Serum Levels of S100b and NSE Proteins in Patients with Non-Transfusion-Dependent Thalassemia as Biomarkers of Brain Ischemia and Cerebral Vasculopathy
Abstract
:1. Introduction
2. Results
3. Discussion
4. Material and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Musallam, K.M.; Rivella, S.; Vichinsky, E.; Rachmilewitz, E.A. Non-transfusion-dependent thalassemias. Haematologica 2013, 98, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Poggiali, E.; Taher, A.T.; Musallam, K.M. Hypercoagulability in beta-thalassemia: A status quo. Expert Rev. Hematol. 2012, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Isma’eel, H.; Mehio, G.; Bignamini, D.; Kattamis, A.; Rachmilewitz, E.A.; Cappellini, M.D. Prevalence of thromboembolic events among 8860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb. Haemost. 2006, 96, 488–491. [Google Scholar] [PubMed]
- DeBaun, M.R.; Gordon, M.; McKinstry, R.C.; Noetzel, M.J.; White, D.A.; Sarnaik, S.A.; Meier, E.R.; Howard, T.H.; Majumdar, S.; Inusa, B.P.; et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N. Engl. J. Med. 2014, 371, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Haghpanah, S.; Karimi, M. Cerebral thrombosis in patients with beta-thalassemia: A systematic review. Blood Coagul. Fibrinolysis 2012, 23, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bagheri, H.; Rastgu, F.; Rachmilewitz, E.A. Magnetic resonance imaging to determine the incidence of brain ischaemia in patients with beta-thalassaemia intermedia. Thromb. Haemost. 2010, 103, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Manfre, L.; Giarratano, E.; Maggio, A.; Banco, A.; Vaccaro, G.; Lagalla, R. MR imaging of the brain: Findings in asymptomatic patients with thalassemia intermedia and sickle cell-thalassemia disease. Am. J. Roentgenol. 1999, 173, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Mehio, G.; Isma’eel, H.; Cappellini, M.D. Stroke in thalassemia: A dilemma. Am. J. Hematol. 2008, 83, 343. [Google Scholar] [CrossRef] [PubMed]
- Teli, A.; Economou, M.; Rudolf, J.; Tzovaras, F.; Gourtsa, V.; Kondou, A.; Kontopoulos, E.; Gombakis, N.; Athanassiou-Metaxa, M.; Zafeiriou, D. Subclinical central nervous system involvement and thrombophilic status in young thalassemia intermedia patients of Greek origin. Blood Coagul. Fibrinolysis 2012, 23, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Beydoun, A.; Hourani, R.; Nasreddine, W.; Raad, R.; Koussa, S.; Taher, A.T. Brain magnetic resonance angiography in splenectomized adults with beta-thalassemia intermedia. Eur. J. Haematol. 2011, 87, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Taher, A.T.; Rachmilewitz, E.A. beta-thalassemia intermedia: A clinical perspective. Cold Spring Harb. Perspect. Med. 2012, 2, a013482. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; McKie, V.; Nichols, F.; Carl, E.; Zhang, D.L.; McKie, K.; Figueroa, R.; Litaker, M.; Thompson, W.; Hess, D. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N. Engl. J. Med. 1992, 326, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bulas, D. Screening children for sickle cell vasculopathy: Guidelines for transcranial Doppler evaluation. Pediatr. Radiol. 2005, 35, 235–241. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Kirkham, F.J. Central nervous system complications and management in sickle cell disease. Blood 2016, 127, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.M.; McKinney, J.; Smith, L.; Brisman, J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit. Care 2007, 6, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Adami, C.; Sorci, G.; Blasi, E.; Agneletti, A.L.; Bistoni, F.; Donato, R. S100B expression in and effects on microglia. Glia 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Barone, F.C.; Clark, R.K.; Price, W.J.; White, R.F.; Feuerstein, G.Z.; Storer, B.L.; Ohlstein, E.H. Neuron-specific enolase increases in cerebral and systemic circulation following focal ischemia. Brain Res. 1993, 623, 77–82. [Google Scholar] [CrossRef]
- Casmiro, M.; Maitan, S.; De Pasquale, F.; Cova, V.; Scarpa, E.; Vignatelli, L. NSE Study Group. Cerebrospinal fluid and serum neuron-specific enolase concentrations in a normal population. Eur. J. Neurol. 2005, 12, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Gattaz, W.F.; Lara, D.R.; Elkis, H.; Portela, L.V.; Goncalves, C.A.; Tort, A.B.; Henna, J.; Souza, D.O. Decreased S100-beta protein in schizophrenia: Preliminary evidence. Schizophr. Res. 2000, 43, 91–95. [Google Scholar] [CrossRef]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. NINDS rt-PA Stroke Study Group. Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 2006, 37, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Lara, D.R.; Gama, C.S.; Belmonte-de-Abreu, P.; Portela, L.V.; Goncalves, C.A.; Fonseca, M.; Hauck, S.; Souza, D.O. Increased serum S100B protein in schizophrenia: A study in medication-free patients. J. Psychiatr. Res. 2001, 35, 11–14. [Google Scholar] [CrossRef]
- Wiesmann, M.; Wandinger, K.P.; Missler, U.; Eckhoff, D.; Rothermundt, M.; Arolt, V.; Kirchner, H. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol. Psychiatry 1999, 45, 1508–1511. [Google Scholar] [CrossRef]
- Kato, G.J.; McGowan, V.; Machado, R.F.; Little, J.A.; Taylor, J.T.; Morris, C.R.; Nichols, J.S.; Wang, X.; Poljakovic, M.; Morris, S.M., Jr.; et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006, 107, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J. TCD in sickle cell disease: An important and useful test. Pediatr. Radiol. 2005, 35, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, F.; Verlhac, S.; Chevret, S.; Torres, M.; Coic, L.; Arnaud, C.; Kamdem, A.; Hau, I.; Grazia Neonato, M.; Delacourt, C. G6PD deficiency, absence of alpha-thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood 2008, 112, 4314–4317. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, C.; Metaxotou-Mavromati, A.; Ladis, V.; Tsiarta, H.; Laskari, S.; Kanavakis, E. The clinical phenotype of beta and delta beta thalassemias in Greece. Eur. J. Pediatr. 1982, 139, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.H.; Bulas, D.I. Transcranial Doppler imaging in children: Sickle cell screening and beyond. Pediatr. Radiol. 2005, 35, 54–65. [Google Scholar] [CrossRef] [PubMed]
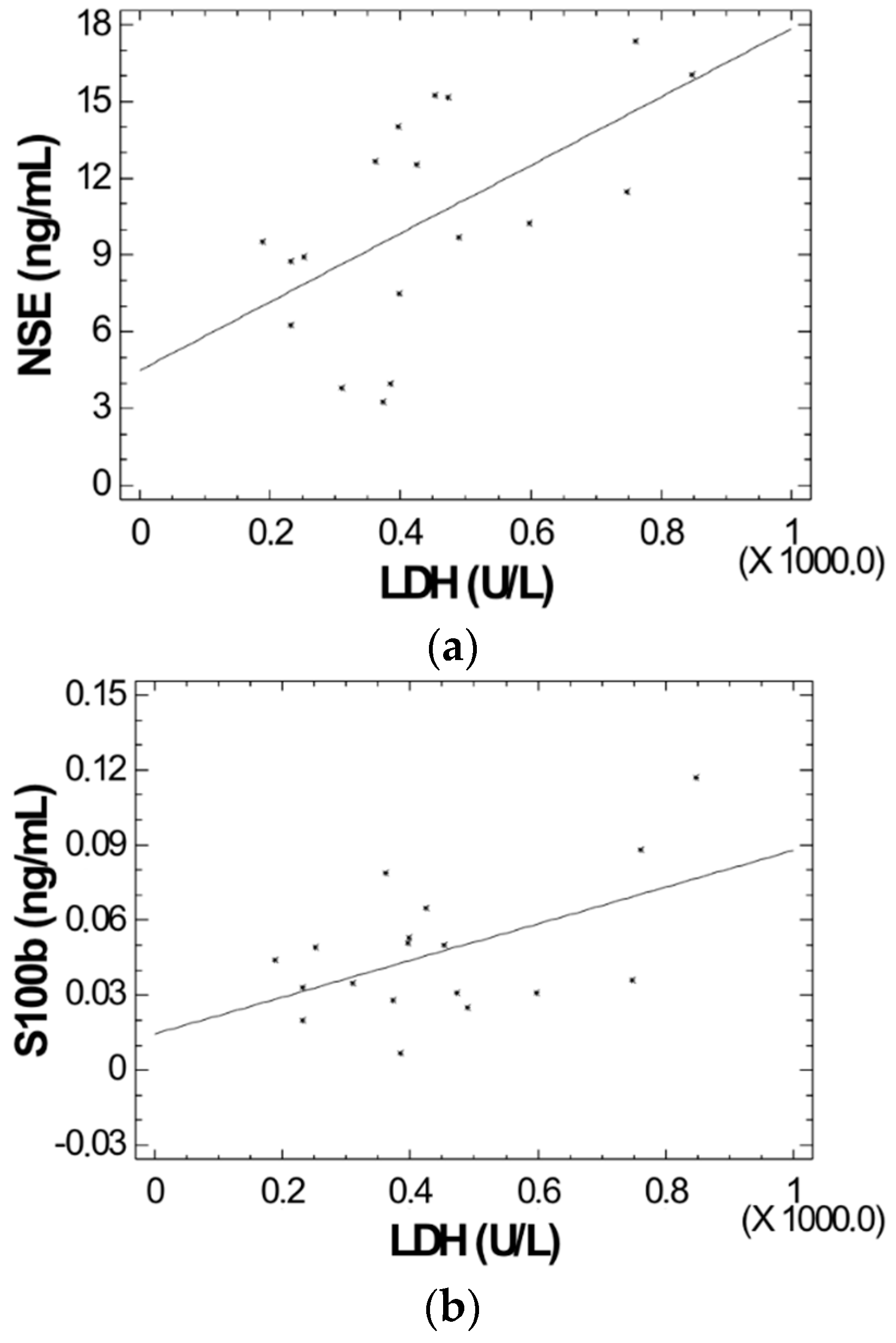
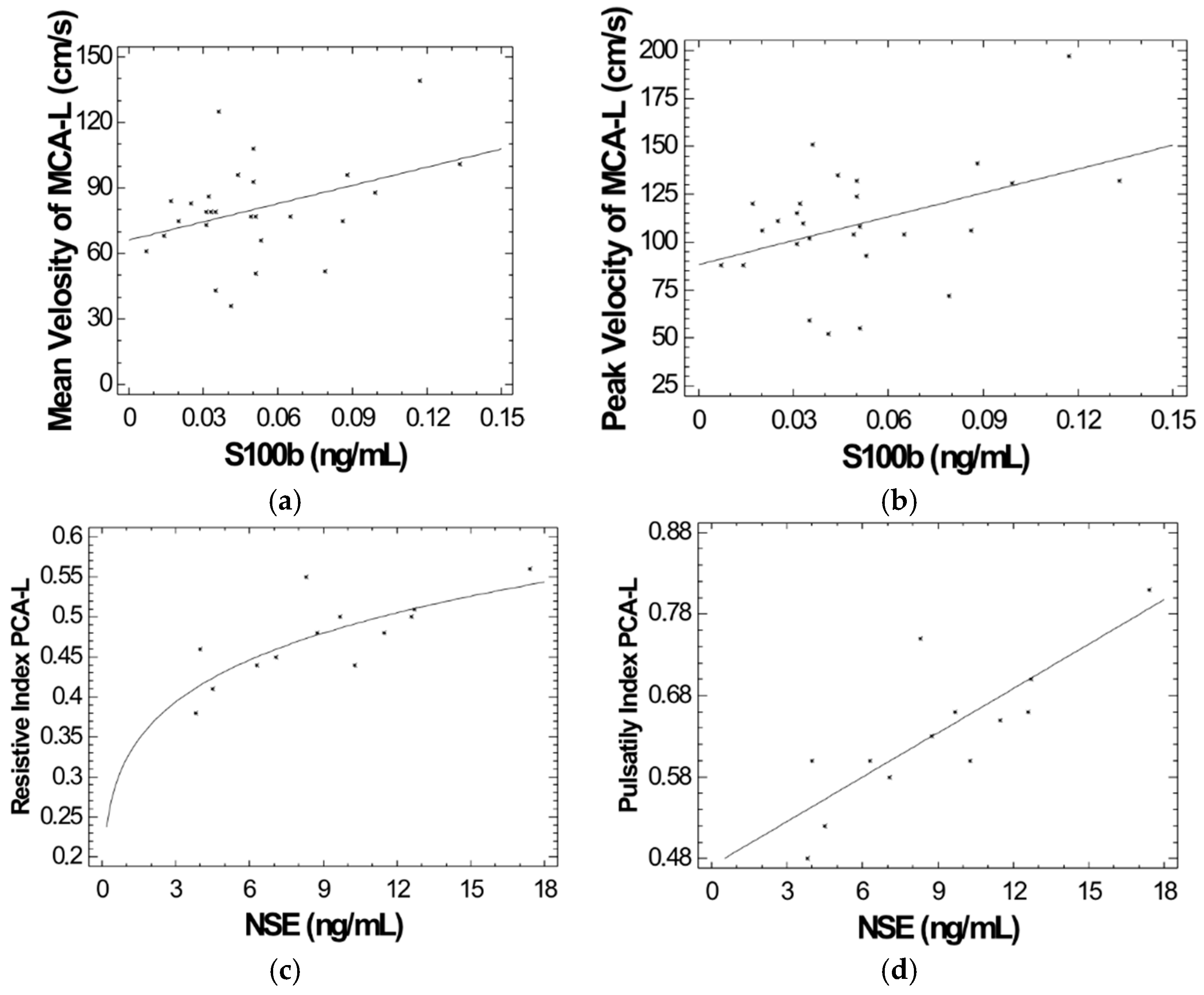
| Patients | NSE (ng/mL) | S100B (ng/mL) | Controls | NSE (ng/mL) | S100B (ng/mL) |
|---|---|---|---|---|---|
| P1 | 4.5 | 0.014 | C1 | 6.94 | 0.104 |
| P2 | 7.07 | 0.035 | C2 | 4.45 | 0.059 |
| P3 | 8.75 | 0.033 | C3 | 2.86 | 0.048 |
| P4 | 16.07 | 0.117 | C4 | 2.91 | 0.059 |
| P5 | 15.25 | 0.050 | C5 | 7.49 | 0.027 |
| P6 | 17.38 | 0.088 | C6 | 7.98 | 0.046 |
| P7 | 9.68 | 0.025 | C7 | 5.69 | 0.042 |
| P8 | 3.00 | 0.05 | C8 | 6.12 | 0.032 |
| P9 | 3.7 | 0.017 | C9 | 8.67 | 0.050 |
| P10 | 4.00 | 0.007 | C10 | 12.77 | 0.075 |
| P11 | 3.81 | 0.035 | C11 | 14.55 | 0.051 |
| P12 | 3.27 | 0.028 | C12 | 4.44 | 0.074 |
| P13 | 7.50 | 0.053 | C13 | 13.95 | 0.049 |
| P14 | 14.03 | 0.051 | C14 | 15.72 | 0.059 |
| P15 | 12.54 | 0.065 | C15 | 15.54 | 0.070 |
| P16 | 15.1 | 0.041 | C16 | 9.74 | 0.030 |
| P17 | 5.13 | 0.086 | C17 | 15.82 | 0.027 |
| P18 | 7.48 | 0.099 | C18 | 13.91 | 0.068 |
| P19 | 11.3 | 0.051 | C19 | 13.35 | 0.027 |
| P20 | 11.48 | 0.049 | C20 | 14.96 | 0.082 |
| P21 | 8.94 | 0.031 | C21 | 6.48 | 0.027 |
| P22 | 10.26 | 0.044 | C22 | 12.71 | 0.053 |
| P23 | 15.15 | 0.133 | C23 | 17.10 | 0.082 |
| P24 | 9.52 | 0.079 | C24 | 10.22 | 0.075 |
| P25 | 14.56 | 0.020 | C25 | 4.70 | 0.056 |
| P26 | 12.68 | 0.032 | C26 | 10.38 | 0.083 |
| P27 | 12.57 | 0.032 | |||
| P28 | 6.28 | 0.036 | |||
| P29 | 14.60 | 0.031 | |||
| P30 | 8.3 | 0.051 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanavaki, A.; Spengos, K.; Moraki, M.; Delaporta, P.; Kariyannis, C.; Papassotiriou, I.; Kattamis, A. Serum Levels of S100b and NSE Proteins in Patients with Non-Transfusion-Dependent Thalassemia as Biomarkers of Brain Ischemia and Cerebral Vasculopathy. Int. J. Mol. Sci. 2017, 18, 2724. https://doi.org/10.3390/ijms18122724
Kanavaki A, Spengos K, Moraki M, Delaporta P, Kariyannis C, Papassotiriou I, Kattamis A. Serum Levels of S100b and NSE Proteins in Patients with Non-Transfusion-Dependent Thalassemia as Biomarkers of Brain Ischemia and Cerebral Vasculopathy. International Journal of Molecular Sciences. 2017; 18(12):2724. https://doi.org/10.3390/ijms18122724
Chicago/Turabian StyleKanavaki, Aikaterini, Konstantinos Spengos, Maria Moraki, Polyxeni Delaporta, Catherine Kariyannis, Ioannis Papassotiriou, and Antonis Kattamis. 2017. "Serum Levels of S100b and NSE Proteins in Patients with Non-Transfusion-Dependent Thalassemia as Biomarkers of Brain Ischemia and Cerebral Vasculopathy" International Journal of Molecular Sciences 18, no. 12: 2724. https://doi.org/10.3390/ijms18122724





