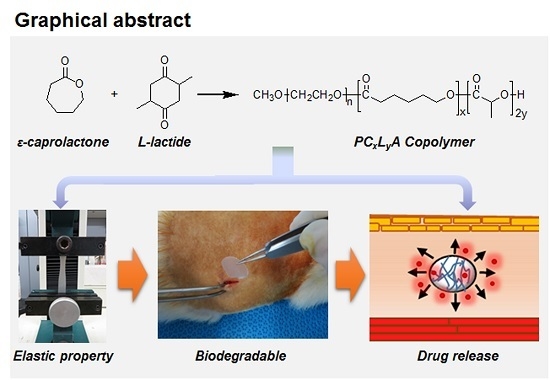
Preparation of Biodegradable and Elastic Poly(ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of PCxLyA Copolymers
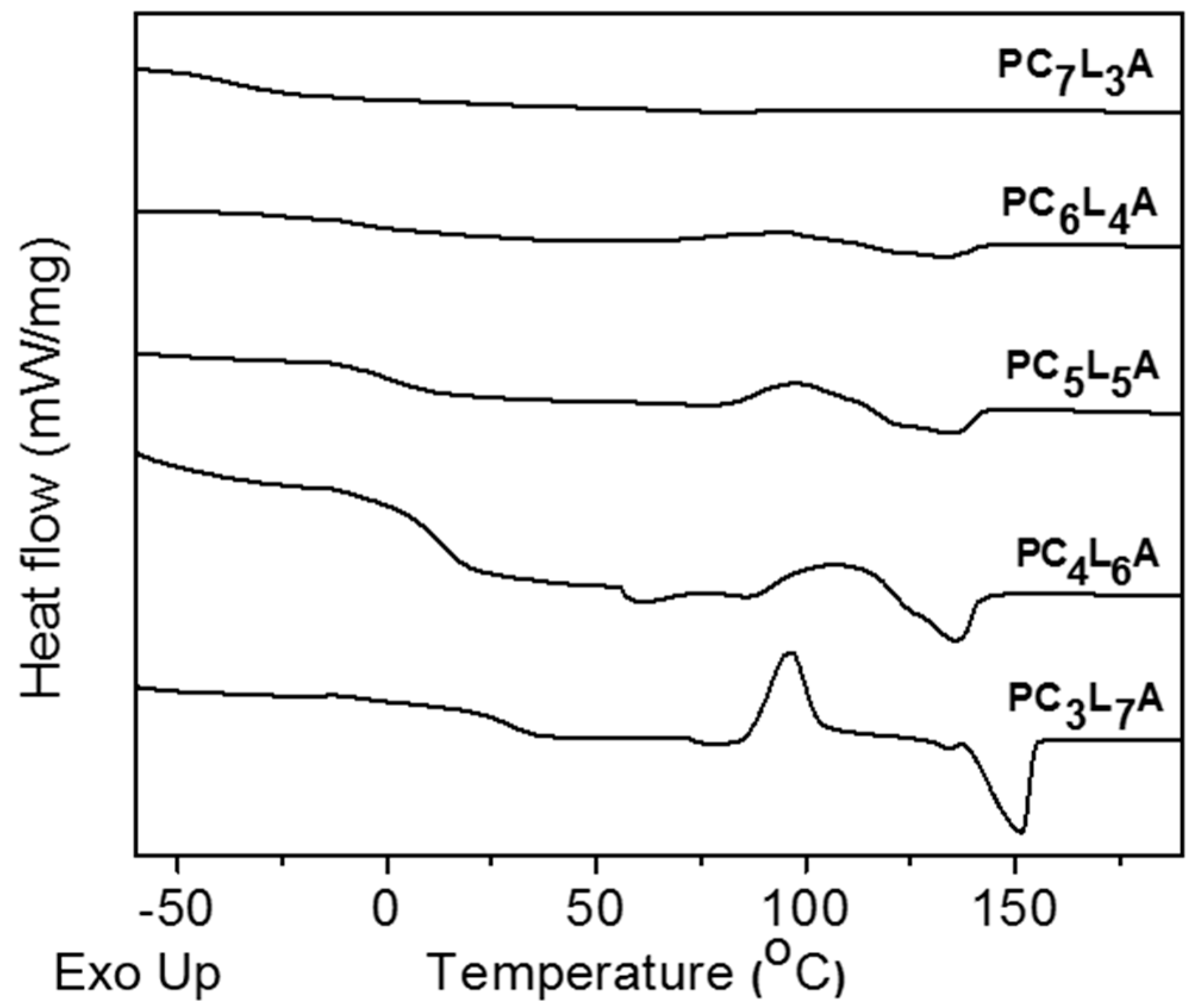
2.2. Thermal Properties of PCxLyA Copolymers
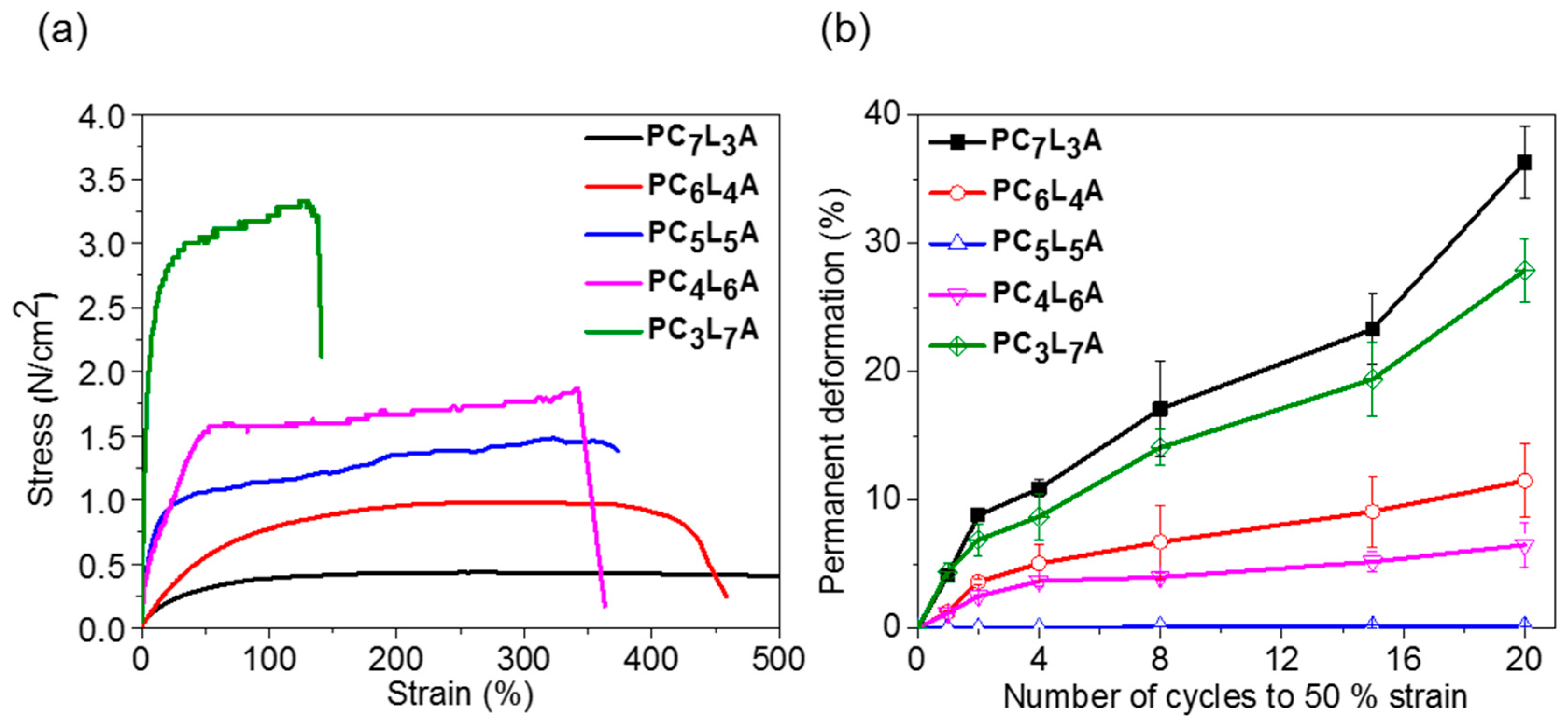
2.3. Mechanical Properties of PCxLyA Copolymers
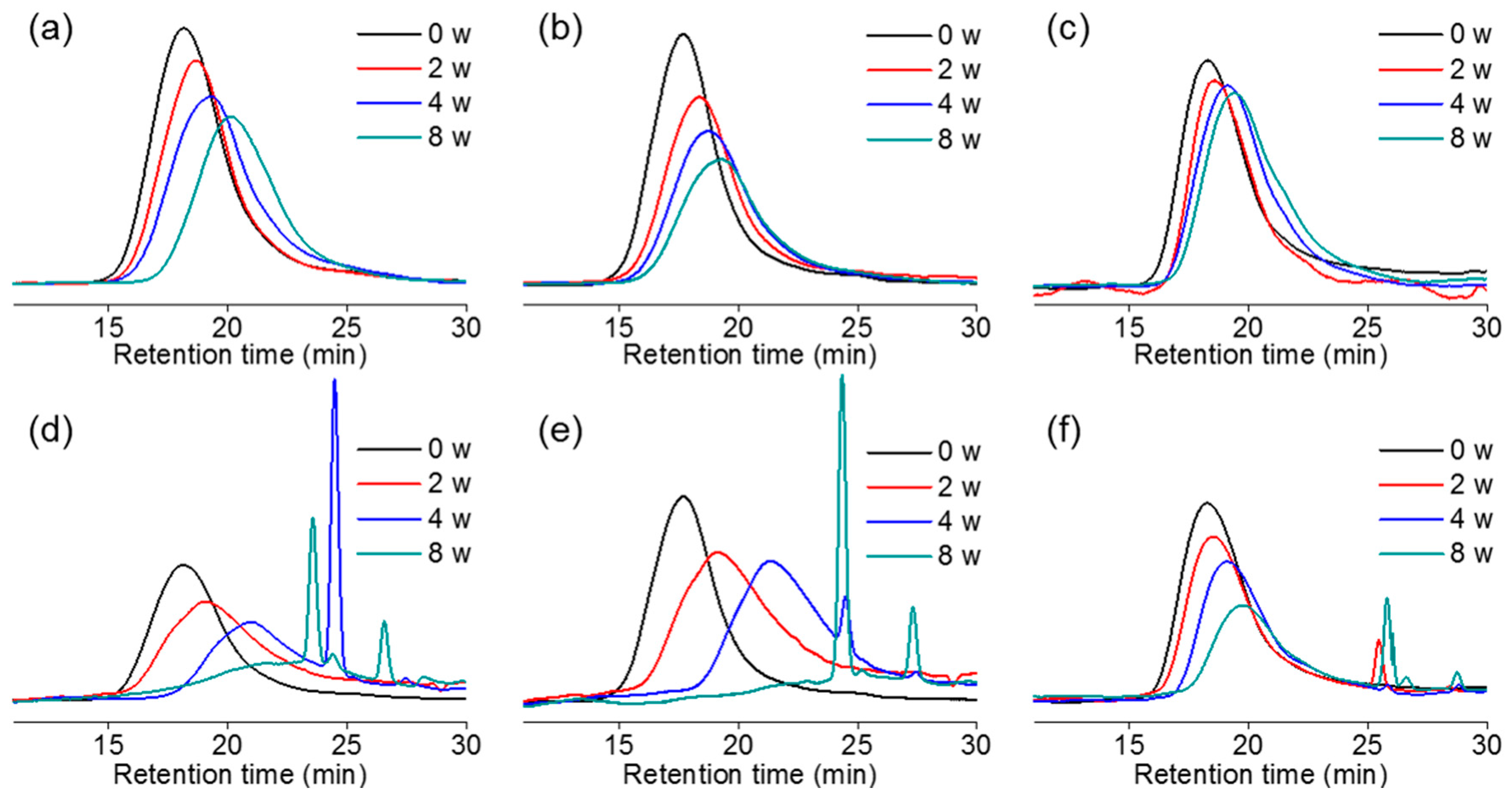
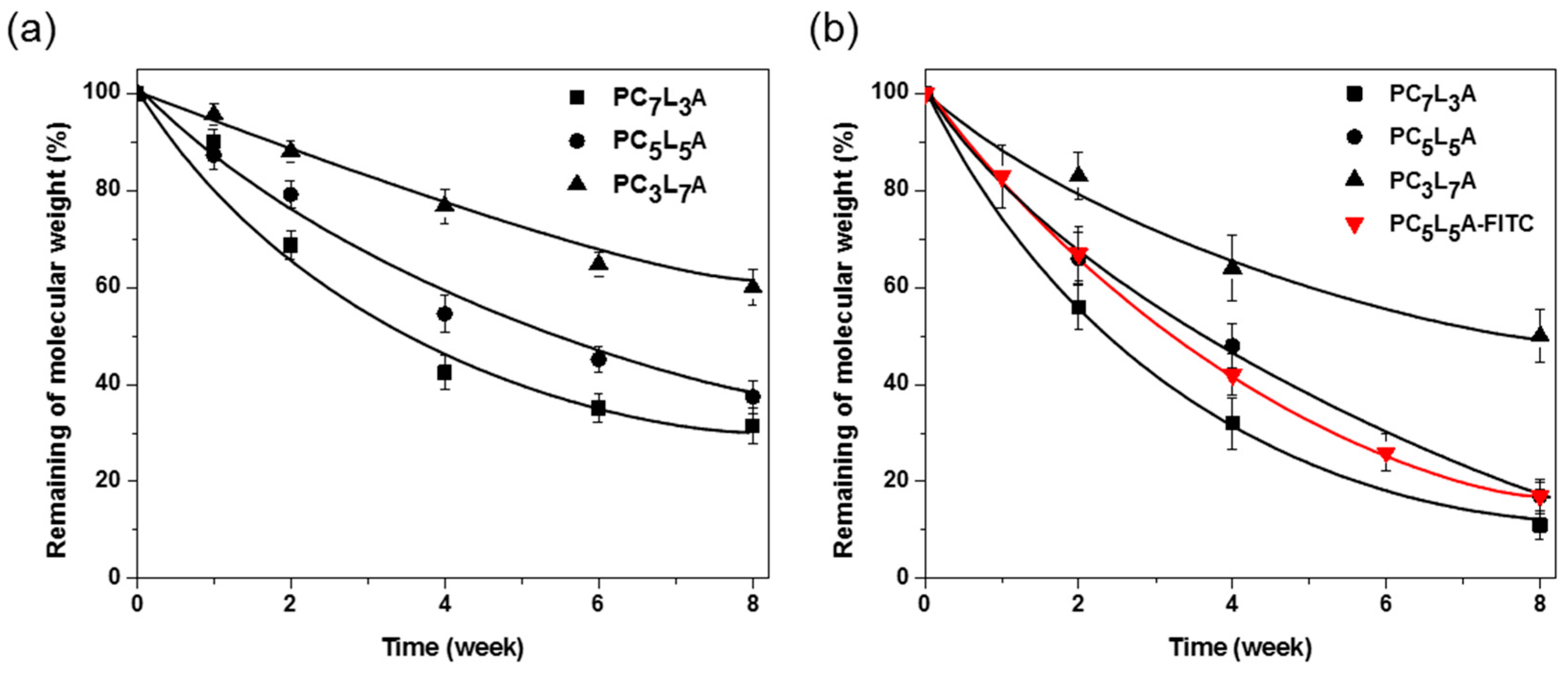
2.4. In Vitro and In Vivo Degradation of PCxLyA Copolymers
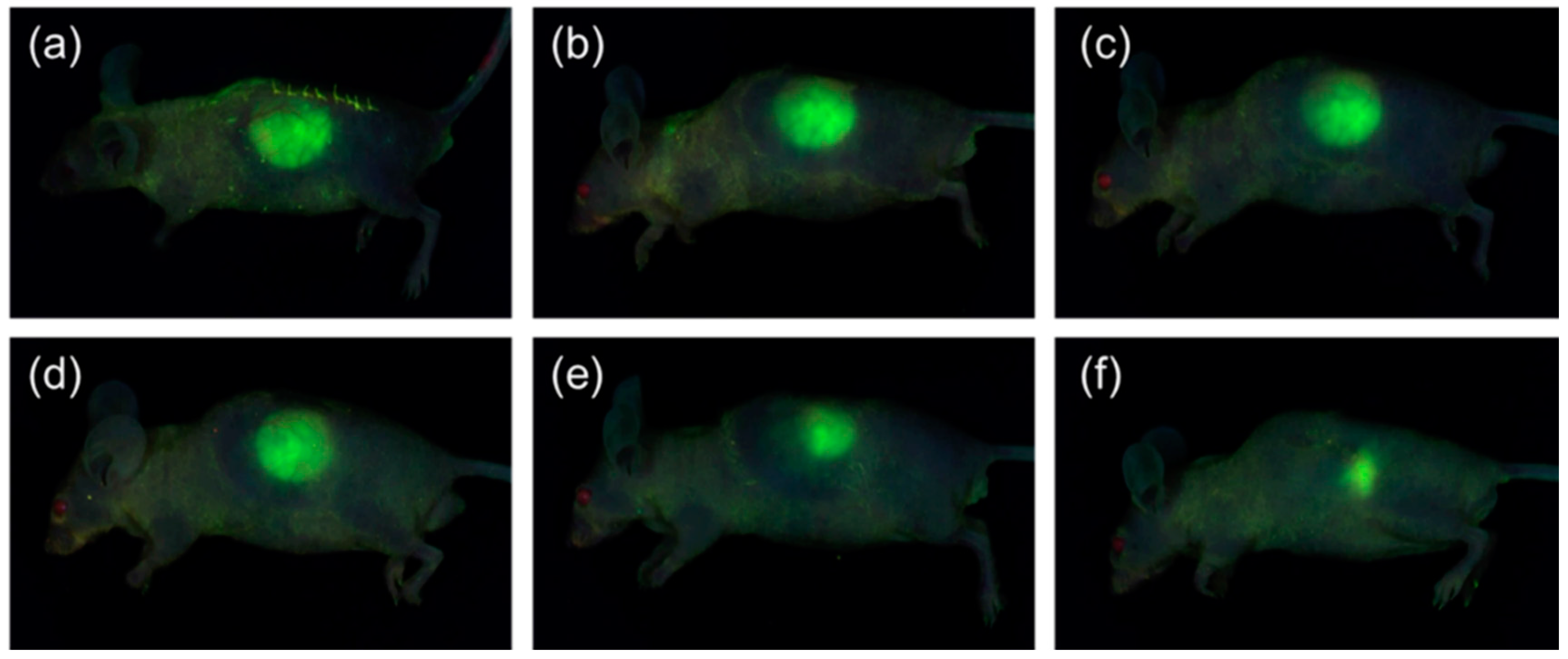
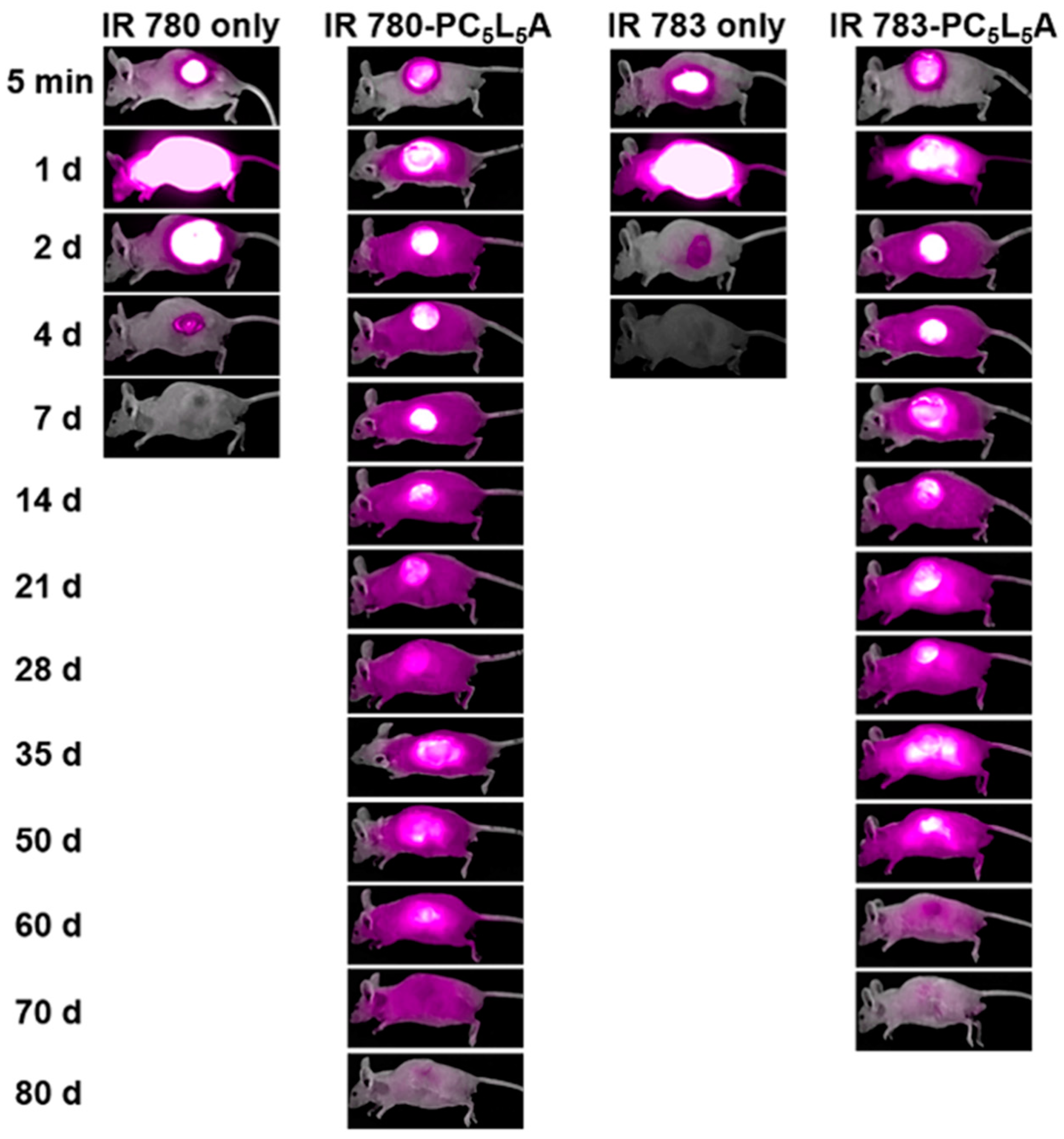
2.5. In Vivo Fluorescence Imaging
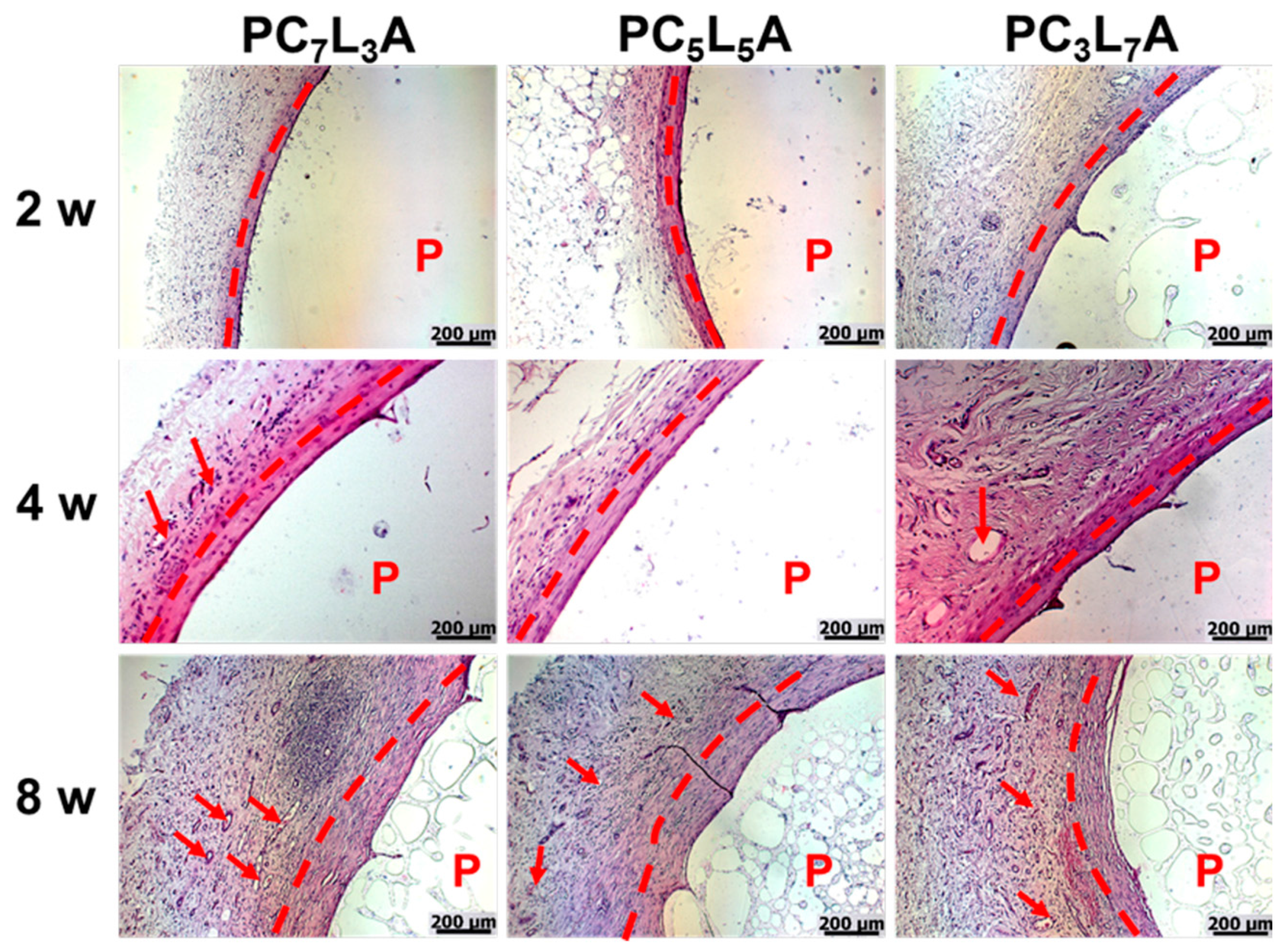
2.6. Histological Analysis
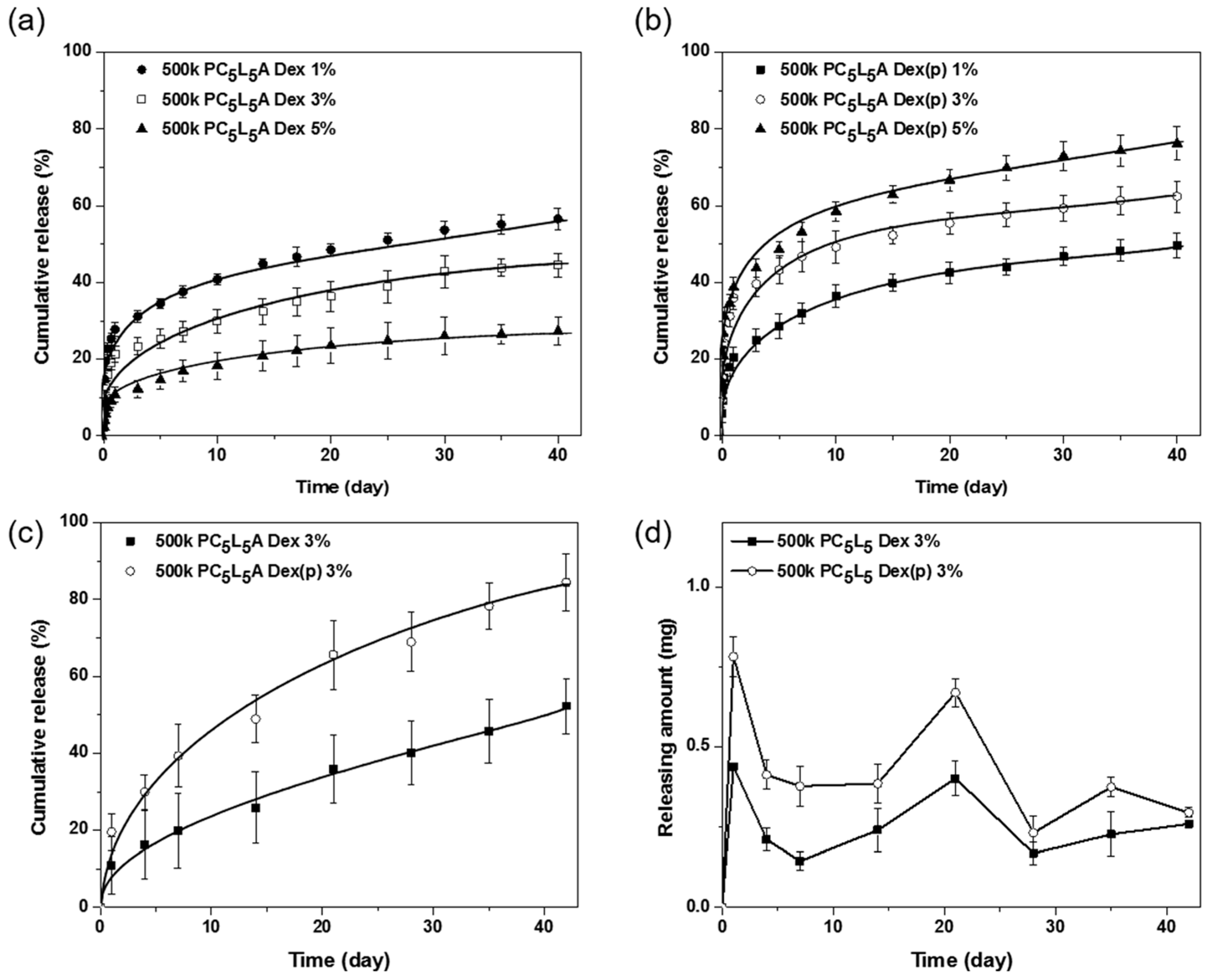
2.7. Drug Release from Drug-Loaded PCxLyA Copolymers
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of PCxLyA Copolymers
3.4. Synthesis of PCxLyA Copolymer with Fluorescein-Isothiocyanate (FITC) (PC5L5A-FITC)
3.5. Preparation of PCxLyA Films
3.6. Mechanical Testing of PCxLyA Films
3.7. In Vitro Degradation Test
3.8. In Vivo Implantation
3.9. Histological Analysis
3.10. Preparation of Drug-Loaded Polymer Films
3.11. In Vitro Release
3.12. In Vivo Release
3.13. In Vivo Fluorescence Imaging
3.14. In Vivo Near-Infrared (NIR) Imaging
3.15. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1337–1397. [Google Scholar] [CrossRef] [PubMed]
- Hur, F.W.; Park, M.; Lee, J.Y.; Kim, M.H.; Lee, S.; Park, C.G.; Kim, S.N.; Min, H.S.; Min, H.J.; Chai, J.H.; et al. Bioabsorbable bone plates enabled with local, sustained delivery of alendronate for bone regeneration. J. Control. Release 2016, 222, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, Y.Y.; Lee, H.B.; Moon, S.Y.; Ku, S.Y.; Kim, M.S. In Vivo Osteogenic differentiation of human embryoid bodies in an injectable in situ-forming hydrogel. Materials 2013, 6, 2978–2988. [Google Scholar] [CrossRef]
- Pan, J.; Liu, N.; Sun, H.; Xu, F. Preparation and Characterization of Electrospun PLCL/Poloxamer Nanofibers and Dextran/Gelatin Hydrogels for Skin Tissue Engineering. PLoS ONE 2014, 9, e112885. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.W.; Kwon, D.Y.; Lee, B.N.; Kwon, J.S.; Park, J.H.; Lee, J.H.; Kim, J.H.; Lee, I.W.; Shin, J.W.; Lee, H.B.; et al. Evaluation of small intestine submucosa and poly(caprolactone-co-lactide) conduits for peripheral nerve regeneration. Tissue Eng. Part A 2015, 21, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kwon, J.S.; Park, S.H.; Park, J.H.; Jang, S.H.; Tin, X.Y.; Yun, J.H.; Kim, J.H.; Min, B.H.; Lee, J.H.; et al. A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci. Rep. 2015, 5, 12721. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8. [Google Scholar] [CrossRef]
- Kwon, J.S.; Yoon, S.M.; Kwon, D.Y.; Kim, D.Y.; Tai, G.Z.; Jin, L.M.; Song, B.; Lee, B.; Kim, J.H.; Han, D.K.; et al. Injectable in situ-forming hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 3314–3321. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Chen, H.; Zhang, S.; Xiong, C.J. Preparation and characterization of biodegradable thermoplastic Elastomers (PLCA/PLGA blends). Polym. Res. 2010, 17, 77–82. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, W.; Chen, D.; Xiong, C.; Pang, X. Nonisothermal crystallization behaviour of poly(ρ-dioxanone) and poly(l-lactic acid) blends. Bull. Mater. Sci. 2015, 38, 517–523. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, W.; Bei, J.; Wang, S. Preparation of poly(lactide-co-glycolide-co-caprolactone) nanoparticles and their degradation behavior in aqueous solution. Polym. Degrad. Stab. 2006, 91, 1929–1936. [Google Scholar] [CrossRef]
- Fukushima, K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2014, 4, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, G. Mechanical and thermal properties of conventional and microcellular injection molded poly(lactic acid)/poly(ε-caprolactone) blends. J. Mech. Behav. Biomed. Mater. 2016, 53, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Fang, W.; Qiao, C.; Li, T. Effect of hard segment architecture on shape memory properties of polycaprolactone-based polyurethane containing azobenzene. J. Mater. Sci. 2016, 51, 2727–2738. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Anthamatten, M. Body temperature triggered shape-memory polymers with high elastic energy storage capacity. J. Polym. Sci. Part B Polym. Phys. 2016. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled release of multiple epidermal induction factors through core-shell nanofibers for skin regeneration. Eur. J. Pharm. Biopharm. 2013, 85, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Xiaomin, H.; Wei, F.; Bei, F.; Hao, W.; Zhenling, L.; Meng, Y.; Wei, W.; Jinghao, Z. Electrospun collagen-poly(l-lactic acid-co-ε-caprolactone) membranes for cartilage tissue engineering. Regen. Med. 2013, 8, 425–436. [Google Scholar]
- Zhang, D.; Ni, N.; Chen, J.; Yao, Q.; Shen, B.; Zhang, Y.; Zhu, M.; Wang, Z.; Ruan, J.; Wang, J. Electrospun SF/PLCL nanofibrous membrane: A potential scaffold for retinal progenitor cell proliferation and dirrferentiation. Sci. Rep. 2015, 5, 14326. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Han, C.C.; Yang, G. Preparation and characterization of PLLA/P(CL-b-LLA) blends by an in situ ring-opening polymerization. Polymer 2004, 45, 8231–8237. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, H.Y.; Park, S.H.; Park, J.H.; Kim, J.H.; Min, B.H.; Kim, M.S. Preparation and Evaluation of Dexamethasone-Loaded Electrospun Nanofiber Sheets as a Sustained Drug Delivery System. Materials 2016, 9, 175. [Google Scholar] [CrossRef]
- London, N.J.; Chiang, A.; Heller, J.A. The dexamethasone drug delivery system: Indications and evidence. Adv. Ther. 2011, 28, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Son, A.R.; Kim, D.Y.; Park, S.H.; Jang, J.Y.; Kim, K.; Kim, B.J.; Yin, X.Y.; Kim, J.H.; Min, B.H.; Han, D.K.; et al. Direct chemotherapeutic dual drug delivery through intra-articular injection for synergistic enhancement of rheumatoid arthritis treatment. Sci. Rep. 2015, 5, 14713. [Google Scholar]
- Yan, S.; Xiaoqiang, L.; Lianjiang, T.; Chen, H.; Xiumel, M. Poly(l-lactide-co-ε-caprolactone) electrospun nanofibers for encapsulating and sustained release proteins. Polymer 2009, 50, 4212–4219. [Google Scholar] [CrossRef]
- Stebbins, N.D.; Faiq, J.J.; Yu, W.; Guliyev, R.; Uhrich, K.E. Polyactives: Controlled and sustained bioactive release via hydrolytic degradation. Biomater. Sci. 2015, 8, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Silverajah, V.S.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.; Hassan, H.A. Mechanical, thermal and morphological properties of poly(lactic acid)/Epoxidized palm olein blend. Molecules 2012, 12, 11729–11747. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Jimenez, A.; Gonzalez-Martinez, C.; Chiralt, A. Influence of plasticizers on thermal properties and crystallization behavior of poly(lactic acid) films obtained by compression moulding. Polym. Int. 2015, 10. [Google Scholar] [CrossRef]
- Kim, B.S.; Nikolovski, J.; Bonadio, J.; Mooney, D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat. Biotechnol. 1999, 10, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Mooney, D.J. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J. Biomech. Eng. 2000, 122, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]



| Polymer | Molar Ratio (%) (CL/LA) a | Yield (%) b | Mn a | MW/Mn c |
|---|---|---|---|---|
| PC7L3A | 70/30 | 90 | 530,000 | 1.7 |
| PC6L4A | 61/39 | 89 | 516,000 | 1.6 |
| PC5L5A | 51/49 | 92 | 498,000 | 1.4 |
| PC4L6A | 38/62 | 93 | 521,000 | 1.7 |
| PC3L7A | 28/73 | 91 | 512,000 | 1.5 |
| Polymer | Tg (°C) a | Tm (°C) | ΔH (J·g−1) | Xc b |
|---|---|---|---|---|
| PC7L3A | −31.7 | - | - | 7.9 |
| PC6L4A | −2.8 | 132.3 | 1.3 | 8.7 |
| PC5L5A | 1.7 | 134 | 5.9 | 9.6 |
| PC4L6A | 12.25 | 136 | 13.2 | 12.7 |
| PC3L7A | 31.7 | 153.6 | 26.8 | 19.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lee, B.K.; Park, S.H.; Kim, M.G.; Lee, J.W.; Lee, H.Y.; Lee, H.B.; Kim, J.H.; Kim, M.S. Preparation of Biodegradable and Elastic Poly(ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier. Int. J. Mol. Sci. 2017, 18, 671. https://doi.org/10.3390/ijms18030671
Park JH, Lee BK, Park SH, Kim MG, Lee JW, Lee HY, Lee HB, Kim JH, Kim MS. Preparation of Biodegradable and Elastic Poly(ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier. International Journal of Molecular Sciences. 2017; 18(3):671. https://doi.org/10.3390/ijms18030671
Chicago/Turabian StylePark, Ji Hoon, Bo Keun Lee, Seung Hun Park, Mal Geum Kim, Jin Woo Lee, Hye Yun Lee, Hai Bang Lee, Jae Ho Kim, and Moon Suk Kim. 2017. "Preparation of Biodegradable and Elastic Poly(ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier" International Journal of Molecular Sciences 18, no. 3: 671. https://doi.org/10.3390/ijms18030671