinflammation reduced CD68+ cells at infarct zone | Improved functional recovery Increased cell proliferation Reduced neuroinflammation | |||
| [68] | TBI | Rat | Wistar | m | n | i.v. | 100 µg | 1 | Reduced neuroinflammation reduced CD68+ cells at infarct zone | Improved functional recovery Increased cell proliferation Reduced neuroinflammation | |||
| [61] | TBI | Mouse | C57BL/6 | m | y | i.v. | 30 µg | 1 | Suppressing Neuroinflammation | Rescue cognitive impairments | |||
| [73] | Brain injury | Rat | Wistar | n.d. | y | i.p. | 1 × 108 CE/kg BW | 1 | Modulated inflammatory responses | Improved cognitive function Reduced cellular degeneration | |||
| [72] | Preterm brain injury | Sheep | Texel | n.d. | y | i.v. | 2 × 107 CE | 2 | IBA-1 | Increased immunoreactivity | Decreased structural injury Functional neuroprotective effects Improved function | ||
| [70] | Cerebral apoplexy | Rat | n.d. | n.d. | y | i.v. | 2.4 × 104 EVs | 3 | Reduced quantity of B-cells, NK cells, and T-cells all increased; neuroinflammation (fewer CD68+ cells in infarct zone) attenuated immunosuppression (reduced numbers of activated T-cells) | Identical effect of MSCs and MSC-EVs Increased neuron survival | |||
| [71] | SCI | Rat | SD | male | n | i.v. | 100 µg | 1 | OPC A2B5 CNP-ase | Improved functional recovery Increased angiogenesis | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. https://doi.org/10.3390/ijms18071450
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. International Journal of Molecular Sciences. 2017; 18(7):1450. https://doi.org/10.3390/ijms18071450
Chicago/Turabian StyleBörger, Verena, Michel Bremer, Rita Ferrer-Tur, Lena Gockeln, Oumaima Stambouli, Amina Becic, and Bernd Giebel. 2017. "Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents" International Journal of Molecular Sciences 18, no. 7: 1450. https://doi.org/10.3390/ijms18071450





