The Role of PAR2 in TGF-β1-Induced ERK Activation and Cell Motility
Abstract
:1. Introduction
2. Results
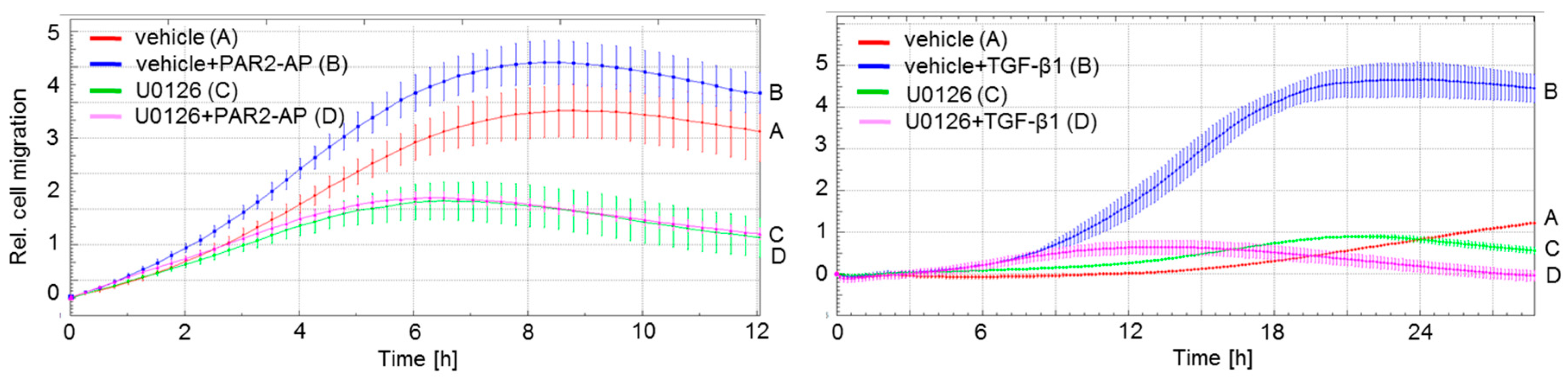
2.1. ERK Activation Is Required for Both PAR2–AP- and TGF-β1-Mediated Cell Migration
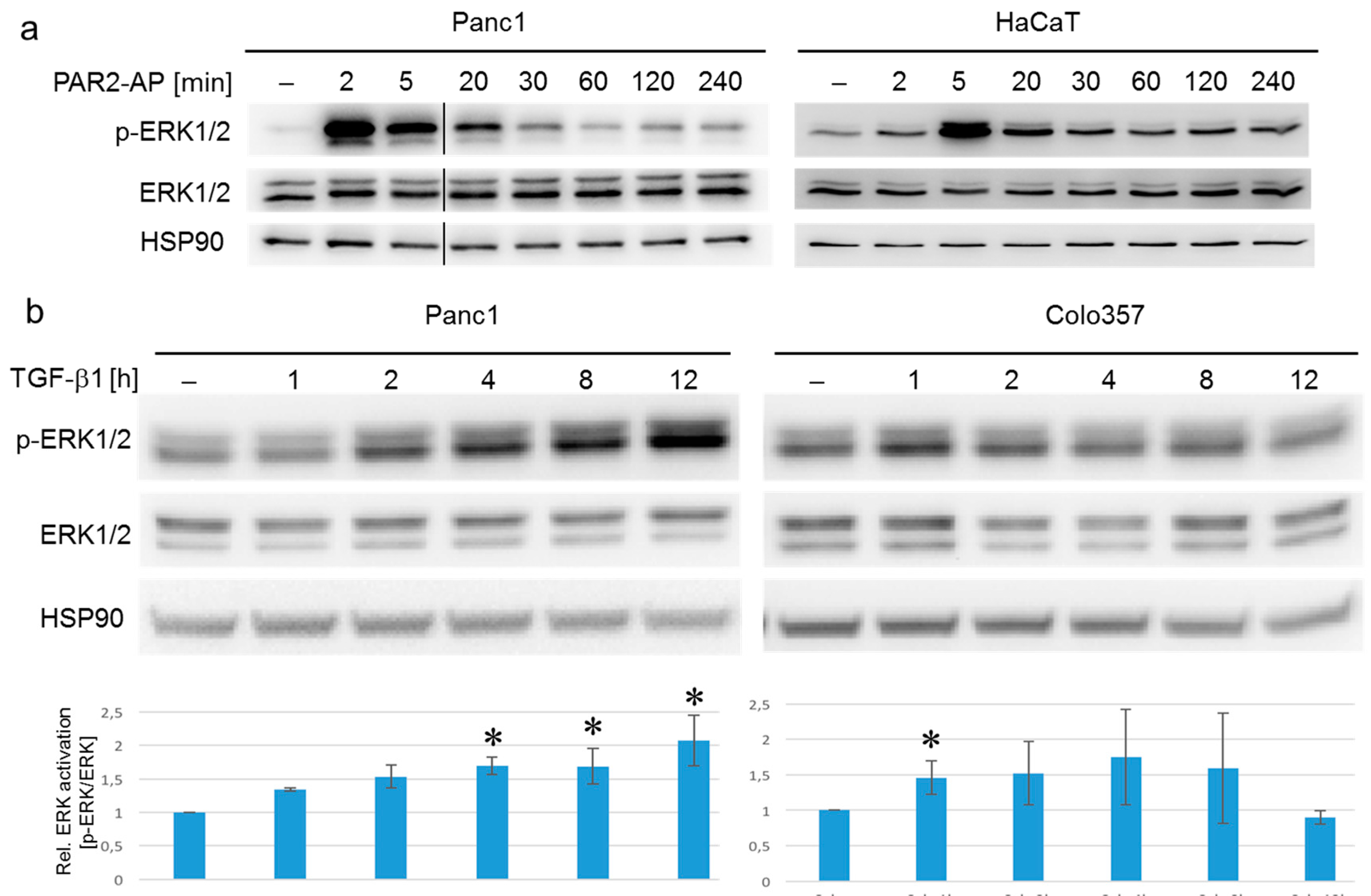
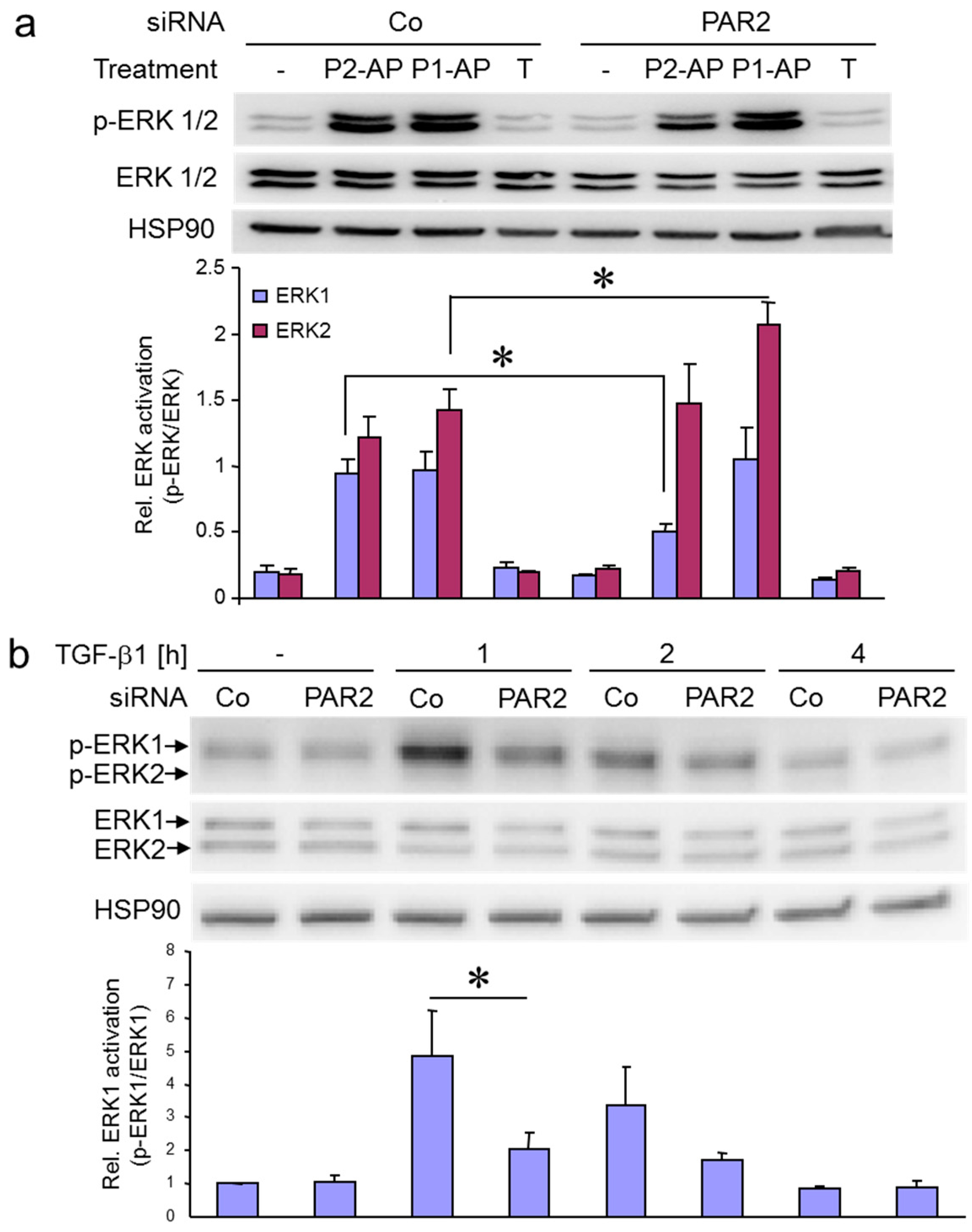
2.2. ERK Is Activated by PAR2–AP and TGF-β1 with Different Kinetics
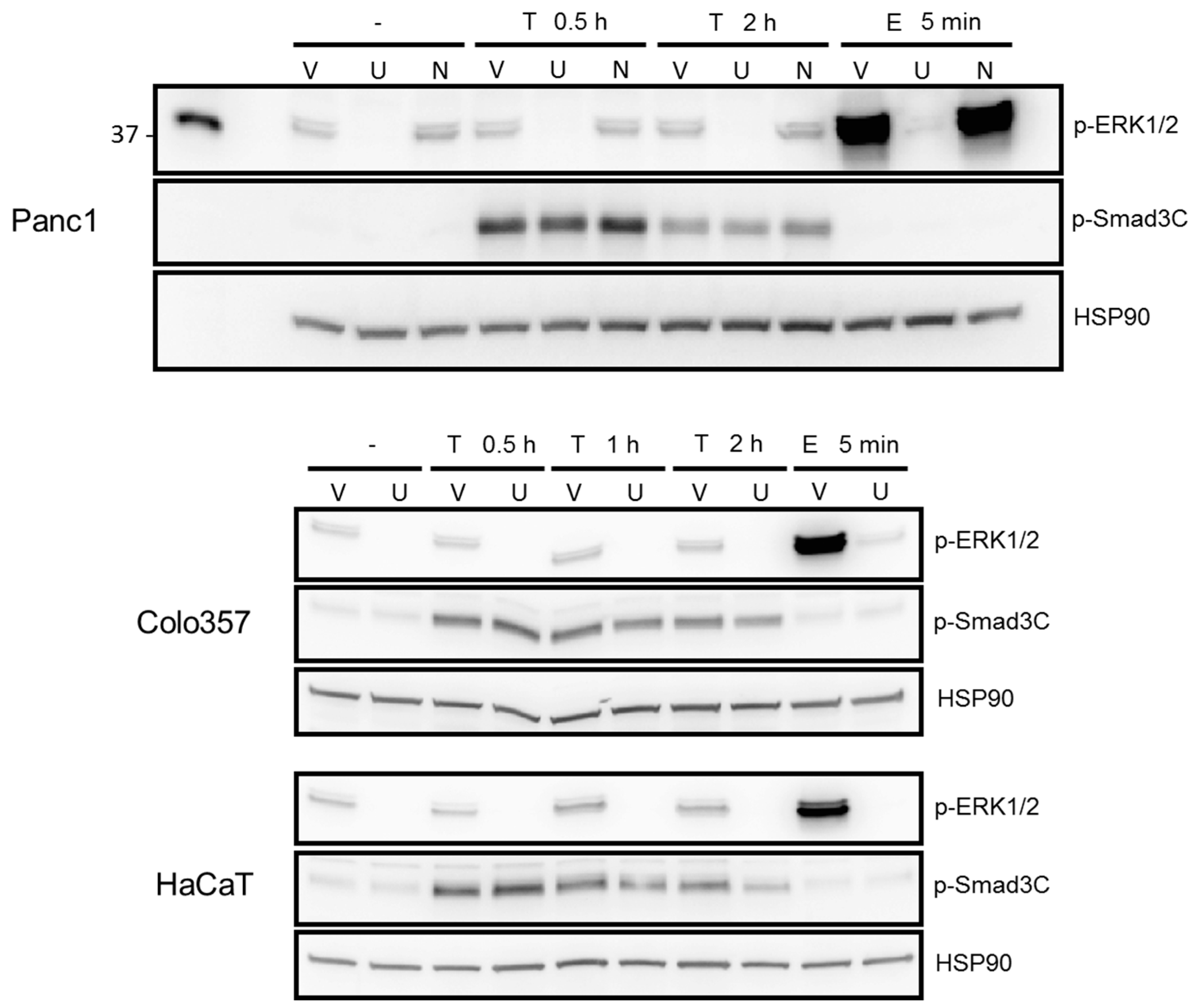
2.3. ERK but Not SMAD Activation in Response to TGF-β1 Was Blocked by the MEK Inhibitor U0126
2.4. Both PAR2-AP- and TGF-β1-Induced ERK Activation are Dependent on PAR2 Protein Expression
2.5. Treatment with High Concentrations of GB88 Increases Basal and TGF-β1-Induced ERK Activation and Cell Migration
2.6. PAR2 and ALK5 Can Be Co-Immunoprecipitated
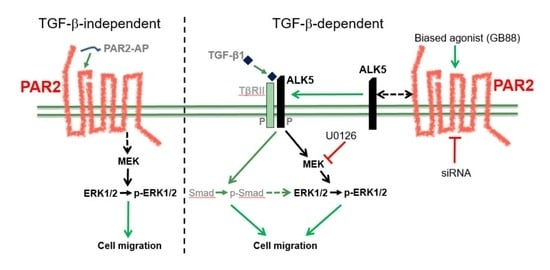
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Transient Transfection of siRNAs
4.4. Immunoblot Analysis and Co-Immunoprecipitation
4.5. Migration Assays
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALK5 | Activin receptor-like kinase 5 |
| AP | Agonistic peptide |
| ERK | Extracellular signal-regulated kinase |
| PAR2 | Proteinase-activated receptor 2 |
| TGF-β | Transforming growth factor-β |
References
- Neuzillet, C.; de Gramont, A.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014, 5, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Mechanisms of TGFβ-Induced Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Ehata, S.; Koinuma, D. Tumor-promoting functions of transforming growth factor-β in progression of cancer. Ups. J. Med. Sci. 2012, 117, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Miyazono, K. TGFbeta signaling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massagué, J. Roles of TGFbeta in metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, N.; Lee, C. Vicious cycle of TGF-β signaling in tumor progression and metastasis. Am. J. Clin. Exp. Urol. 2014, 2, 149–155. [Google Scholar] [PubMed]
- Lee, M.K.; Pardoux, C.; Hall, M.C.; Lee, P.S.; Warburton, D.; Qing, J.; Smith, S.M.; Derynck, R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007, 26, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Diaz, A.M.; Torres, C.; Mangan, R.J.; DeCant, B.; McKinney, R.; Tsao, M.S.; Lowy, A.; Munshi, H.G.; Jung, B.; et al. TGFβ engages MEK/ERK to differentially regulate benign and malignant pancreas cell function. Oncogene 2017, 36, 4336–4348. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.; Radu, M.; Doré, J.J. TGF-beta induced Erk phosphorylation of smad linker region regulates smad signaling. PLoS ONE 2012, 7, e42513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellenrieder, V.; Hendler, S.F.; Boeck, W.; Seufferlein, T.; Menke, A.; Ruhland, C.; Adler, G.; Gress, T.M. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001, 61, 4222–4228. [Google Scholar] [PubMed]
- Giehl, K.; Imamichi, Y.; Menke, A. Smad4-independent TGF-beta signaling in tumor cell migration. Cells Tissues Organs 2007, 185, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.N.; Ramachandran, R.; Yau, M.K.; Suen, J.Y.; Fairlie, D.P.; Hollenberg, M.D.; Hooper, J.D. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 2011, 130, 248–282. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Noorbakhsh, F.; Defea, K.; Hollenberg, M.D. Targeting proteinase-activated receptors: Therapeutic potential and challenges. Nat. Rev. Drug Discov. 2012, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Gieseler, F.; Ungefroren, H.; Settmacher, U.; Hollenberg, M.D.; Kaufmann, R. Proteinase-activated receptors (PARs)—Focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun. Signal 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yau, M.K.; Kok, W.M.; Lim, J.; Wu, K.C.; Liu, L.; Hill, T.A.; Suen, J.Y.; Fairlie, D.P. Biased Signaling by Agonists of Protease Activated Receptor 2. ACS Chem. Biol. 2017, 12, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Mihara, K.; Chung, H.; Renaux, B.; Lau, C.S.; Muruve, D.A.; DeFea, K.A.; Bouvier, M.; Hollenberg, M.D. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J. Biol. Chem. 2011, 286, 24638–24648. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, O.; Nishimura, S.; Matsunami, M.; Aoki, Y.; Nishikawa, H.; Ishikura, H.; Kawabata, A. Phosphorylation of ERK in the spinal dorsal horn following pancreatic pronociceptive stimuli with proteinase-activated receptor-2 agonists and hydrogen sulfide in rats: Evidence for involvement of distinct mechanisms. J. Neurosci. Res. 2010, 88, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.M.; Georgakopoulos, A.; Robakis, N.K. Presenilin 1 promotes trypsin-induced neuroprotection via the PAR2/ERK signaling pathway. Effects of presenilin 1 FAD mutations. Neurobiol. Aging 2016, 42, 41–49. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.L.; Traynelis, S.F.; Hepler, J.R. PAR1 and PAR2 couple to overlapping and distinct sets of G proteins and linked signaling pathways to differentially regulate cell physiology. Mol. Pharmacol. 2010, 77, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhou, H.; Hu, L.; Mu, Y.; Wu, Y. Involvement of PKCα activation in TF/VIIa/PAR2-induced proliferation, migration, and survival of colon cancer cell SW620. Tumour Biol. 2013, 34, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhou, H.; Wu, Y.; Zhou, F.; Xu, G.; Wen, H.; Zhang, X. Involvement of ERK1/2/NF-κB signal transduction pathway in TF/FVIIa/PAR2-induced proliferation and migration of colon cancer cell SW620. Tumour Biol. 2011, 32, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xia, L.; Zhou, H.; Wu, B.; Mu, Y.; Wu, Y.; Yan, J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCα and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumour Biol. 2013, 34, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Zeeh, F.; Witte, D.; Gädeken, T.; Rauch, B.H.; Grage-Griebenow, E.; Leinung, N.; Fromm, S.J.; Stölting, S.; Mihara, K.; Kaufmann, R.; et al. Proteinase-activated receptor 2 promotes TGF-β-dependent cell motility in pancreatic cancer cells by sustaining expression of the TGF-β type I receptor ALK5. Oncotarget 2016, 7, 41095–41109. [Google Scholar] [CrossRef] [PubMed]
- Witte, D.; Zeeh, F.; Gädeken, T.; Gieseler, F.; Rauch, B.H.; Settmacher, U.; Kaufmann, R.; Lehnert, H.; Ungefroren, H. Proteinase-Activated Receptor 2 Is a Novel Regulator of TGF-β Signaling in Pancreatic Cancer. J. Clin. Med. 2016, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H.; Witte, D.; Mihara, K.; Rauch, B.H.; Henklein, P.; Johren, O.; Bonni, S.; Settmacher, U.; Lehnert, H.; Hollenberg, M.D.; et al. TGF-β1/ALK5-mediated cell migration is dependent on the protein PAR2 but not on PAR2-stimulated Gq-calcium signaling. Mol. Pharmacol. 2017, 92, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, L.; Liang, Z.; Song, W.; Liu, Y.; Wang, Y.; Sun, B.; Zhang, B.; Cao, W. Transforming growth factor-β1 induces EMT by the transactivation of epidermal growth factor signaling through HA/CD44 in lung and breast cancer cells. Int. J. Mol. Med. 2015, 36, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rattenholl, A.; Seeliger, S.; Buddenkotte, J.; Schön, M.; Schön, M.P.; Ständer, S.; Vergnolle, N.; Steinhoff, M. Proteinase-activated receptor-2 (PAR2): A tumor suppressor in skin carcinogenesis. J. Investig. Dermatol. 2007, 127, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Ramachandran, R.; Hollenberg, M.D.; Muruve, D.A. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-β receptor signaling pathways contributes to renal fibrosis. J. Biol. Chem. 2013, 288, 37319–37331. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Chen, K.D.; Wang, C.C.; Huang, K.T.; Wu, C.H.; Kuo, I.Y.; Chen, L.Y.; Hu, T.H.; Goto, S.; Nakano, T.; et al. Factor VII promotes hepatocellular carcinoma progression through ERK-TSC signaling. Cell Death Discov. 2015, 1, 15051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamperl, H.; Plattfaut, C.; Freund, A.; Quecke, T.; Theophil, F.; Gieseler, F. Extracellular vesicles from malignant effusions induce tumor cell migration: Inhibitory effect of LMWH tinzaparin. Cell Biol. Int. 2016, 40, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Witte, D.; Otterbein, H.; Förster, M.; Giehl, K.; Zeiser, R.; Lehnert, H.; Ungefroren, H. Negative regulation of TGF-β1-induced MKK6-p38 and MEK-ERK signalling and epithelial-mesenchymal transition by Rac1b. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Suen, J.Y.; Barry, G.D.; Lohman, R.J.; Halili, M.A.; Cotterell, A.J.; Le, G.T.; Fairlie, D.P. Modulating human proteinase activated receptor 2 with a novel antagonist (GB88) and agonist (GB110). Br. J. Pharmacol. 2012, 165, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Suen, J.Y.; Cotterell, A.; Lohman, R.J.; Lim, J.; Han, A.; Yau, M.K.; Liu, L.; Cooper, M.A.; Vesey, D.A.; Fairlie, D.P. Pathway-selective antagonism of proteinase activated receptor 2. Br. J. Pharmacol. 2014, 171, 4112–4124. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Nhu, Q.M.; Toshchakov, V.Y.; Piao, W.; Medvedev, A.E.; Hollenberg, M.D.; Fasano, A.; Vogel, S.N. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: A novel paradigm for receptor cooperativity. J. Biol. Chem. 2008, 283, 24314–24325. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.J.; Sandhu, S.S.; Wijesuriya, S.J.; Hollenberg, M.D. Glycosylation of human proteinase-activated receptor-2 (hPAR2): Role in cell surface expression and signalling. Biochem. J. 2002, 368, 495–505. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungefroren, H.; Witte, D.; Fiedler, C.; Gädeken, T.; Kaufmann, R.; Lehnert, H.; Gieseler, F.; Rauch, B.H. The Role of PAR2 in TGF-β1-Induced ERK Activation and Cell Motility. Int. J. Mol. Sci. 2017, 18, 2776. https://doi.org/10.3390/ijms18122776
Ungefroren H, Witte D, Fiedler C, Gädeken T, Kaufmann R, Lehnert H, Gieseler F, Rauch BH. The Role of PAR2 in TGF-β1-Induced ERK Activation and Cell Motility. International Journal of Molecular Sciences. 2017; 18(12):2776. https://doi.org/10.3390/ijms18122776
Chicago/Turabian StyleUngefroren, Hendrik, David Witte, Christian Fiedler, Thomas Gädeken, Roland Kaufmann, Hendrik Lehnert, Frank Gieseler, and Bernhard H. Rauch. 2017. "The Role of PAR2 in TGF-β1-Induced ERK Activation and Cell Motility" International Journal of Molecular Sciences 18, no. 12: 2776. https://doi.org/10.3390/ijms18122776