A New Chemical Pathway Yielding A-Type Vitisins in Red Wines
Abstract
:1. Introduction
2. Results and Discussion
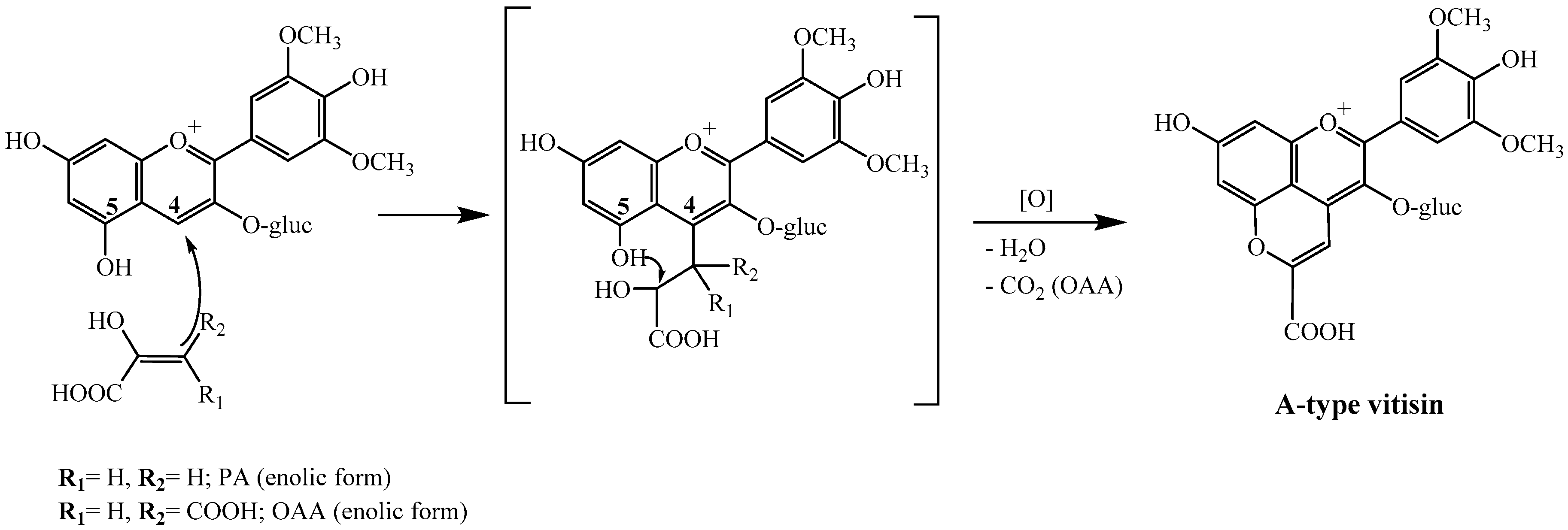
2.1. Reactivity of Anthocyanins with Oxaloacetic Acid (OAA) and Pyruvic Acid (PA)
2.2. Detection and Quantification of OAA and PA in Grape Must
3. Materials and Methods
3.1. Reagents
3.2. Grape Must Samples
3.3. Malvidin-3-O-glucoside (Mv-3-glc)
3.4. Reactivity of Mv-3-glc with PA and OAA
3.5. High Performance Liquid Chromatography (HPLC–DAD) Analysis
3.6. LC-MS Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PA | Pyruvic acid |
| OAA | Oxaloacetic acid |
| Mv-3-glc | Malvidin-3-O-glucoside |
| Dp-3-glc | Delphinidin-3-O-glucoside |
| Cy-3-glc | Cyanidin-3-O-glucoside |
| Pt-3-glc | Petunidin-3-O-glucoside |
| Pn-3-glc | Peonidin-3-O-glucoside |
| W-BF | White grape must—Before fermentation |
| W-MF | White grape must—Middle of fermentation |
| W-EF | White grape must—End of fermentation |
| R-BF | Red grape must—Before fermentation |
| R-MF | Red grape must—Middle of fermentation |
| R-EF | Red grape must—End of fermentation |
References
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Gomez-Alonso, S.; Blanco-Vega, D.; Victoria Gomez, M.; Hermosin-Gutierrez, I. Synthesis, isolation, structure elucidation, and color properties of 10-acetyl-pyranoanthocyanins. J. Agric. Food Chem. 2012, 60, 12210–12223. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Santos-Buelga, C.; Silva, A.M.S.; Mateus, N.; de Freitas, V. Isolation and structural characterization of new anthocyanin-derived yellow pigments in aged red wines. J. Agric. Food Chem. 2006, 54, 9598–9603. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Silva, A.M.S.; Vercauteren, J.; de Freitas, V. Occurrence of anthocyanin-derived pigments in red wines. J. Agric. Food Chem. 2001, 49, 4836–4840. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Radler, F. Yeasts—Metabolism of organic acids. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publisher: Camberwell, VIC, Australia, 1992. [Google Scholar]
- Romero, C.; Bakker, J. Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. Int. J. Food Sci. Technol. 2000, 35, 129–140. [Google Scholar] [CrossRef]
- Morata, A.; Calderon, F.; Gonzalez, M.C.; Gomez-Cordoves, M.C.; Suarez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Burns, T.R.; Osborne, J.P. Impact of malolactic fermentation on the color and color stability of pinot noir and merlot wine. Am. J. Enol. Vitic. 2013, 64, 370–377. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; de Freitas, V. Network of carboxypyranomalvidin-3-O-glucoside (vitisin A) equilibrium forms in aqueous solution. Tetrahedron Lett. 2013, 54, 5106–5110. [Google Scholar] [CrossRef]
- Oliveira, J.; Fernandes, V.; Miranda, C.; Santos-Buelga, C.; Silva, A.; de Freitas, V.; Mateus, N. Color properties of four cyanidin-pyruvic acid adducts. J. Agric. Food Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Oliveira, J.; Santos-Buelga, C.; Silva, A.M.S.; de Freitas, V. NMR structure characterization of a new vinylpyranoanthocyanin-catechin pigment (a portisin). Tetrahedron Lett. 2004, 45, 3455–3457. [Google Scholar] [CrossRef]
- Oliveira, J.; de Freitas, V.; Silva, A.M.S.; Mateus, N. Reaction between hydroxycinnamic acids and anthocyanin-pyruvic acid adducts yielding new portisins. J. Agric. Food Chem. 2007, 55, 6349–6356. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Azevedo, J.; Silva, A.M.S.; Teixeira, N.; Cruz, L.; Mateus, N.; de Freitas, V. Pyranoanthocyanin dimers: A new family of turquoise blue anthocyanin-derived pigments found in Port wine. J. Agric. Food Chem. 2010, 58, 5154–5159. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Oliveira, J.; Silva, A.M.S.; Mateus, N.; de Freitas, V. Oxovitisins: A new class of neutral pyranone-anthocyanin derivatives in red wines. J. Agric. Food Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Organic acids in wines. In Handbook of Enology: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The grape and its maturation. In Handbook of Enology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 241–297. [Google Scholar]
- Shapiro, F.; Silanikove, N. Rapid and accurate determination of malate, citrate, pyruvate and oxaloacetate by enzymatic reactions coupled to formation of a fluorochromophore: Application in colorful juices and fermentable food (yogurt, wine) analysis. Food Chem. 2011, 129, 608–613. [Google Scholar] [CrossRef]
- Noguchi, K.; Mizukoshi, T.; Miyano, H.; Yamada, N. Development of a new LC-MS/MS method for the quantification of keto acids. Chromatography 2014, 35, 117–123. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Araújo, P.; Fernandes, A.; de Freitas, V.; Oliveira, J. Detection of A-Type Vitisins in Red Grape Must at the Beggining of Fermentation; Instituto de Ciências, Tecnologias e Agroambiente da Universidade do Porto/Rede de Química e Tecnologia: Porto, Portugal, 2017. [Google Scholar]
- Oliveira, J.; da Silva, M.A.; Jorge Parola, A.; Mateus, N.; Brás, N.F.; Ramos, M.J.; de Freitas, V. Structural characterization of a A-type linked trimeric anthocyanin derived pigment occurring in a young Port wine. Food Chem. 2013, 141, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
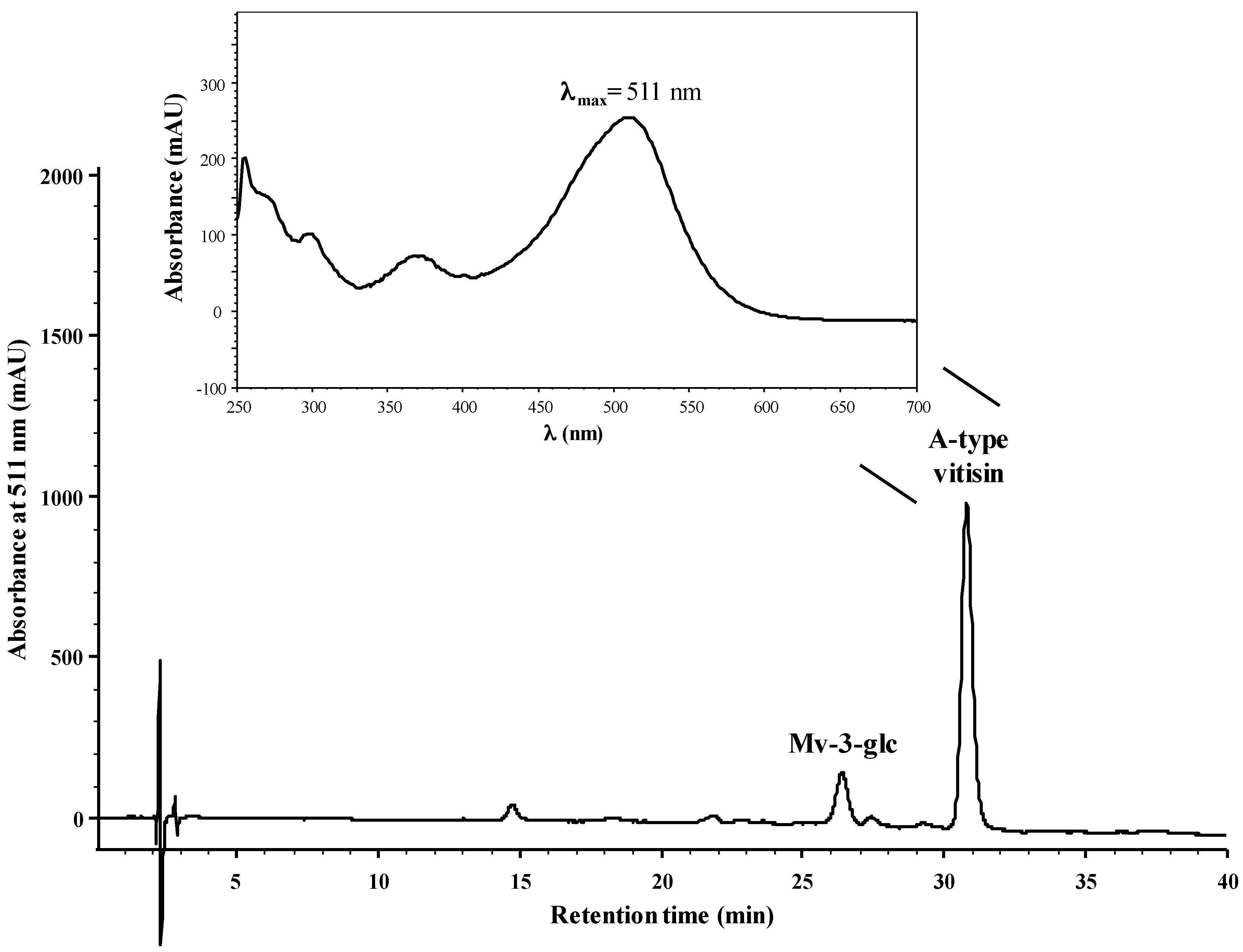

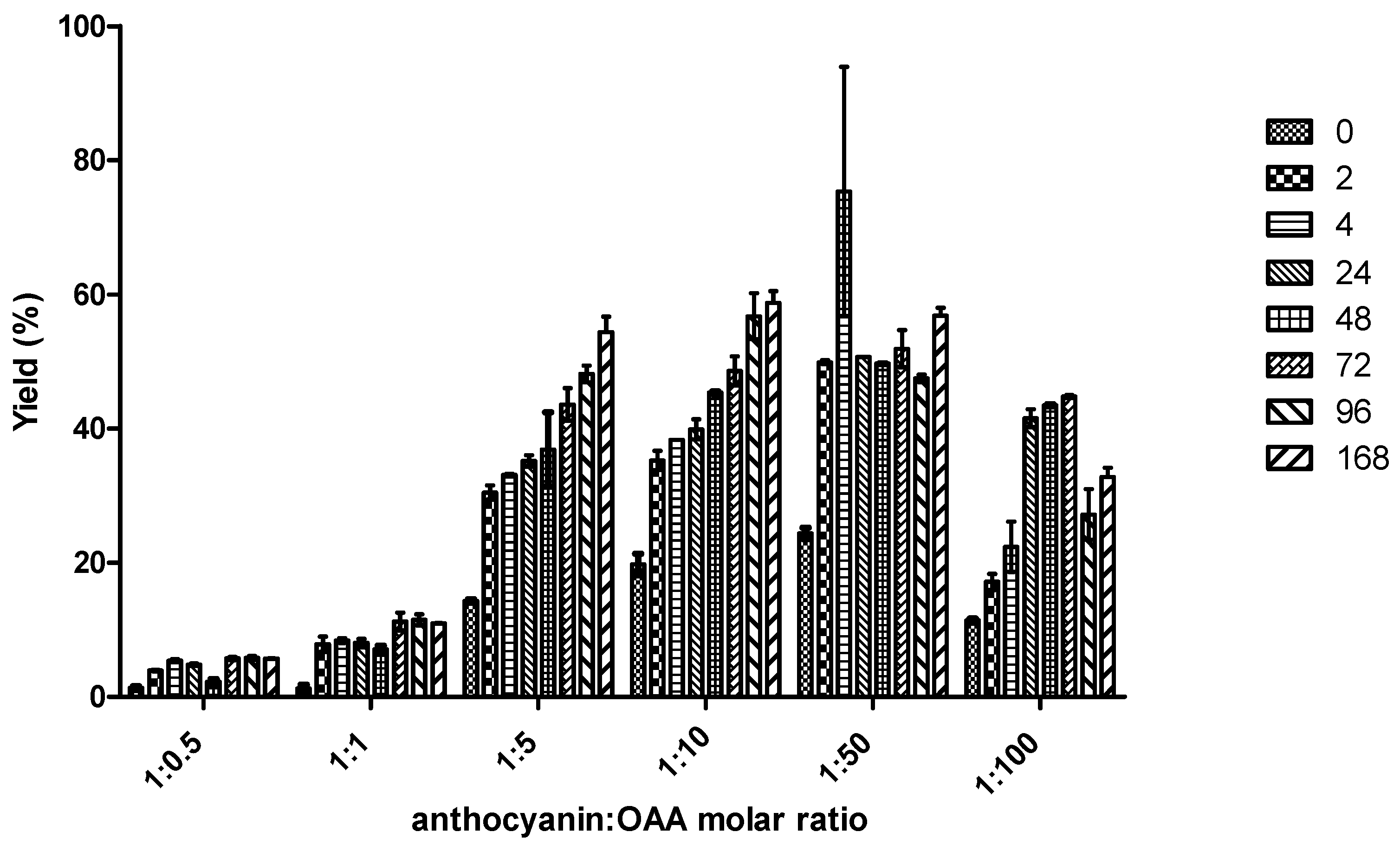
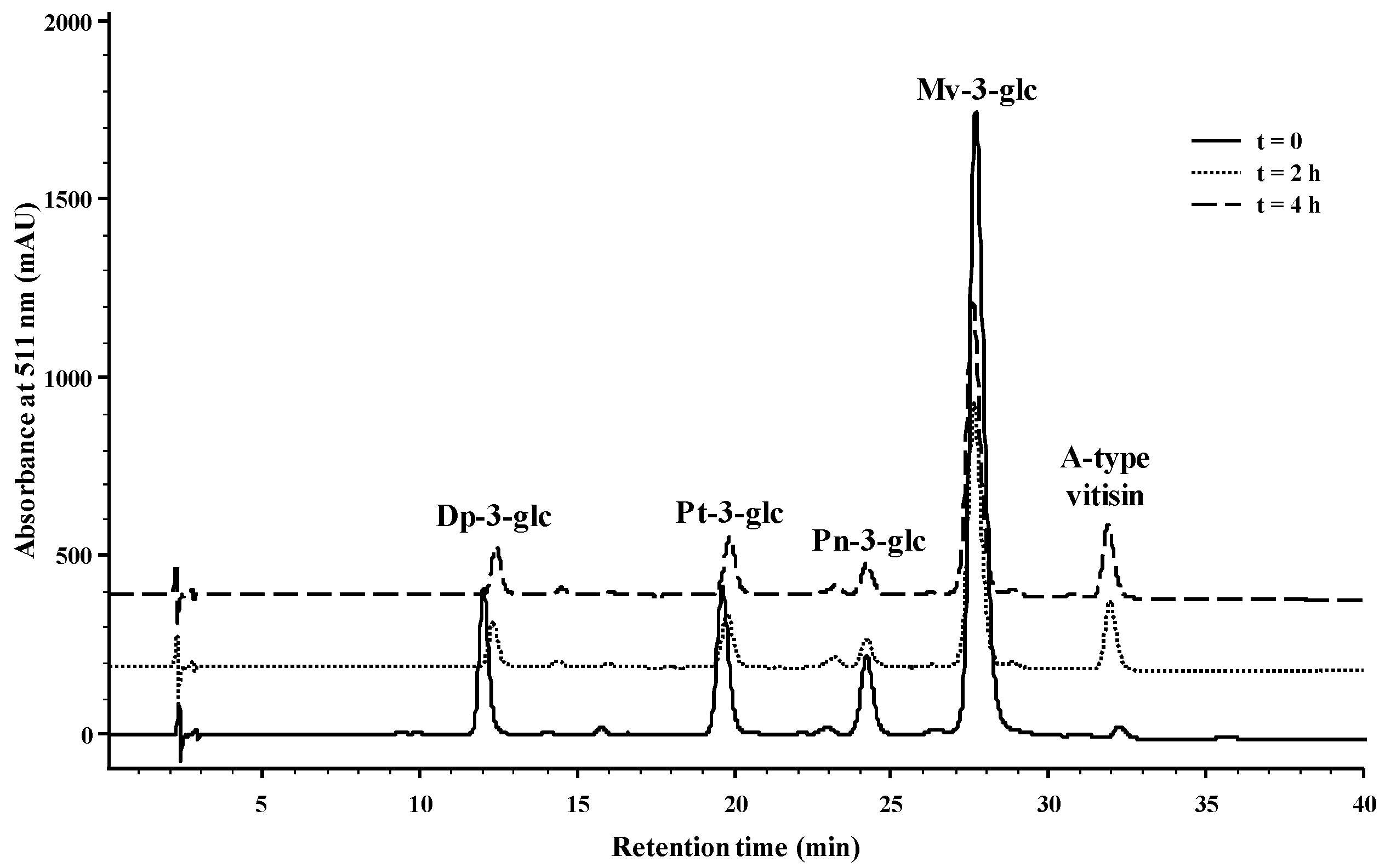


| Organic Acid | OAA | PA | ||||
|---|---|---|---|---|---|---|
| pH | 1.5 | 2.6 | 3.5 | 1.5 | 2.6 | 3.5 |
| Yield (%) | 5.4 ± 0.6 a | 59 ± 3 b | 48 ± 3 c | 0.8 ± 0.1 a | 44 ± 2 c | 20.9 ± 0.5 d |
| Type of Must | Samples | Sugar Concentration (g/L) | OAA (mg/L) | PA (mg/L) |
|---|---|---|---|---|
| Red grape must samples | R-BF | 180 | <0.03 a | 46.40 ± 0.02 c |
| R-MF | 109 | 5.5 ± 0.2 b | 243 ± 28 f | |
| R-EF | 2 | 6.1 ± 0.9 b | 250 ± 13 f | |
| White grape must samples | W-BF | 213 | 14.0 ± 0.8 c | 224 ± 16 f,g |
| W-MF | 95 | 11.4 ± 0.2 d | 320 ± 26 h | |
| W-EF | 0.07 | 13.0 ± 0.1 c | 163 ± 25g e,o |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, P.; Fernandes, A.; De Freitas, V.; Oliveira, J. A New Chemical Pathway Yielding A-Type Vitisins in Red Wines. Int. J. Mol. Sci. 2017, 18, 762. https://doi.org/10.3390/ijms18040762
Araújo P, Fernandes A, De Freitas V, Oliveira J. A New Chemical Pathway Yielding A-Type Vitisins in Red Wines. International Journal of Molecular Sciences. 2017; 18(4):762. https://doi.org/10.3390/ijms18040762
Chicago/Turabian StyleAraújo, Paula, Ana Fernandes, Victor De Freitas, and Joana Oliveira. 2017. "A New Chemical Pathway Yielding A-Type Vitisins in Red Wines" International Journal of Molecular Sciences 18, no. 4: 762. https://doi.org/10.3390/ijms18040762
APA StyleAraújo, P., Fernandes, A., De Freitas, V., & Oliveira, J. (2017). A New Chemical Pathway Yielding A-Type Vitisins in Red Wines. International Journal of Molecular Sciences, 18(4), 762. https://doi.org/10.3390/ijms18040762







