Galectins and Carcinogenesis: Their Role in Head and Neck Carcinomas and Thyroid Carcinomas
Abstract
:1. Introduction
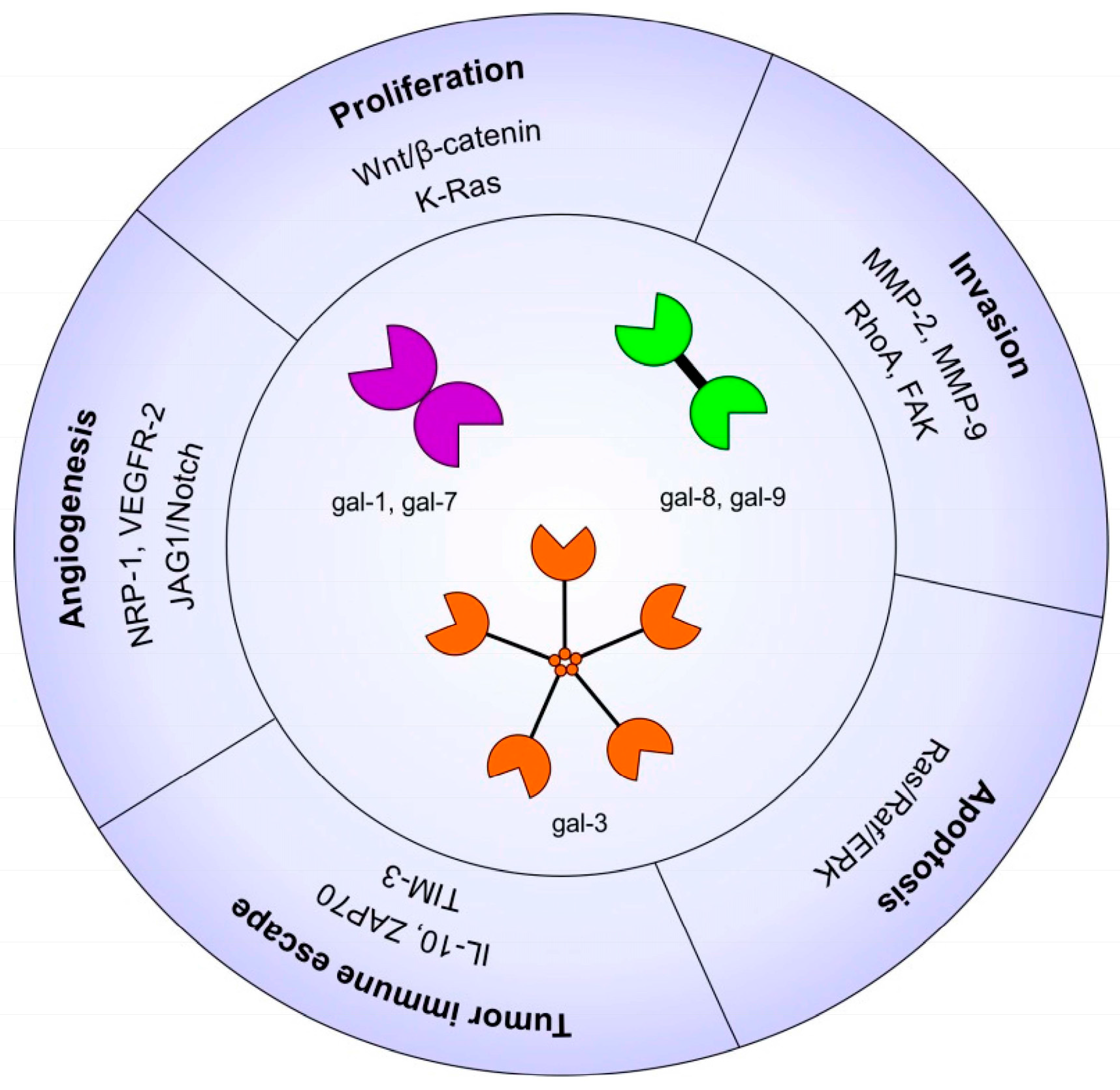
2. Galectins in Head and Neck Cancer and Thyroid Cancer
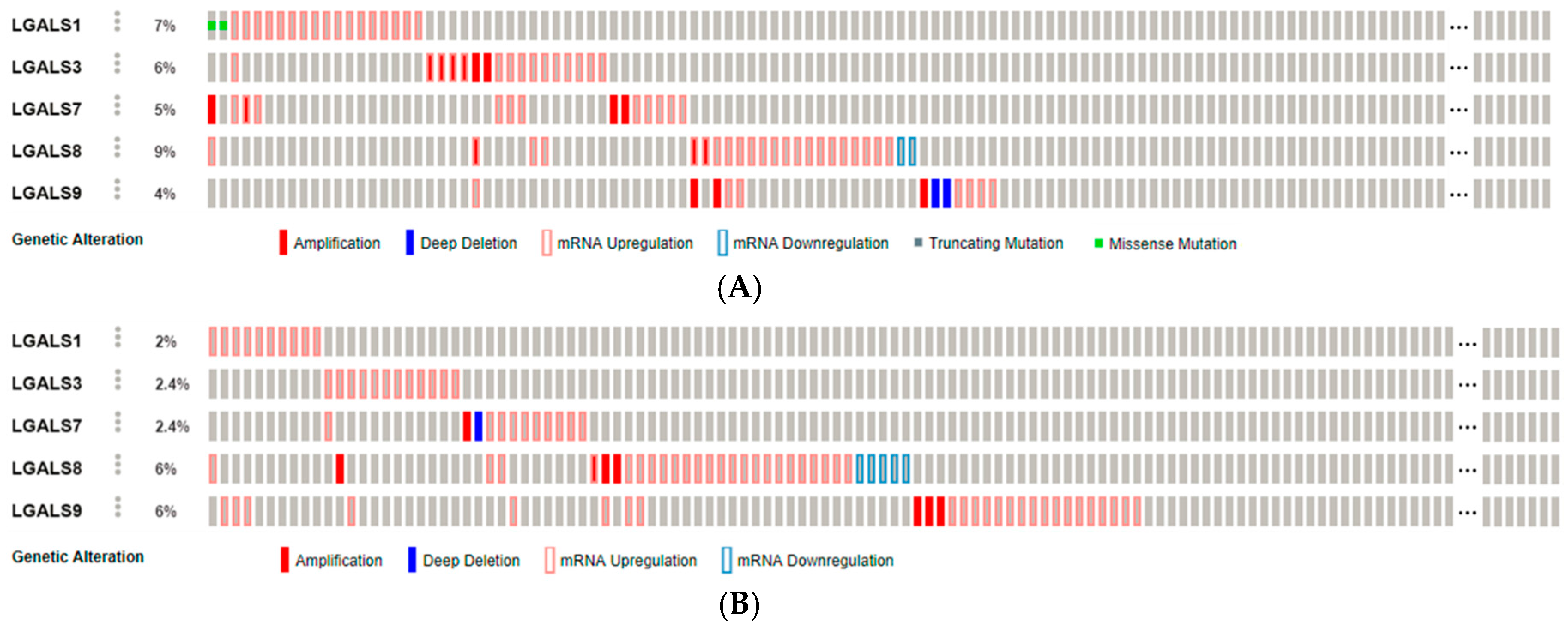
2.1. Galectins in Carcinogenesis
2.2. Galectins and Patient Prognosis
2.3. Galectins and Cell Proliferation/Survival
2.4. Galectins and Cell Migration/Invasion
2.5. Galectins and Angiogenesis
2.6. Galectins and Tumor Immune Escape
2.7. Clinical Potential of Galectin Inhibition
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Duray, A.; Descamps, G.; Decaestecker, C.; Remmelink, M.; Sirtaine, N.; Lechien, J.; Ernoux-Neufcoeur, P.; Bletard, N.; Somja, J.; Depuydt, C.E.; et al. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope 2012, 122, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Gupta, R.; Ashford, B.; Jabbour, J.; Clark, J.R. Oropharyngeal cancer and human papilloma virus: Evolving diagnostic and management paradigms. ANZ J. Surg. 2015. [Google Scholar] [CrossRef] [PubMed]
- Descamps, G.; Karaca, Y.; Lechien, J.R.; Kindt, N.; Decaestecker, C.; Remmelink, M.; Larsimont, D.; Andry, G.; Hassid, S.; Rodriguez, A.; et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Sipos, J.A.; Mazzaferri, E.L. Thyroid cancer epidemiology and prognostic variables. Clin. Oncol. R. Coll. Radiol. 2010, 22, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Pazaitou-Panayiotou, K.; Polyzos, S.A.; Mantzoros, C.S. Obesity and thyroid cancer: Epidemiologic associations and underlying mechanisms. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Grafi-Cohen, M.; Kraiem, Z.; Kloog, Y. Galectin-3 promotes chronic activation of K-Ras and differentiation block in malignant thyroid carcinomas. Mol. Cancer Ther. 2010, 9, 2208–2219. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Tang, W.; Huang, C.; Tang, C.; Xu, J.; Wang, H. Galectin-3 may serve as a potential marker for diagnosis and prognosis in papillary thyroid carcinoma: A meta-analysis. OncoTargets Ther. 2016, 9, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Arcolia, V.; Journe, F.; Wattier, A.; Leteurtre, E.; Renaud, F.; Gabius, H.-J.; Remmelink, M.; Decaestecker, C.; Rodriguez, A.; Boutry, S.; et al. Galectin-1 is a diagnostic marker involved in thyroid cancer progression. Int. J. Oncol. 2017, 51, 760–770. [Google Scholar] [CrossRef] [PubMed]
- cBioPortal for Cancer Genomics. Available online: http://www.cbioportal.org/ (accessed on 15 December 2017).
- Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Sola Gallego, J.J.; Lotan, R.; El-Naggar, A.K. Differential expression of galectin-1 and galectin-3 in benign and malignant salivary gland neoplasms. Int. J. Oncol. 2000, 17, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Remmelink, M.; de Leval, L.; Decaestecker, C.; Duray, A.; Crompot, E.; Sirtaine, N.; André, S.; Kaltner, H.; Leroy, X.; Gabius, H.-J.; Saussez, S. Quantitative immunohistochemical fingerprinting of adhesion/growth-regulatory galectins in salivary gland tumours: Divergent profiles with diagnostic potential. Histopathology 2011, 58, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-M.; Dong, J.-H.; Chen, L.-L.; Zhang, H.-D. Increased expression of galectin-1 is associated with human oral squamous cell carcinoma development. Oncol. Rep. 2009, 21, 983–987. [Google Scholar] [PubMed]
- De Vasconcelos Carvalho, M.; Pereira, J.D.S.; Alves, P.M.; da Silveira, E.J.D.; de Souza, L.B.; Queiroz, L.M.G. Alterations in the immunoexpression of galectins-1, -3 and -7 between different grades of oral epithelial dysplasia. J. Oral Pathol. Med. 2013, 42, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sharma, S.C.; Das, S.N. Galectin-1 and galectin-3: Plausible tumour markers for oral squamous cell carcinoma and suitable targets for screening high-risk population. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 442, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-P.; Chen, S.-W.; Zhuang, S.-M.; Li, H.; Song, M. Galectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol. Oncol. Res. 2013, 19, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Decaestecker, C.; Lorfevre, F.; Chevalier, D.; Mortuaire, G.; Kaltner, H.; André, S.; Toubeau, G.; Gabius, H.-J.; Leroy, X. Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology 2008, 52, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-E.; Tan, T.; Li, C.; Chen, Z.-C.; Ruan, L.; Wang, H.-H.; Su, T.; Zhang, P.-F.; Xiao, Z.-Q. Identification of Galectin-1 as a novel biomarker in nasopharyngeal carcinoma by proteomic analysis. Oncol. Rep. 2010, 24, 495–500. [Google Scholar] [PubMed]
- Duray, A.; De Maesschalck, T.; Decaestecker, C.; Remmelink, M.; Chantrain, G.; Neiveyans, J.; Horoi, M.; Leroy, X.; Gabius, H.-J.; Saussez, S. Galectin fingerprinting in naso-sinusal diseases. Oncol. Rep. 2014, 32, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Thyroid Cancer Study Group Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Italian Thyroid Cancer Study Group (ITCSG) Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- Than, T.H.; Swethadri, G.K.; Wong, J.; Ahmad, T.; Jamil, D.; Maganlal, R.K.; Hamdi, M.M.; Abdullah, M.S. Expression of Galectin-3 and Galectin-7 in thyroid malignancy as potential diagnostic indicators. Singap. Med. J. 2008, 49, 333–338. [Google Scholar] [PubMed]
- Saussez, S.; Glinoer, D.; Chantrain, G.; Pattou, F.; Carnaille, B.; André, S.; Gabius, H.-J.; Laurent, G. Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid Off. J. Am. Thyroid Assoc. 2008, 18, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.-T.; Shi, G.; Cao, H.; Nelson, D.W.; Wang, Y.; Chen, E.Y.; Zhao, S.; Kong, C.; Richardson, D.; O’Byrne, K.J.; et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8932–8941. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Decaestecker, C.; Lorfevre, F.; Cucu, D.-R.; Mortuaire, G.; Chevalier, D.; Wacreniez, A.; Kaltner, H.; André, S.; Toubeau, G.; Camby, I.; et al. High level of galectin-1 expression is a negative prognostic predictor of recurrence in laryngeal squamous cell carcinomas. Int. J. Oncol. 2007, 30, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-L.; Li, C.-F.; Lin, C.; Lin, Y.-S. Galectin-1 overexpression in nasopharyngeal carcinoma: Effect on survival. Acta Otolaryngol. (Stockh.) 2014, 134, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kishino, M.; Sato, S.; Hirose, K.; Sakai, M.; Fukuda, Y.; Murakami, S.; Toyosawa, S. Galectin-1 expression is associated with tumour immunity and prognosis in gingival squamous cell carcinoma. J. Clin. Pathol. 2017, 70, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Inohara, H.; Akahani, S.; Yoshii, T.; Takenaka, Y.; Yoshida, J.; Hattori, K.; Tomiyama, Y.; Raz, A.; Kubo, T. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 4635–4640. [Google Scholar]
- Teymoortash, A.; Pientka, A.; Schrader, C.; Tiemann, M.; Werner, J.A. Expression of galectin-3 in adenoid cystic carcinoma of the head and neck and its relationship with distant metastasis. J. Cancer Res. Clin. Oncol. 2006, 132, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Lorfevre, F.; Lequeux, T.; Laurent, G.; Chantrain, G.; Vertongen, F.; Toubeau, G.; Decaestecker, C.; Kiss, R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008, 44, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Cucu, D.-R.; Decaestecker, C.; Chevalier, D.; Kaltner, H.; André, S.; Wacreniez, A.; Toubeau, G.; Camby, I.; Gabius, H.-J.; Kiss, R. Galectin 7 (p53-induced gene 1): A new prognostic predictor of recurrence and survival in stage IV hypopharyngeal cancer. Ann. Surg. Oncol. 2006, 13, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Piantelli, M.; Iacobelli, S.; Almadori, G.; Iezzi, M.; Tinari, N.; Natoli, C.; Cadoni, G.; Lauriola, L.; Ranelletti, F.O. Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J. Clin. Oncol. 2002, 20, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Plzák, J.; Betka, J.; Smetana, K.; Chovanec, M.; Kaltner, H.; André, S.; Kodet, R.; Gabius, H.-J. Galectin-3—An emerging prognostic indicator in advanced head and neck carcinoma. Eur. J. Cancer 2004, 40, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Thyroid Cancer Survival Rates, by Type and Stage. Available online: https://www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 27 October 2017).
- Kim, E.S.; Lim, D.J.; Lee, K.; Jung, C.K.; Bae, J.S.; Jung, S.L.; Baek, K.H.; Lee, J.M.; Moon, S.D.; Kang, M.I.; et al. Absence of galectin-3 immunostaining in fine-needle aspiration cytology specimens from papillary thyroid carcinoma is associated with favorable pathological indices. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, J.B. Prognostic value of epidermal growth factor receptor, p53 and galectin-3 expression in papillary thyroid carcinoma. J. Int. Med. Res. 2013, 41, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Rossi, G.; Coscio, G.D.; Iervasi, G.; Nicolini, A.; Carpi, F.; Mechanick, J.I.; Bartolazzi, A. Galectin-3 detection on large-needle aspiration biopsy improves preoperative selection of thyroid nodules: A prospective cohort study. Ann. Med. 2010, 42, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sumana, B.S.; Shashidhar, S.; Shivarudrappa, A.S. Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. J. Clin. Diagn. Res. 2015, 9, EC07-11. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandria, C.; Braesch-Andersen, S.; Bejo, K.; Reder, S.; Blechert, B.; Schwaiger, M.; Bartolazzi, A. Noninvasive In Vivo Imaging and Biologic Characterization of Thyroid Tumors by ImmunoPET Targeting of Galectin-3. Cancer Res. 2016, 76, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Arcolia, V.; Journe, F.; Renaud, F.; Leteurtre, E.; Gabius, H.-J.; Remmelink, M.; Saussez, S. Combination of galectin-3, CK19 and HBME-1 immunostaining improves the diagnosis of thyroid cancer. Oncol. Lett. 2017, 14, 4183–4189. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Tinari, N.; Semeraro, M.L.; Natoli, C.; Iacobelli, S.; Malorni, W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000, 473, 311–315. [Google Scholar] [CrossRef]
- Yoshii, T.; Fukumori, T.; Honjo, Y.; Inohara, H.; Kim, H.-R.C.; Raz, A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J. Biol. Chem. 2002, 277, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, N.; Xie, J.; Luo, H.; Zhang, J.; Deng, G.; Li, Y.; Zhang, J. Gene silencing of galectin-3 changes the biological behavior of Eca109 human esophageal cancer cells. Mol. Med. Rep. 2016, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Sun, J.; Li, J.; Gu, X.; Xu, W. Antitumor effects of galectin-3 inhibition in human renal carcinoma cells. Exp. Biol. Med. 2016, 241, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Zhang, T.; Hogan, V.; Raz, A. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget 2014, 5, 9992–10001. [Google Scholar] [CrossRef] [PubMed]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-I.; Whang, E.E.; Donner, D.B.; Jiang, X.; Price, B.D.; Carothers, A.M.; Delaine, T.; Leffler, H.; Nilsson, U.J.; Nose, V.; et al. Galectin-3 targeted therapy with a small molecule inhibitor activates apoptosis and enhances both chemosensitivity and radiosensitivity in papillary thyroid cancer. Mol. Cancer Res. 2009, 7, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Menachem, A.; Bodner, O.; Pastor, J.; Raz, A.; Kloog, Y. Inhibition of malignant thyroid carcinoma cell proliferation by Ras and galectin-3 inhibitors. Cell Death Discov. 2015, 1, 15047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, P.; Shi, B.; Zhou, M.; Jiang, H.; Zhang, H.; Pan, X.; Gao, H.; Sun, H.; Li, Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014, 5, e991. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Dong, X.-W.; Guo, X.-L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Shimura, T.; Yajima, T.; Kubo, N.; Araki, K.; Tsutsumi, S.; Suzuki, H.; Kuwano, H.; Raz, A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin. Int. J. Cancer 2011, 129, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Mendoza, P.; Silva, P.; Quest, A.F.; Torres, V.A. A novel caveolin-1/p85α/Rab5/Tiam1/Rac1 signaling axis in tumor cell migration and invasion. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.; Wiseman, S.M.; Meng, F.; Kasaian, K.; Strugnell, S.; Mofid, A.; Gown, A.; Jones, S.J.M.; Nabi, I.R. Coordinated expression of galectin-3 and caveolin-1 in thyroid cancer. J. Pathol. 2012, 228, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zou, R.; Chen, Z.; Fan, B.; Wang, Z.; Wang, Y.; Yin, X.; Zhang, D.; Tong, L.; Yang, F.; et al. Silencing RhoA inhibits migration and invasion through Wnt/β-catenin pathway and growth through cell cycle regulation in human tongue cancer. Acta Biochim. Biophys. Sin. 2014, 46, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Xu, F.; Yin, Y.; Zhu, L.; Zhou, F. Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim. Biophys. Sin. 2016, 48, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Hong, H.-C.; Hong, T.-M.; Chiang, W.-F.; Jin, Y.-T.; Chen, Y.-L. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin. Cancer Res. 2011, 17, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Hong, T.-M.; Cheng, H.-W.; Pan, S.-H.; Liang, Y.-R.; Hong, H.-C.; Chiang, W.-F.; Wong, T.-Y.; Shieh, D.-B.; Shiau, A.-L.; et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol. Cancer Res. 2009, 7, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rizqiawan, A.; Tobiume, K.; Okui, G.; Yamamoto, K.; Shigeishi, H.; Ono, S.; Shimasue, H.; Takechi, M.; Higashikawa, K.; Kamata, N. Autocrine galectin-1 promotes collective cell migration of squamous cell carcinoma cells through up-regulation of distinct integrins. Biochem. Biophys. Res. Commun. 2013, 441, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Seilhamer, J.J.; Pang, M.; Levine, W.B.; Beynon, D.; Berliner, J.A. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj. J. 1995, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.J.L.; Postel, R.; Brandwijk, R.J.M.G.E.; Dings, R.P.M.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.H.; Ying, N.W.; Wu, M.H.; Chiang, W.F.; Hsu, C.L.; Wong, T.Y.; Jin, Y.T.; Hong, T.M.; Chen, Y.L. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008, 27, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- D’Haene, N.; Sauvage, S.; Maris, C.; Adanja, I.; Le Mercier, M.; Decaestecker, C.; Baum, L.; Salmon, I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS ONE 2013, 8, e67029. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.-G. Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.I.; Liu, F.-T.; Panjwani, N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J. Exp. Med. 2010, 207, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.N.; Sheldon, H.; Pereira, J.X.; Paluch, C.; Bridges, E.M.; El-Cheikh, M.C.; Harris, A.L.; Bernardes, E.S. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget 2017, 8, 49484–49501. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Ramhorst, R.E.; Rubinstein, N.; Corigliano, A.; Daroqui, M.C.; Kier-Joffé, E.B.; Fainboim, L. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002, 9, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.; Alvarez, M.; Zwirner, N.W.; Toscano, M.A.; Ilarregui, J.M.; Bravo, A.; Mordoh, J.; Fainboim, L.; Podhajcer, O.L.; Rabinovich, G.A. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004, 5, 241–251. [Google Scholar] [CrossRef]
- Kovács-Sólyom, F.; Blaskó, A.; Fajka-Boja, R.; Katona, R.L.; Végh, L.; Novák, J.; Szebeni, G.J.; Krenács, L.; Uher, F.; Tubak, V.; Kiss, R.; Monostori, E. Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol. Lett. 2010, 127, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Opperman, M.J.; Barthel, S.R.; Hays, D.; Schatton, T.; Zhan, Q.; He, X.; Matta, K.L.; Supko, J.G.; Frank, M.H.; et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. J. Investig. Dermatol. 2012, 132, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.-R.C.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar] [PubMed]
- Peng, W.; Wang, H.Y.; Miyahara, Y.; Peng, G.; Wang, R.-F. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008, 68, 7228–7236. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Habuchi, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, H.; Geng, J.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J. Biol. Chem. 2014, 289, 33311–33319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Oomizu, S.; Sakata, K.-M.; Sakata, A.; Arikawa, T.; Watanabe, K.; Ito, K.; Takeshita, K.; Niki, T.; Saita, N.; et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008, 127, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Severson, J.J.; Serracino, H.S.; Mateescu, V.; Raeburn, C.D.; McIntyre, R.C.; Sams, S.B.; Haugen, B.R.; French, J.D. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol. Res. 2015, 3, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Klyosov, A.; Zomer, E.; Platt, D. DAVANAT® (GM-CT-01) and Colon Cancer: Preclinical and Clinical (Phase I and II) Studies. In Glycobiology and Drug Design; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1102, pp. 89–130. ISBN 978-0-8412-2765-1. [Google Scholar]
- Demotte, N.; Bigirimana, R.; Wieërs, G.; Stroobant, V.; Squifflet, J.-L.; Carrasco, J.; Thielemans, K.; Baurain, J.-F.; Van Der Smissen, P.; Courtoy, P.J.; van der Bruggen, P. A short treatment with galactomannan GM-CT-01 corrects the functions of freshly isolated human tumor-infiltrating lymphocytes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Redmond, W. Immunotherapy plus a galectin-3 inhibitor improves anti-tumor immunity: Insights from mice and a first-in-human phase I clinical trial. In Proceedings of the GTCbio 9th Immunotherapeutics & Immunomonitoring Conference, San Diego, CA, USA, 2 February 2017. [Google Scholar]

| Type of Tissues | Gal-1 | Gal-3 | Gal-7 | Gal-8 | Gal-9 | Ref. |
|---|---|---|---|---|---|---|
| Salivary gland carcinomas | *  | **  |  |  | [19,20] | |
| Oral cavity carcinoma |  |  | [21,22,23,24] | |||
| Laryngeal carcinoma |  |  | [25] | |||
| Hypopharyngeal carcinoma |  |  | [25] | |||
| Naso-pharyngeal carcinoma |  |  |  | [26,27] | ||
| Thyroid carcinomas |  |  | No change | [14,28,29,30,31] |
| Gal-1 | Gal-3 | Gal-9 | ||||
|---|---|---|---|---|---|---|
| HNSCC | TC | HNSCC | TC | HNSCC | TC | |
| Cell proliferation | [22] | [14] | [23] | [54,56] | ||
| Apoptosis | [53,55,56] | |||||
| Cell migration/invasion | [64,65] | [14] | [23,62] | [61] | ||
| Angiogenesis | [69] | |||||
| Tumor immune escape | [34] | [85] | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindt, N.; Journe, F.; Ghanem, G.E.; Saussez, S. Galectins and Carcinogenesis: Their Role in Head and Neck Carcinomas and Thyroid Carcinomas. Int. J. Mol. Sci. 2017, 18, 2745. https://doi.org/10.3390/ijms18122745
Kindt N, Journe F, Ghanem GE, Saussez S. Galectins and Carcinogenesis: Their Role in Head and Neck Carcinomas and Thyroid Carcinomas. International Journal of Molecular Sciences. 2017; 18(12):2745. https://doi.org/10.3390/ijms18122745
Chicago/Turabian StyleKindt, Nadège, Fabrice Journe, Ghanem E. Ghanem, and Sven Saussez. 2017. "Galectins and Carcinogenesis: Their Role in Head and Neck Carcinomas and Thyroid Carcinomas" International Journal of Molecular Sciences 18, no. 12: 2745. https://doi.org/10.3390/ijms18122745




