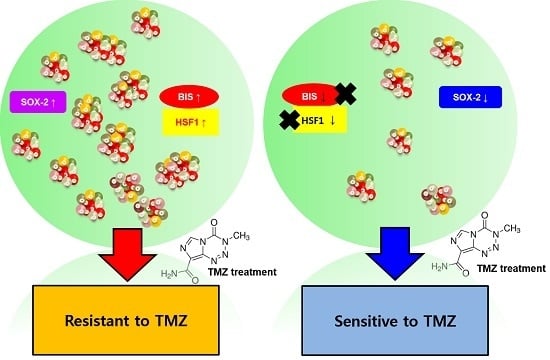
Heat Shock Factor 1 Depletion Sensitizes A172 Glioblastoma Cells to Temozolomide via Suppression of Cancer Stem Cell-Like Properties
Abstract
:1. Introduction
2. Results
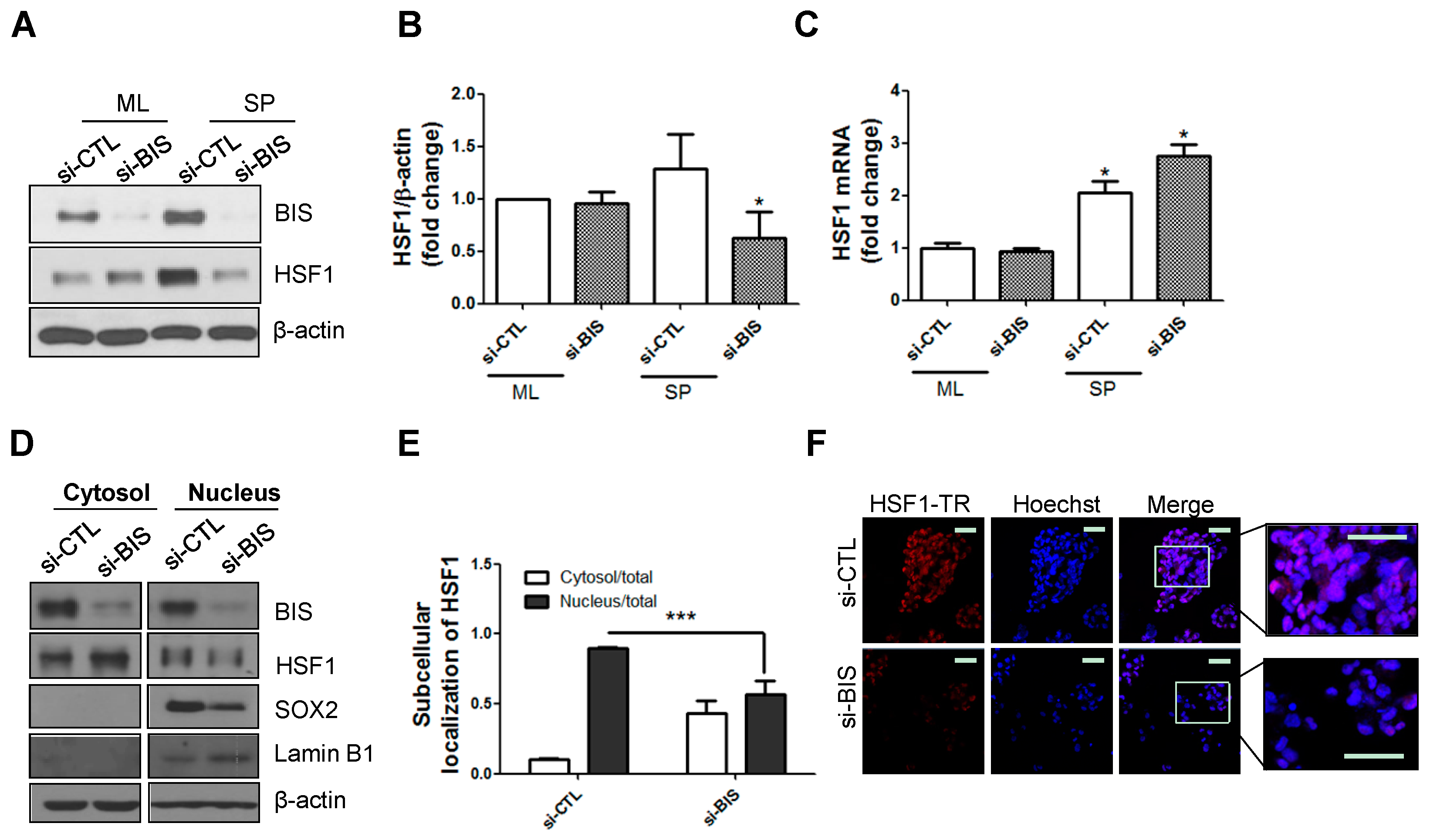
2.1. BIS Depletion Decreased Protein Levels of HSF1 as Well as Its Nuclear Localization in A172 Glioblastoma Cells under SP-Forming Conditions
2.2. mRNA and Protein Levels of HSF1 Increased in SP-Forming Conditions and HSF1 Knockdown Inhibited SP Formation and Matrix Metalloprotease 2 (MMP2) Activity in A172 Cells
2.3. Transcription of HSF1 Target Genes Was Not Affected by Depletion of BIS or HSF1 in SP-Forming Conditions
2.4. SP A172 Cells Were Less Sensitive to TMZ Treatment Than Cells Cultured in the ML
2.5. HSF1 Depletion Increased TMZ Sensitivity and Was Accompanied by a Decrease in SP Formation and SOX2 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Small Interfering RNA (siRNA) Transfection
4.2. Sphere-Formation Assay
4.3. Western Blot
4.4. Subcellular Fractionation
4.5. Confocal Microscopy
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Gelatin Zymography
4.8. Cell Viability Assay
4.9. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BIS | Bcl-2 (B-cell lymphoma 2) interacting cell death suppressor |
| CSC | Cancer stem cell |
| DTT | Dithiothreitol |
| HSEs | Heat shock elements |
| HSF1 | Heat shock factor 1 |
| HSPs | Heat shock proteins |
| GBM | Glioblastoma multiforme |
| GSCs | Glioblastoma stem cells |
| si-CTL | control siRNA |
| siRNA | Small interfering RNA |
| SP | Sphere |
| MMP2 | Matrix metalloprotease 2 |
| PARP | Poly(ADP-ribose) polymerase |
| SOX2 | Sex determining region Y (SRY)-box 2 |
| TMZ | Temozolomide |
| MAP1LC3B | Microtubule-associated protein 1 light chain 3 beta |
| MCL-1 | Myeloid leukemia cell differentiation protein |
| MGMT | O6-methylguanine DNA methyltransferase |
| ML | Monolayer |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
References
- Messaoudi, K.; Clavreul, A.; Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today 2015, 20, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Chuma, M.; Sakamoto, N.; Nakai, A.; Hige, S.; Nakanishi, M.; Natsuizaka, M.; Suda, G.; Sho, T.; Hatanaka, K.; Matsuno, Y.; et al. Heat shock factor 1 accelerates hepatocellular carcinoma development by activating nuclear factor-kappaB/mitogen-activated protein kinase. Carcinogenesis 2014, 35, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.; Hu, R.; Lin, N.U.; Mendillo, M.L.; Collins, L.C.; Hankinson, S.E.; Schnitt, S.J.; Whitesell, L.; Tamimi, R.M.; Lindquist, S.; et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 18378–18383. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lee, C.W.; Witt, A.; Thakkar, A.; Ince, T.A. Heat shock factor 1 induces cancer stem cell phenotype in breast cancer cell lines. Breast Cancer Res. Treat. 2015, 153, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Takahashi, T.; Yasuhara, N.; Inazawa, J.; Kamada, S.; Tsujimoto, Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 1999, 18, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Basile, A.; Falco, A.; d'Avenia, M.; Festa, M.; Graziano, V.; De Laurenzi, V.; Arra, C.; Pascale, M.; Turco, M.C. Role of BAG3 protein in leukemia cell survival and response to therapy. Biochim. Biophys. Acta 2012, 1826, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Im, C.N.; Youn, D.Y.; Yun, H.H.; Lee, J.H. BIS is Induced by Oxidative Stress via Activation of HSF1. Korean J. Physiol. 2014, 18, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Rosati, A.; Lerose, R.; de Nicola, S.; Turco, M.C.; Pascale, M. Bag3 gene expression is regulated by heat shock factor 1. J. Cell. Physiol. 2008, 215, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.X.; Zhang, H.Y.; Meng, X.; Gao, Y.Y.; Zou, R.L.; Liu, B.Q.; Guan, Y.; Wang, H.Q. Proteasome inhibitor MG132 induces BAG3 expression through activation of heat shock factor 1. J. Cell. Physiol. 2009, 218, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.T.; Marnett, L.J. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J. Biol. Chem. 2009, 284, 9176–9183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.N.; Cheng, L.; Tu, S.; Guo, S.J.; Le, H.Y.; Xiong, Q.; Mo, R.; Li, C.Y.; Jeong, J.S.; et al. Bcl2-associated athanogene 3 interactome analysis reveals a new role in modulating proteasome activity. Mol. Cell. Proteom. 2013, 12, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Ahn, S.G.; Kim, S.A. BAG3 affects the nucleocytoplasmic shuttling of HSF1 upon heat stress. Biochem. Biophys. Res. Commun. 2015, 464, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, Y.S.; Yun, H.H.; Im, C.N.; Ko, J.H.; Lee, J.H. ERK-mediated phosphorylation of BIS regulates nuclear translocation of HSF1 under oxidative stress. Exp. Mol. Med. 2016, 48, e260. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Yun, H.H.; Yoo, H.J.; Park, M.J.; Lee, J.H. Enhancement of SOX-2 expression and ROS accumulation by culture of A172 glioblastoma cells under non-adherent culture conditions. Oncol. Rep. 2015, 34, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Yun, H.H.; Song, B.H.; Youn, D.Y.; Cui, M.N.; Kim, H.S.; Park, K.S.; Lee, J.H. BIS-mediated STAT3 stabilization regulates glioblastoma stem cell-like phenotypes. Oncotarget. 2016, 7, 35056–35070. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Shimamura, M.; Mitsutake, N.; Nagayama, Y. SNAIL Induces Epithelial-to-Mesenchymal Transition and Cancer Stem Cell-Like Properties in Aldehyde Dehydroghenase-Negative Thyroid Cancer Cells. Thyroid 2013, 23, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, P.; Da Pozzo, E.; Bendinelli, S.; Costa, B.; Nuti, E.; Casalini, F.; Orlandini, E.; Da Settimo, F.; Rossello, A.; Martini, C. Inhibition of metalloproteinases derived from tumours: New insights in the treatment of human glioblastoma. Neuroscience 2010, 168, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007, 130, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, M.E.; Georgescu, A.M.; Purcaru, S.O.; Artene, S.A.; Emami, G.H.; Boldeanu, M.V.; Tache, D.E.; Dricu, A. Cancer Stem Cells: Biological Functions and Therapeutically Targeting. Int. J. Mol. Sci. 2014, 15, 8169–8185. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; Zhou, F.L.; Shen, Y.; Liao, D.F.; Cao, D.L. Apoptotic Death of Cancer Stem Cells for Cancer Therapy. Int. J. Mol. Sci. 2014, 15, 8335–8351. [Google Scholar] [CrossRef] [PubMed]
- Gentilella, A.; Khalili, K. BAG3 expression in glioblastoma cells promotes accumulation of ubiquitinated clients in an Hsp70-dependent manner. J. Biol. Chem. 2011, 286, 9205–9215. [Google Scholar] [CrossRef] [PubMed]
- Garros-Regulez, L.; Aldaz, P.; Arrizabalaga, O.; Moncho-Amor, V.; Carrasco-Garcia, E.; Manterola, L.; Moreno-Cugnon, L.; Barrena, C.; Villanua, J.; Ruiz, I.; et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin. Ther. Target 2016, 20, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Kang, N.Y.; Ha, H.H.; Bi, X.; Lee, J.J.; Park, S.J.; Lee, S.Y.; Vendrell, M.; Kim, Y.K.; Lee, J.S.; et al. A fluorescent rosamine compound selectively stains pluripotent stem cells. Angew. Chem. Int. Ed. Engl. 2010, 49, 7497–7500. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N. Targeting glioblastoma stem cells (GSCs) with peroxisome proliferator-activated receptor gamma (PPARγ) ligands. IUBMB Life 2016, 68, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Erice, O.; Smith, M.P.; White, R.; Goicoechea, I.; Barriuso, J.; Jones, C.; Margison, G.P.; Acosta, J.C.; Wellbrock, C.; Arozarena, I. MGMT Expression Predicts PARP-Mediated Resistance to Temozolomide. Mol. Cancer Ther. 2015, 14, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Wang, L.; Yachi, K.; Mahabir, R.; Narita, T.; Itoh, T.; Tanino, M.; Kimura, T.; Nishihara, H.; Tanaka, S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol. Cancer Ther. 2012, 11, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Villalva, C.; Cortes, U.; Wager, M.; Tourani, J.M.; Rivet, P.; Marquant, C.; Martin, S.; Turhan, A.G.; Karayan-Tapon, L. O6-Methylguanine-Methyltransferase (MGMT) Promoter Methylation Status in Glioma Stem-Like Cells is Correlated to Temozolomide Sensitivity Under Differentiation-Promoting Conditions. Int. J. Mol. Sci. 2012, 13, 6983–6994. [Google Scholar] [CrossRef] [PubMed]
- Boiani, M.; Daniel, C.; Liu, X.; Hogarty, M.D.; Marnett, L.J. The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J. Biol. Chem. 2013, 288, 6980–6990. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.N.; Yun, H.H.; Lee, N.E.; Kim, H.Y.; Im, C.N.; Kim, Y.S.; Lee, J.H. Depletion of BIS sensitizes A549 cells to treatment with cisplatin. Mol. Cell. Toxicol. 2016, 12, 63–71. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Lopez-Crisosto, C.; Pena-Oyarzun, D.; Salas, D.; Parra, V.; Quiroga, C.; Morawe, T.; Chiong, M.; Behl, C.; Lavandero, S. BAG3 regulates total MAP1LC3B protein levels through a translational but not transcriptional mechanism. Autophagy 2016, 12, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, J.S.; Lee, H.; Jeong, J.; Yun, H.H.; Kim, H.Y.; Ko, Y.G.; Lee, J.H. Myosin heavy chain is stabilized by BCL-2 interacting cell death suppressor (BIS) in skeletal muscle. Exp. Mol. Med. 2016, 48, e225. [Google Scholar] [CrossRef] [PubMed]
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 Drives a Transcriptional Program Distinct from Heat Shock to Support Highly Malignant Human Cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Liu, P.C.; Santoro, N.; Thiele, D.J. Conservation of a stress response: Human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997, 16, 6466–6477. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Nguyen, L.; Nguyen, H.C.; Vieusseux, J.L.; Chai, R.C.; Christophi, C.; Fifis, T.; Kouspou, M.M.; Price, J.T. Heat stress induces epithelial plasticity and cell migration independent of heat shock factor 1. Cell. Stress Chaperones 2012, 17, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Ishiwata, T.; Yoshimura, H.; Hagio, M.; Arai, T. Inhibition of nestin suppresses stem cell phenotype of glioblastomas through the alteration of post-translational modification of heat shock protein HSPA8/HSC71. Cancer Lett. 2015, 357, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Kim, B.M.; Moon, E.Y.; Hong, D.W.; Park, J.W.; Hong, S.H. Characterization of H460R, a Radioresistant Human Lung Cancer Cell Line, and Involvement of Syntrophin β2 (SNTB2) in Radioresistance. Genom. Inform. 2013, 11, 245–253. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequence (5'-3') |
|---|---|
| BIS | Forward: 5′-GGAATTCGCATGAGCGCCGCCACCCACTCG-3′ |
| Reverse: 5′-CTCGAGCTACGGTGCTGCTGGGTTACCAGG-3′ | |
| SOX2 | Forward: 5′-TACCTCTTCCTCCCACTCCA-3′ |
| Reverse: 5′-ACTCTCCTCTTTTGCACCCC-3′ | |
| HSF1 | Forward: 5′-GGCCATGAAGCATGAGAATG -3′ |
| Reverse: 5′-GTTCAGCATCAGGGGGATCT-3′ | |
| HSPA1A | Forward: 5′-AGCTGGAGCAGGTGTGTAAC-3′ |
| Reverse: 5′-CAGCAATCTTGGAAAGGCCC-3′ | |
| HSPA8 | Forward: 5′-TAACCCCATCATCAGCGGAC-3′ |
| Reverse: 5′-TCCCAACAGTCCACCTCAAAG-3′ | |
| HSP27 | Forward: 5′-TGACGGTCAAGACCAAGGAT-3′ |
| Reverse: 5′-ATGGTGATCTCGTTGGACTG-3′ | |
| MGMT | Forward: 5′-CCGTTTGCGACTTGGTACTT-3′ |
| Reverse: 5′-CCTTGCCCAGGAGCTTTATTT-3′ | |
| β-ACTIN | Forward: 5′-AGTACTCCGTGTGGATCGGC-3′ |
| Reverse: 5′-GCTGATCCACATCTGCTGGA-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, C.-N.; Yun, H.H.; Lee, J.-H. Heat Shock Factor 1 Depletion Sensitizes A172 Glioblastoma Cells to Temozolomide via Suppression of Cancer Stem Cell-Like Properties. Int. J. Mol. Sci. 2017, 18, 468. https://doi.org/10.3390/ijms18020468
Im C-N, Yun HH, Lee J-H. Heat Shock Factor 1 Depletion Sensitizes A172 Glioblastoma Cells to Temozolomide via Suppression of Cancer Stem Cell-Like Properties. International Journal of Molecular Sciences. 2017; 18(2):468. https://doi.org/10.3390/ijms18020468
Chicago/Turabian StyleIm, Chang-Nim, Hye Hyeon Yun, and Jeong-Hwa Lee. 2017. "Heat Shock Factor 1 Depletion Sensitizes A172 Glioblastoma Cells to Temozolomide via Suppression of Cancer Stem Cell-Like Properties" International Journal of Molecular Sciences 18, no. 2: 468. https://doi.org/10.3390/ijms18020468