The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia
Abstract
:1. Introduction
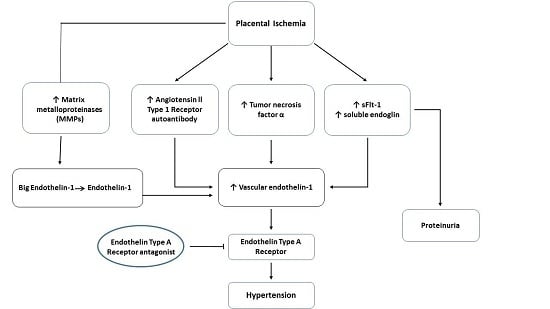
2. The Pathogenesis of Preeclampsia
3. The Endothelin System in Preeclampsia
4. ETA Receptor Antagonism in Animal Models Used to Study the Pathophysiology of PE
5. The ETA Receptor as a Potential Therapeutic Target in Preeclampsia?
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| sFlt-1 | Soluble fms-like tyrosine kinase 1 |
| TNF-α | Tumor necrosis factor-α |
| AT1-AA | Angiotensin II type 1 receptor autoantibody |
| ET-1 | Endothelin-1 |
| PlGF | Placental growth factor |
| sEng | Soluble Endoglin |
| bET-1 | Big Endothelin-1 |
| MMP | Matrix Metalloproteinase |
| RUPP | Reduced Uterine Perfusion Pressure |
| ETA | Endothelin Type A receptor |
| ETB | Endothelin Type B receptor |
| PAR-1 | Protease-activated receptor 1 |
| MAP | Mean Arterial Pressure |
| UARI | Uterine Artery Resistance Index |
| NP | Normal Pregnant |
| BP | Blood Pressure |
| VEGF | Vascular Endothelial Growth Factor |
References
- Roberts, J.M.; Taylor, R.N.; Goldfien, A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am. J. Hypertens. 1991, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.P.; Alexander, B.T.; Llinas, M.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 2002, 9, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Palei, A.C.; Spradley, F.T.; Warrington, J.P.; George, E.M.; Granger, J.P. Pathophysiology of hypertension in pre-eclampsia: A lesson in integrative physiology. Acta Physiol. 2013, 208, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A.; Robertson, W.B.; Dixon, H.G. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet. Gynecol. Annu. 1972, 1, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Ryan, M.J.; LaMarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 541–550. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.D.; Gilbert, J.; Granger, J.P. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension 2008, 51, 982–988. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.D.; Ryan, M.J.; Gilbert, J.S.; Murphy, S.R.; Granger, J.P. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr. Hypertens. Rep. 2007, 9, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; LaMarca, B.B.; Cockrell, K.; Granger, J.P. Role of endothelin in mediating soluble Fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 2010, 55, 394–398. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Granger, J.P. Endothelin: Key mediator of hypertension in preeclampsia. Am. J. Hypertens. 2011, 24, 964–969. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Granger, J.P. Linking placental ischemia and hypertension in preeclampsia: Role of endothelin 1. Hypertension 2012, 60, 507–511. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Palei, A.C.; Granger, J.P. Endothelin as a final common pathway in the pathophysiology of preeclampsia: Therapeutic implications. Curr. Opin. Nephrol. Hypertens. 2012, 21, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.P.; Abram, S.; Stec, D.; Chandler, D.; LaMarca, B. Endothelin, the kidney, and hypertension. Curr. Hypertens. Rep. 2006, 8, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Orisio, S.; Gaspari, F.; Frusca, T.; Amuso, G.; Remuzzi, G. Evidence against a pathogenetic role for endothelin in pre-eclampsia. Br. J. Obstet. Gynaecol. 1992, 99, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannis, D.S.; O’Brien, W.F.; Krammer, J.; Benoit, R. Potential role of endothelin-1 in normal and hypertensive pregnancies. Am. J. Obstet. Gynecol. 1991, 165, 1711–1716. [Google Scholar] [CrossRef]
- Taylor, R.N.; Varma, M.; Teng, N.N.; Roberts, J.M. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J. Clin. Endocrinol. Metab. 1990, 71, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, K.; Saleh, L.; Lankhorst, S.; Smilde, J.E.; van Ingen, M.M.; Garrelds, I.M.; Friesema, E.C.; Russcher, H.; van den Meiracker, A.H.; Visser, W.; et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 2015, 65, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The relationship between circulating endothelin-1, soluble Fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J. Hum. Hypertens. 2012, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Rinewalt, A.N.; Cockrell, K.L.; Massey, M.B.; Bennett, W.A.; Granger, J.P. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 2001, 37, 485–489. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.; Parrish, M.; Ray, L.F.; Murphy, S.R.; Roberts, L.; Glover, P.; Wallukat, G.; Wenzel, K.; Cockrell, K.; Martin, J.N., Jr.; et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1–AA) in pregnant rats: Role of endothelin-1. Hypertension 2009, 54, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Abdalvand, A.; Morton, J.S.; Bourque, S.L.; Quon, A.L.; Davidge, S.T. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 2013, 61, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Nugent, W.H.; Mishra, N.; Strauss, J.F., 3rd; Walsh, S.W. Matrix metalloproteinase 1 causes vasoconstriction and enhances vessel reactivity to angiotensin ii via protease-Activated receptor 1. Reprod. Sci. 2016, 23, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Llinas, M.T.; Kruckeberg, W.C.; Granger, J.P. l-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension 2004, 43, 832–836. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Granger, J.P.; Roberts, J.M. Chapter 10-animal models for investigating pathophysiological mechanisms of preeclampsia. In Chesley's Hypertensive Disorders in Pregnancy, 4th ed.; Taylor, R., Roberts, J.M., Cunningham, F.G., Lindheimer, M.D., Eds.; Academic Press: Tokyo, Japan, 2014; pp. 209–220. [Google Scholar]
- Granger, J.P.; LaMarca, B.B.; Cockrell, K.; Sedeek, M.; Balzi, C.; Chandler, D.; Bennett, W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 2006, 122, 383–392. [Google Scholar] [PubMed]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Crews, J.K.; Herrington, J.N.; Granger, J.P.; Khalil, R.A. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 2000, 35, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; LaMarca, B.B.; Fournier, L.; Bain, J.; Cockrell, K.; Granger, J.P. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension 2006, 47, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Tam Tam, K.B.; George, E.; Cockrell, K.; Arany, M.; Speed, J.; Martin, J.N., Jr.; Lamarca, B.; Granger, J.P. Endothelin type a receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am. J. Obstet. Gynecol. 2011, 204, 330.e1–330.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, D.; Hu, Y.; Wang, Z.; Paradis, A.; Mata-Greenwood, E.; Zhang, L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 2013, 62, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kappers, M.H.; Smedts, F.M.; Horn, T.; van Esch, J.H.; Sleijfer, S.; Leijten, F.; Wesseling, S.; Strevens, H.; Jan Danser, A.H.; van den Meiracker, A.H. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011, 58, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kappers, M.H.; de Beer, V.J.; Zhou, Z.; Danser, A.H.; Sleijfer, S.; Duncker, D.J.; van den Meiracker, A.H.; Merkus, D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension 2012, 59, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.B.; Cockrell, K.; Sullivan, E.; Bennett, W.; Granger, J.P. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 2005, 46, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Parchim, N.F.; Iriyama, T.; Luo, R.; Zhao, C.; Liu, C.; Irani, R.A.; Zhang, W.; Ning, C.; Zhang, Y.; et al. Excess light contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 2014, 63, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Irani, R.A.; Dai, Y.; Blackwell, S.C.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J. Immunol. 2011, 186, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, M.Q.; Li, W.; Reslan, O.M.; Yu, P.; Mata, K.M.; Khalil, R.A. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension 2014, 64, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.E.; Hosoda, K.; Richardson, J.A.; Williams, S.C.; Yanagisawa, H.; Kuwaki, T.; Kumada, M.; Hammer, R.E.; Yanagisawa, M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 1998, 125, 813–824. [Google Scholar] [PubMed]
- Kingman, M.; Ruggiero, R.; Torres, F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin. Pharmacother. 2009, 10, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Muramatsu, I. Pharmacological knockout of endothelin ETA receptors. Life Sci. 2003, 74, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Thaete, L.G.; Khan, S.; Synowiec, S.; Dayton, B.D.; Bauch, J.; Neerhof, M.G. Endothelin receptor antagonist has limited access to the fetal compartment during chronic maternal administration late in pregnancy. Life Sci. 2012, 91, 583–586. [Google Scholar] [CrossRef] [PubMed]
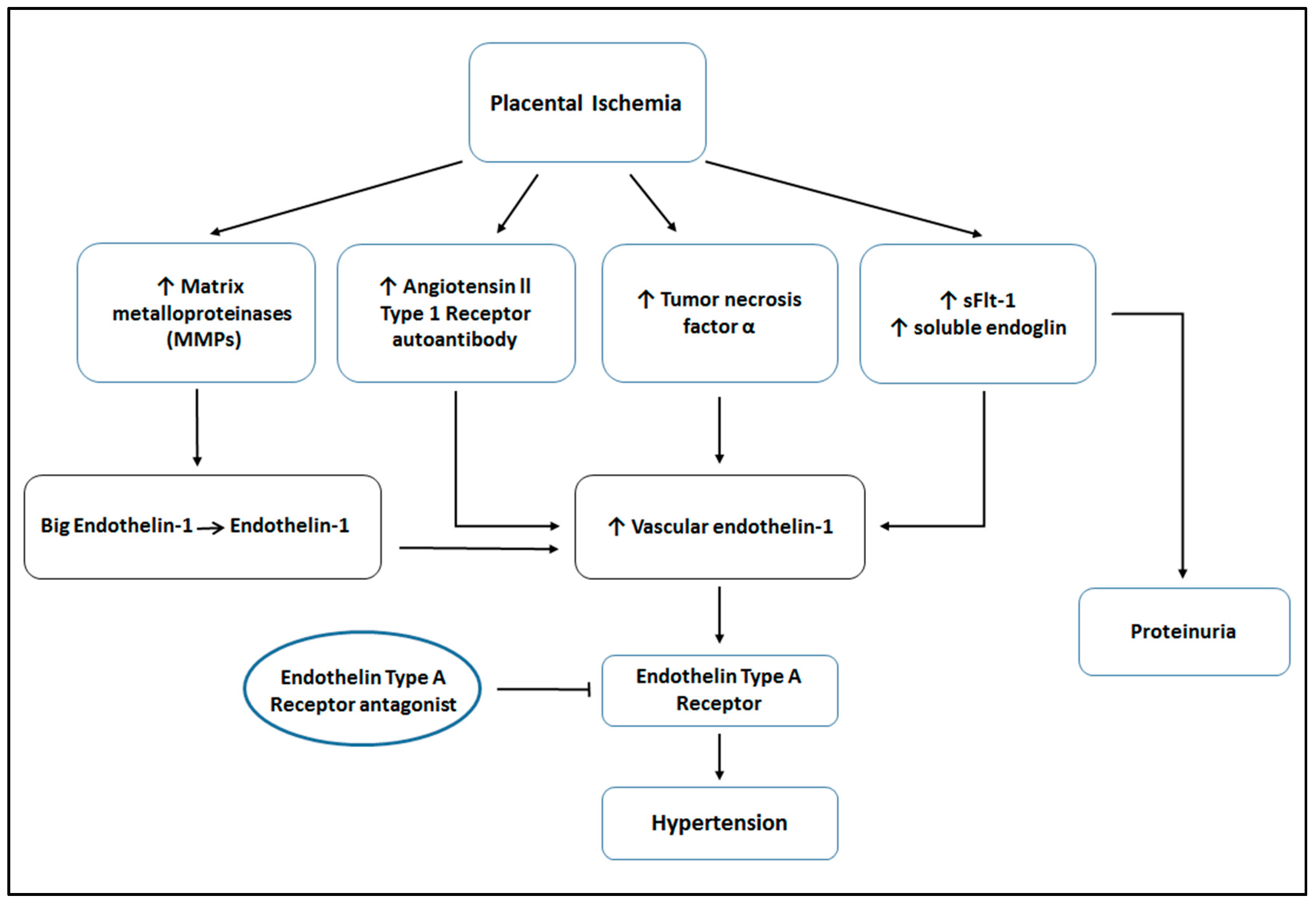
| Animal Model | Response to ETA Receptor Antagonist | References |
|---|---|---|
| RUPP | ↓ MAP, ↓ UARI | [18,28] |
| sFlt-1 infusion | ↓ MAP | [8] |
| TNF-α infusion | ↓ MAP | [7] |
| AT1-AA infusion | ↓ MAP | [19] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakrania, B.; Duncan, J.; Warrington, J.P.; Granger, J.P. The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia. Int. J. Mol. Sci. 2017, 18, 522. https://doi.org/10.3390/ijms18030522
Bakrania B, Duncan J, Warrington JP, Granger JP. The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia. International Journal of Molecular Sciences. 2017; 18(3):522. https://doi.org/10.3390/ijms18030522
Chicago/Turabian StyleBakrania, Bhavisha, Jeremy Duncan, Junie P. Warrington, and Joey P. Granger. 2017. "The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia" International Journal of Molecular Sciences 18, no. 3: 522. https://doi.org/10.3390/ijms18030522