The Interactions of Aquaporins and Mineral Nutrients in Higher Plants
Abstract
:1. Introduction
2. Nitrogen (N)
2.1. Nitrate
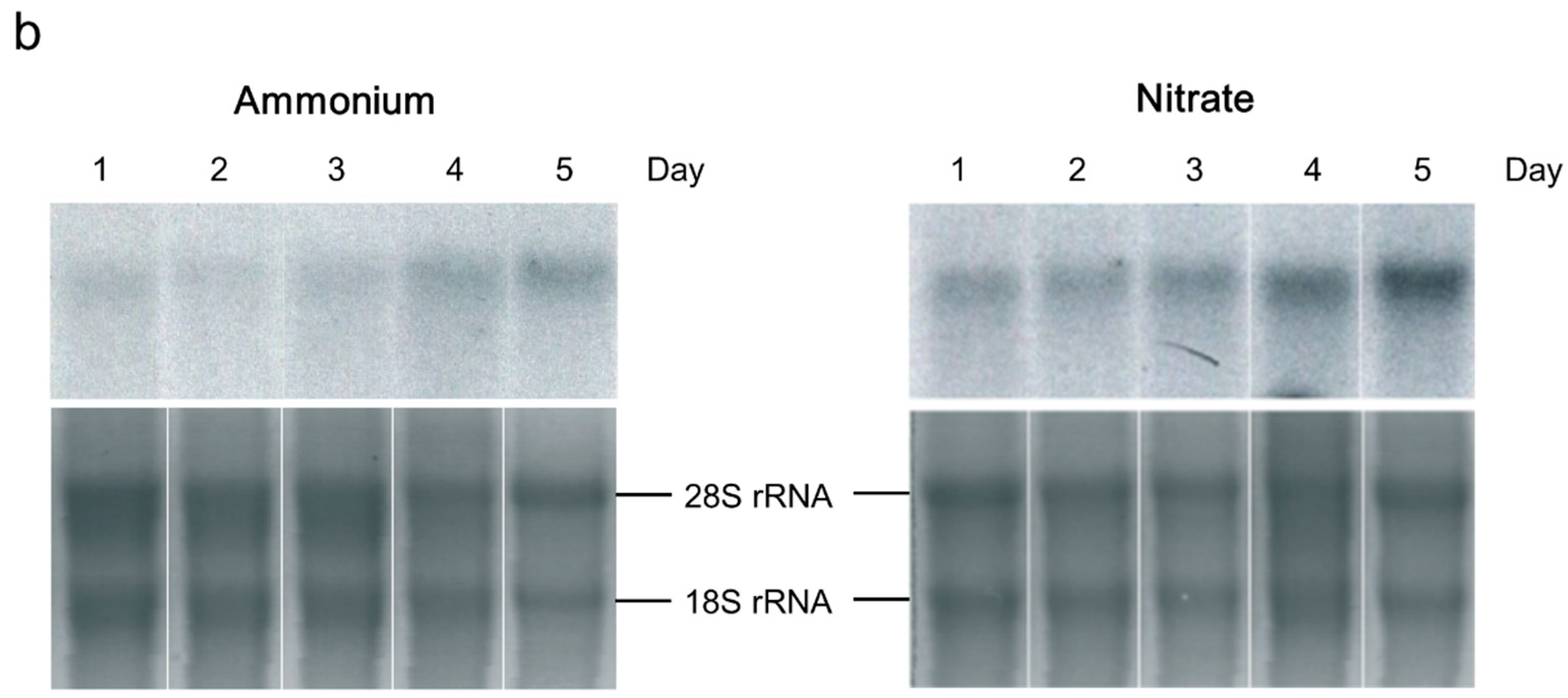
2.2. Ammonia/Ammonium
2.3. Urea
3. Phosphorus (P)
4. Potassium (K)
5. Calcium (Ca)
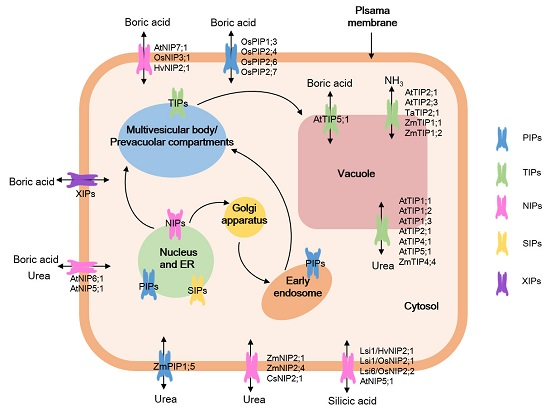
6. Boron (B)
7. Silicon (Si)
8. Conclusions and Future Perspectives
- (1)
- The functions of aquaporins in the transport of other novel putative substrates, such as Mg, S, and other micronutrients, which await further investigation.
- (2)
- New aquaporin subclasses and unknown functions of aquaporins recently discovered in certain plant species should be deciphered.
- (3)
- Investigation of the interactions between water and mineral nutrient transport, as well as interactions between different mineral nutrients regulated by aquaporins will be required.
- (4)
- The role of aquaporins during biotic and abiotic stress, and the relevance of altered aquaporin expression for biotechnological improvement of plant tolerance must be explored.
- (5)
- Aquaporin functions need to be further investigated concerning whole plant physiology, which requires a better understanding of how the various aquaporin transport activities are coupled with plant mineral nutrient transport proteins.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C. Aquaporins and water permeability of plant membranes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 399–429. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Santoni, V.; Luu, D.T.; Wudick, M.M.; Verdoucq, L. The cellular dynamics of plant aquaporin expression and functions. Curr. Opin. Plant Biol. 2009, 12, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Chrispeels, M.J. Aquaporins. A molecular entry into plant water relations. Plant Physiol. 2001, 125, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Plant aquaporins: Roles in water homeostasis, nutrition, and signaling processes. In Transporters and Pumps in Plant Signaling; Geisler, M., Venema, K., Eds.; Springer Berlin Heidelberg: Berlin & Heidelberg, Germany, 2011; pp. 3–36. [Google Scholar]
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Bienert, M.D.; Jahn, T.P.; Boutry, M.; Chaumont, F. Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 2011, 66, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Danielson, J.A.H.; Johanson, U. Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Zelazny, E.; Chaumont, F. Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochim. Biophys. Acta 2006, 1758, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.J.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, W.; Liu, J.H.; Zhang, J.B.; Jia, C.H.; Miao, H.X.; Xu, B.Y.; Jin, Z.Q. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, S.Y.; Hu, W.; Deng, X.M.; Wei, S.Y.; Yang, G.X.; He, G.Y. The wheat aquaporin gene TaAQP7 confers tolerance to cold stress in transgenic tobacco. Z. Naturforsch. C. 2014, 69, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant Cell Physiol. 2012, 53, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; von Wiren, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ago, Y.; Mitani, N.; Li, R.Y.; Su, Y.H.; Yamaji, N.; McGrath, S.P.; Ma, J.F. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010, 186, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Rodriguez-Zas, S.; Aldea, M.; Li, M.; Zhu, J.; Gonzalez, D.O.; Vodkin, L.O.; DeLucia, E.; Clough, S.J. Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol. Plant Microbe Interact. 2005, 18, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.R.; Paek, K.H. Arabidopsis tonoplast proteins TIP1 and TIP2 interact with the cucumber mosaic virus 1a replication protein. J. Gen. Virol. 2006, 87, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Liu, L.H.; Ludewig, U.; Gassert, B.; Frommer, W.B.; von Wiren, N. Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol. 2003, 133, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Guclu, J.; Ripoche, P.; Maurel, C. Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J. 1999, 18, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bertl, A.; Kaldenhoff, R. Function of a separate NH3-pore in Aquaporin TIP2;2 from wheat. FEBS Lett. 2007, 581, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Loque, D.; Ludewig, U.; Yuan, L.X.; von Wiren, N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005, 137, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, L.; Liu, W.; Wang, M.; Gu, M.; Ren, B.; Xu, G.; Shen, Q.; Guo, S. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa) plants. Physiol. Plant. 2016, 156, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Miwa, K.; Takano, J.; Wada, M.; Fujiwara, T. Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant Cell Physiol. 2009, 50, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 is a silicon influx transporter in barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Roberts, D.M. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J. Biol. Chem. 2007, 282, 24209–24218. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schussler, M.D.; Jahn, T.P. Metalloids: Essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem. Sci. 2008, 33, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK; Academic Press: London, UK, 2012. [Google Scholar]
- Hacke, U.G.; Plavcova, L.; Almeida-Rodriguez, A.; King-Jones, S.; Zhou, W.C.; Cooke, J.E.K. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol. 2010, 30, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Sakurai, J.; Hayashi, H.; Murai-Hatano, M. Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 2014, 379, 289–300. [Google Scholar] [CrossRef]
- Ren, B.B.; Wang, M.; Chen, Y.P.; Sun, G.M.; Li, Y.; Shen, Q.R.; Guo, S.W. Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 2015, 396, 397–410. [Google Scholar] [CrossRef]
- Wilkinson, S.; Bacon, M.A.; Davies, W.J. Nitrate signalling to stomata and growing leaves: Interactions with soil drying, ABA, and xylem sap pH in maize. J. Exp. Bot. 2007, 58, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Gloser, V.; Zwieniecki, M.A.; Orians, C.M.; Holbrook, N.M. Dynamic changes in root hydraulic properties in response to nitrate availability. J. Exp. Bot. 2007, 58, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Gorska, A.; Ye, Q.; Holbrook, N.M.; Zwieniecki, M.A. Nitrate control of root hydraulic properties in plants: Translating local information to whole plant response. Plant Physiol. 2008, 148, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.; Bousser, A.; Sissoeff, I.; Roche, O.; Hoarau, J.; Mahe, A. Cloning and characterization of ZmPIP1-5b, an aquaporin transporting water and urea. Plant Sci. 2003, 165, 21–31. [Google Scholar] [CrossRef]
- Wang, Y.H.; Garvin, D.F.; Kochian, L.V. Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiol. 2001, 127, 1323–1323. [Google Scholar] [CrossRef]
- Li, G.; Tillard, P.; Gojon, A.; Maurel, C. Dual regulation of root hydraulic conductivity and plasma membrane aquaporins by plant nitrate accumulation and high-affinity nitrate transporter NRT2.1. Plant Cell Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Nacry, P.; Pervent, M.; Filleur, S.; Diatloff, E.; Mounier, E.; Tillard, P.; Forde, B.G.; Gojon, A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl. Acad. Sci. USA 2006, 103, 19206–19211. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.B.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Kohler, B.; Cao, F.Q.; Liu, L.H. Molecular and physiological aspects of urea transport in higher plants. Plant Sci. 2008, 175, 467–477. [Google Scholar] [CrossRef]
- Barzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.P.; Moller, A.L.B.; Zeuthen, T.; Holm, L.M.; Klaerke, D.A.; Mohsin, B.; Kuhlbrandt, W.; Schjoerring, J.K. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004, 574, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kirscht, A.; Kaptan, S.S.; Bienert, G.P.; Chaumont, F.; Nissen, P.; de Groot, B.L.; Kjellbom, P.; Gourdon, P.; Johanson, U. Crystal structure of an ammonia-permeable aquaporin. PLoS Biol. 2016, 14, e1002411. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, C.M.; Li, Y.R.; Li, Y.; Zhu, Y.Y.; Xu, G.H.; Shen, Q.R.; Kaldenhoff, R.; Kai, L.; Guo, S.W. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci. 2015, 234, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Kaldenhoff, R.; Uehlein, N.; Sattelmacher, B.; Brueck, H. Relationship between water and nitrogen uptake in nitrate- and ammonium-supplied Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 2007, 170, 73–80. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.R.; Wang, Y.; Gao, L.M.; Wang, M.; Chaumont, F.; Shen, Q.R.; Guo, S.W. Root ABA accumulation enhances rice seedling drought tolerance under ammonium supply: Interaction with aquaporins. Front. Plant Sci. 2016, in press. [Google Scholar]
- Soto, G.; Alleva, K.; Mazzella, M.A.; Amodeo, G.; Muschietti, J.P. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett. 2008, 582, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Vatamaniuk, O.K.; Du, X. CsNIP2;1 is a plasma membrane transporter from Cucumis sativus that facilitates urea uptake when expressed in Saccharomyces cerevisiae and Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.L.; Chen, X.L.; Zhou, Y.L.; Yuan, L.X. Isolation and characterization of three maize aquaporin genes, ZmNIP2;1, ZmNIP2;4 and ZmTIP4;4 involved in urea transport. BMB Rep. 2012, 45, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.S.; Roberts, D.M. Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 2005, 44, 16826–16834. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.X.; He, X.L.; Zhao, B.C.; Zhou, C.J.; Liang, Y.Z.; Ge, R.C.; Shen, Y.Z.; Huang, Z.J. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Physiol. 2010, 51, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Menz, J.; Haussermann, I.; Benz, M.; Fujiwara, T.; Ludewig, U. High and low affinity urea root uptake: Involvement of NIP5;1. Plant Cell Physiol. 2015, 56, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D.; Bohnert, H.J.; Maurel, C.; Steudle, E.; Smith, J.A.C. Plant aquaporins: Their molecular biology, biophysics and significance for plant water relations. J. Exp. Bot. 1999, 50, 1055–1071. [Google Scholar] [CrossRef]
- Soto, G.; Fox, R.; Ayub, N.; Alleva, K.; Guaimas, F.; Erijman, E.J.; Mazzella, A.; Amodeo, G.; Muschietti, J. TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J. 2010, 64, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M.; Lovelock, C.E. Photosynthetic characteristics of dwarf and fringe Rhizophora mangle L. in a Belizean mangrove. Plant Cell Environ. 2004, 27, 769–780. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Ball, M.C.; Choat, B.; Engelbrecht, B.M.J.; Holbrook, N.M.; Feller, I.C. Linking physiological processes with mangrove forest structure: Phosphorus deficiency limits canopy development, hydraulic conductivity and photosynthetic carbon gain in dwarf Rhizophora mangle. Plant Cell Environ. 2006, 29, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; Cooke, D.T.; Clarkson, D.T. Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 1996, 199, 372–381. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Shukla, V.K.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 1998, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Vialaret, J.; Li, G.W.; Hem, S.; Prado, K.; Rossignol, M.; Maurel, C.; Santoni, V. Coordinated post-translational responses of aquaporins to abiotic and nutritional stimuli in Arabidopsis roots. Mol. Cell. Proteom. 2013, 12, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Mao, X.T.; Tian, Q.Y.; Li, L.H.; Zhang, W.H. Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environ. Exp. Bot. 2009, 67, 172–177. [Google Scholar] [CrossRef]
- Kamaluddin, M.; Zwiazek, J.J. Ethylene enhances water transport in hypoxic aspen. Plant Physiol. 2002, 128, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Alleva, K.; Niemietz, C.M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Tian, Q.Y.; Zhang, W.H. Ethylene activates a plasma membrane Ca2+-permeable channel in tobacco suspension cells. New Phytol. 2007, 174, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Z.P.; Lei, T.W.; Shao, M.A.; Xue, Q.W. Effects of phosphorus nutrient on the hydraulic conductivity of sorghum (Sorghum vulgare Pers.) seedling roots under water deficiency. J. Integr. Plant Biol. 2005, 47, 421–427. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. Amst. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- He, F.; Zhang, H.Q.; Tang, M. Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 2016, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Shen, Q.R.; Brueck, H. Effects of local nitrogen supply on water uptake of bean plants in a split root system. J. Integr. Plant Biol. 2007, 49, 472–480. [Google Scholar] [CrossRef]
- Hill, A.E.; Shachar Hill, B.; Shachar Hill, Y. What are aquaporins for? J. Membr. Biol. 2004, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M.; Filatov, V.; Herzyk, P.; Krijger, G.C.; Axelsen, K.B.; Chen, S.X.; Green, B.J.; Li, Y.; Madagan, K.L.; Sanchez-Fernandez, R.; et al. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J. 2003, 35, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Breitling, R.; Amtmann, A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; de Rycke, R.; Inze, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, M.; Sutou, E.; Shibasaka, M. Onion root water transport sensitive to water channel and K+ channel inhibitors. Plant Cell Physiol. 2001, 42, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sahr, T.; Voigt, G.; Paretzke, H.G.; Schramel, P.; Ernst, D. Caesium-affected gene expression in Arabidopsis thaliana. New Phytol. 2005, 165, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Sun, W.N.; Su, W.A.; Tang, Z.C. Co-regulation of water channels and potassium channels in rice. Physiol. Plant. 2006, 128, 58–69. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.S.; Shen, Q.R.; Guo, S.W. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Oddo, E.; Inzerillo, S.; La Bella, F.; Grisafi, F.; Salleo, S.; Nardini, A. Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol. 2011, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Melcher, P.J.; Holbrook, N.M. Hydrogel control of xylem hydraulic resistance in plants. Science 2001, 291, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Galmes, J.; Pou, A.; Alsina, M.M.; Tomas, M.; Medrano, H.; Flexas, J. Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): Relationship with ecophysiological status. Planta 2007, 226, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar, T.; Pascaud, F.; Verdeil, J.L.; Torregrosa, L.; Adam-Blondon, A.F.; Thibaud, J.B.; Sentenac, H.; Gaillard, I. A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J. 2010, 61, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.S. Calcium regulation in plant-cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Ionenko, I.F.; Anisimov, A.V.; Karimova, F.G. Water transport in maize roots under the influence of mercuric chloride and water stress: A role of water channels. Biol. Plant. 2006, 50, 74–80. [Google Scholar] [CrossRef]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Characterization of protein phosphatase 2A acting on phosphorylated plasma membrane aquaporin of tulip petals. Biosci. Biotechnol. Biochem. 2004, 68, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Henzler, T. Water channels in plants: Do basic concepts of water transport change? J. Exp. Bot. 1995, 46, 1067–1076. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.F.; Wang, W.F.; Zhang, S.Q.; Xu, B.C. Calcium regulates the cell-to-cell water flow pathway in maize roots during variable water conditions. Plant Physiol. Biochem. 2012, 58, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; CerdÁ, A.; MartÍNez, V. Does calcium ameliorate the negative effect of NaCl on melon root water transport by regulating aquaporin activity? New Phytol. 2000, 145, 439–447. [Google Scholar] [CrossRef]
- Cabanero, F.J.; Martinez-Ballesta, M.C.; Teruel, J.A.; Carvajal, M. New evidence about the relationship between water channel activity and calcium in salinity-stressed pepper plants. Plant Cell Physiol. 2006, 47, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta, M.C.; Cabanero, F.; Olmos, E.; Periago, P.M.; Maurel, C.; Carvajal, M. Two different effects of calcium on aquaporins in salinity-stressed pepper plants. Planta 2008, 228, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Luu, D.T.; Maurel, C. Aquaporins in a challenging environment: Molecular gears for adjusting plant water status. Plant Cell Environ. 2005, 28, 85–96. [Google Scholar] [CrossRef]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004, 135, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Zhang, X.Y.; Tang, Q.L.; Wang, G.X. Extracellular calcium is involved in stomatal movement through the regulation of water channels in broad bean. Plant Growth Regul. 2006, 50, 79–83. [Google Scholar] [CrossRef]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Chrispeels, M.J.; Brown, P.H. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 2000, 124, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.L.; Reid, R.J. The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ. 2009, 32, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Wallace, I.S.; Takano, J.; Roberts, D.M.; Fujiwara, T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 2008, 20, 2860–2875. [Google Scholar] [CrossRef] [PubMed]
- Schnurbusch, T.; Hayes, J.; Hrmova, M.; Baumann, U.; Ramesh, S.A.; Tyerman, S.D.; Langridge, P.; Sutton, T. Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol. 2010, 153, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Fujiwara, T. Channel-mediated boron transport in rice. Plant Cell Physiol. 2007, 48, S227. [Google Scholar]
- Pang, Y.Q.; Li, L.J.; Ren, F.; Lu, P.L.; Wei, P.C.; Cai, J.H.; Xin, L.G.; Zhang, J.A.; Chen, J.; Wang, X.C. Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. J. Genet. Genom. 2010, 37, 389–397. [Google Scholar] [CrossRef]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Musante, C.; White, J.C.; Dhankher, O.P. Enhanced boron tolerance in plants mediated by bidirectional transport through plasma membrane intrinsic proteins. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mosa, K.A.; Chhikara, S.; Musante, C.; White, J.C.; Dhankher, O.P. Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 2014, 239, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, L.N.; Wang, S.W.; Zhang, M.J.; Deng, X.P.; Zhang, S.Q.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.M.; Rivers, R.L.; Zeidel, M.L.; Roberts, D.M. Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry 1999, 38, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2; 1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ding, L.; Gao, L.; Li, Y.; Shen, Q.; Guo, S. The Interactions of Aquaporins and Mineral Nutrients in Higher Plants. Int. J. Mol. Sci. 2016, 17, 1229. https://doi.org/10.3390/ijms17081229
Wang M, Ding L, Gao L, Li Y, Shen Q, Guo S. The Interactions of Aquaporins and Mineral Nutrients in Higher Plants. International Journal of Molecular Sciences. 2016; 17(8):1229. https://doi.org/10.3390/ijms17081229
Chicago/Turabian StyleWang, Min, Lei Ding, Limin Gao, Yingrui Li, Qirong Shen, and Shiwei Guo. 2016. "The Interactions of Aquaporins and Mineral Nutrients in Higher Plants" International Journal of Molecular Sciences 17, no. 8: 1229. https://doi.org/10.3390/ijms17081229