Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Tomato
Abstract
:1. Introduction
2. Results
2.1. Identification of the TCS Genes in Tomato
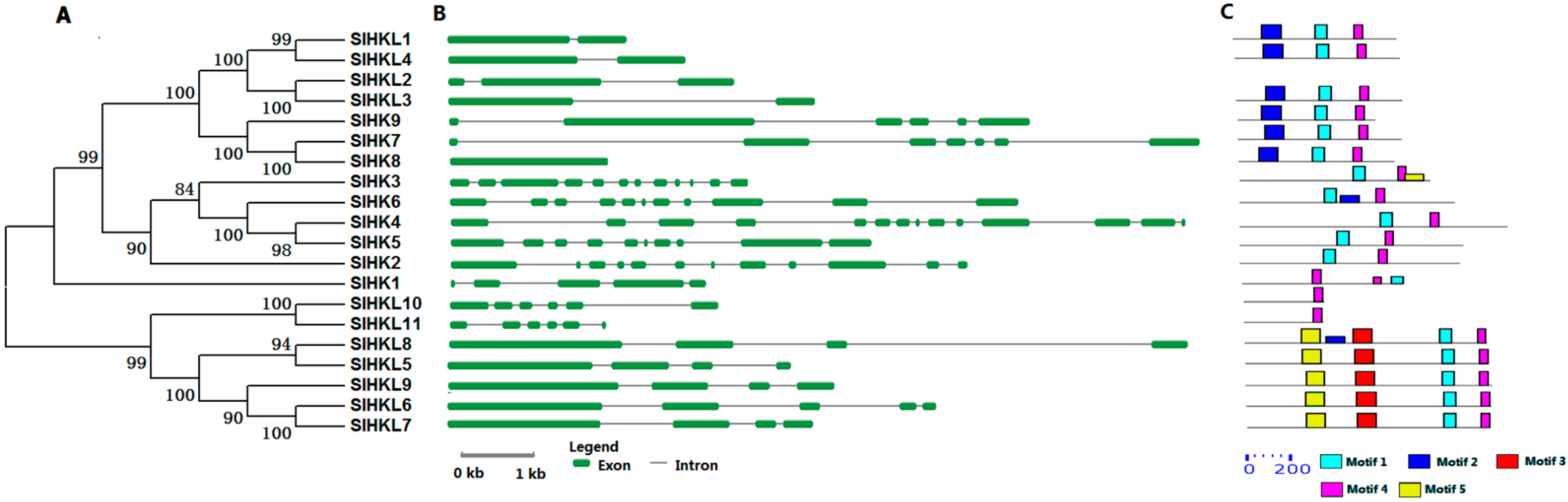
2.2. HK Proteins in Tomato
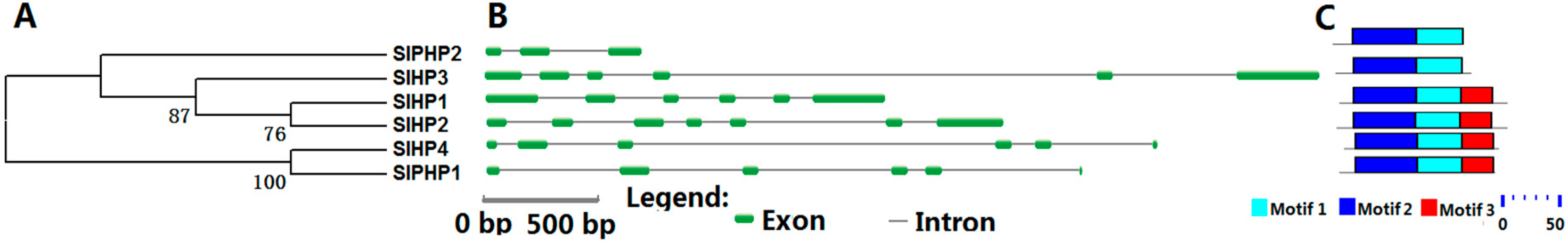
2.3. HP Proteins in Tomato
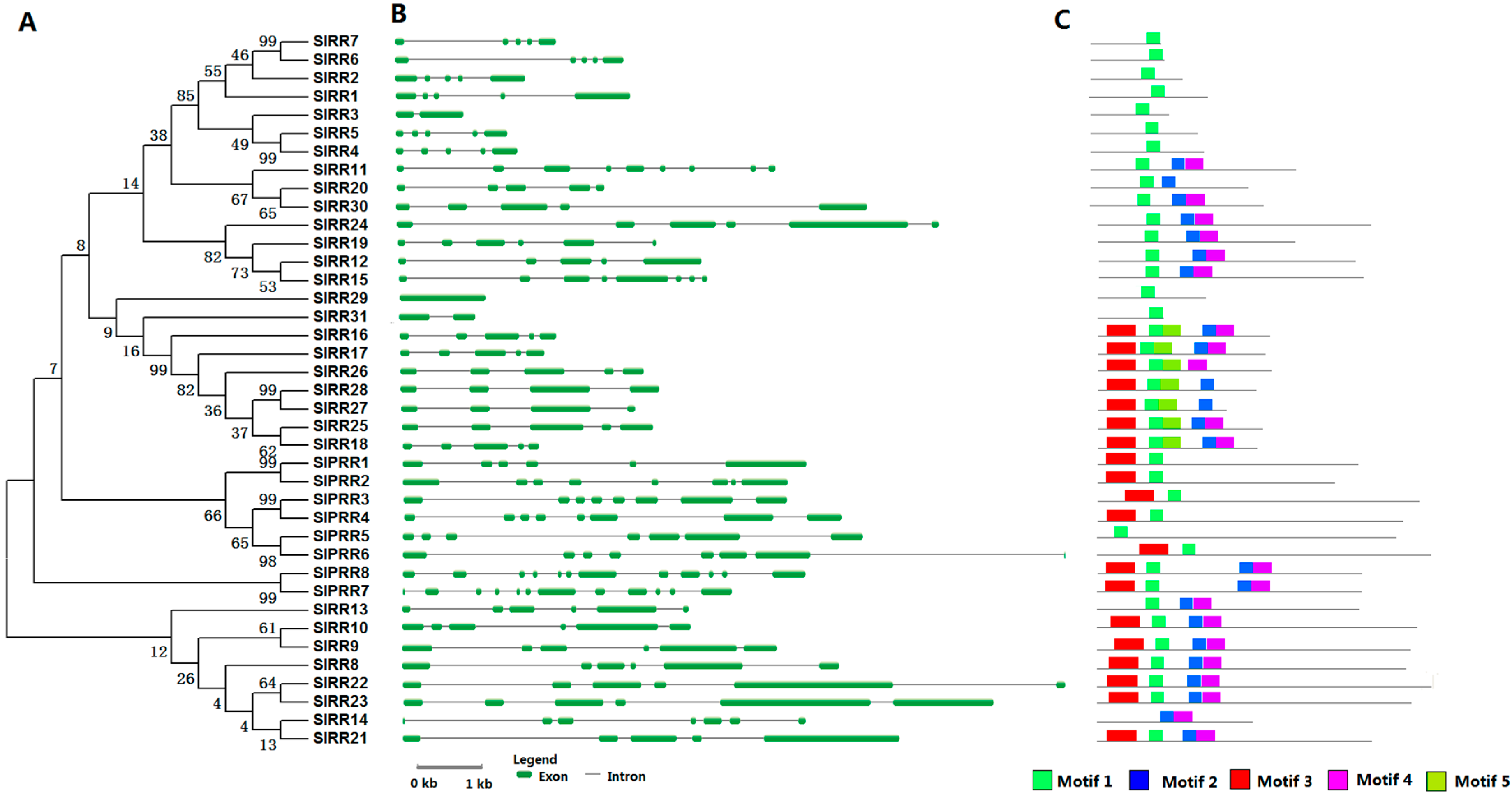
2.4. RR Proteins in Tomato
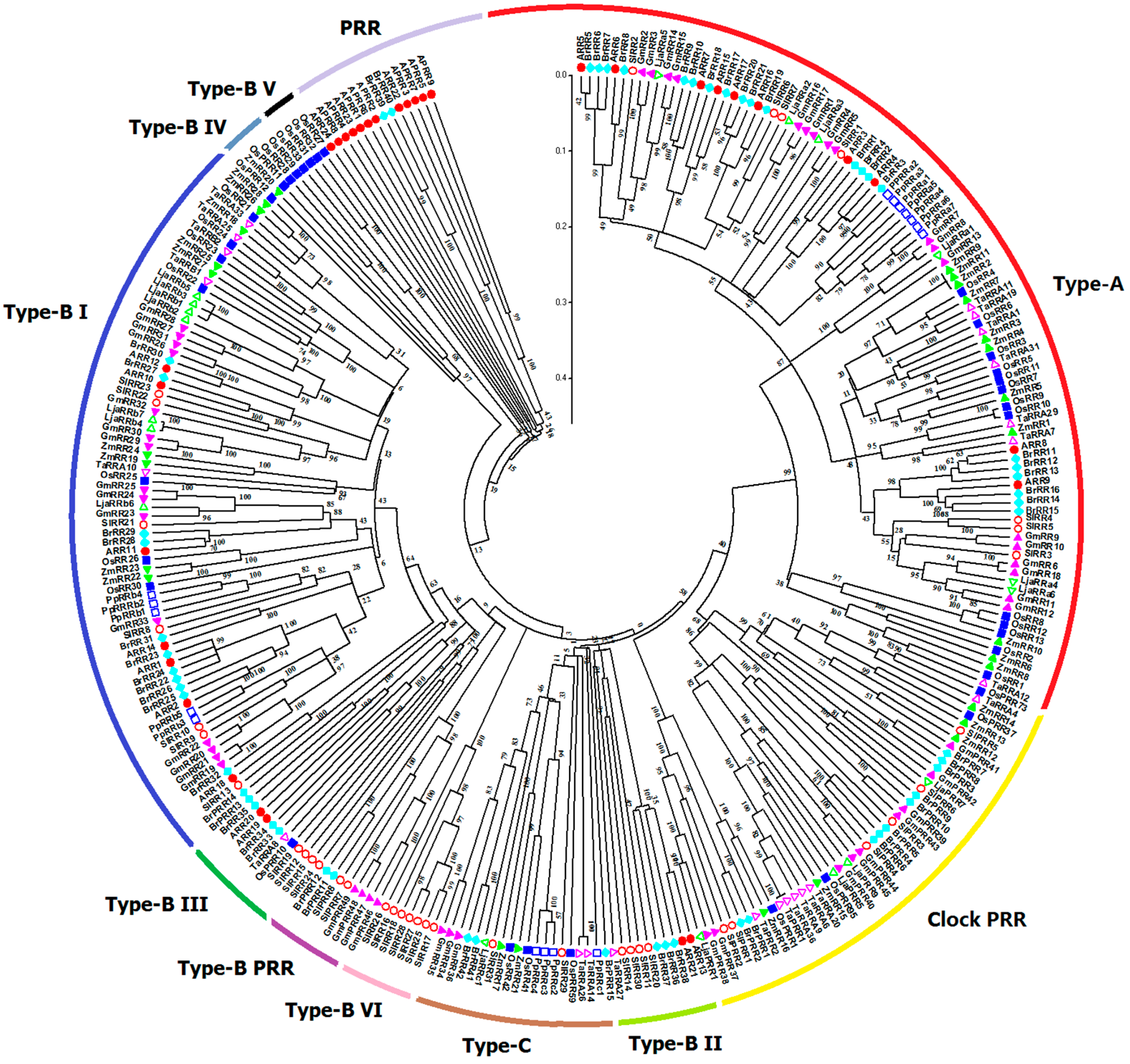
2.5. Phylogenetic Analysis of Plant TCS Proteins
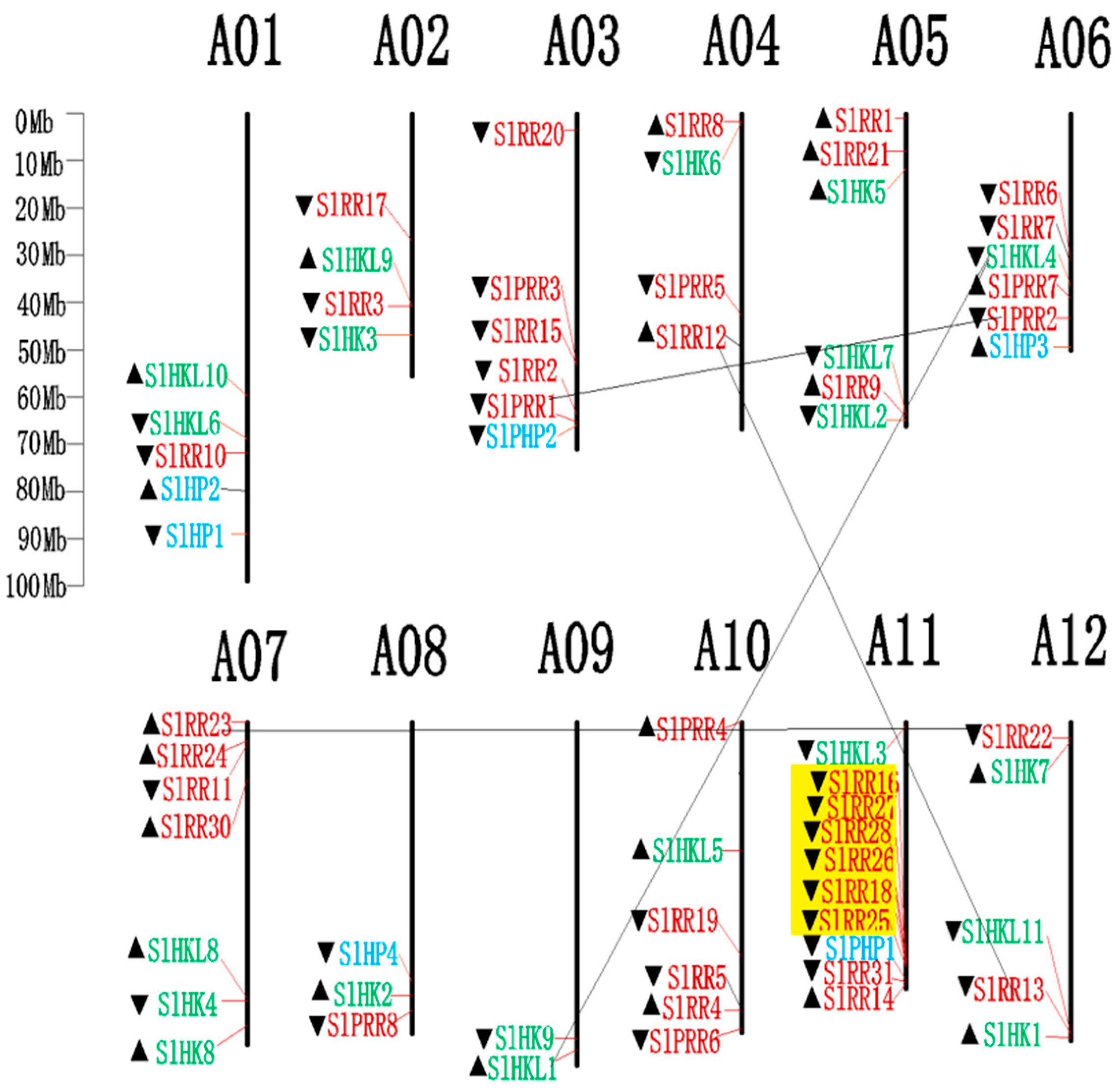
2.6. Genomic Distribution and Gene Duplication of Tomato TCS Members
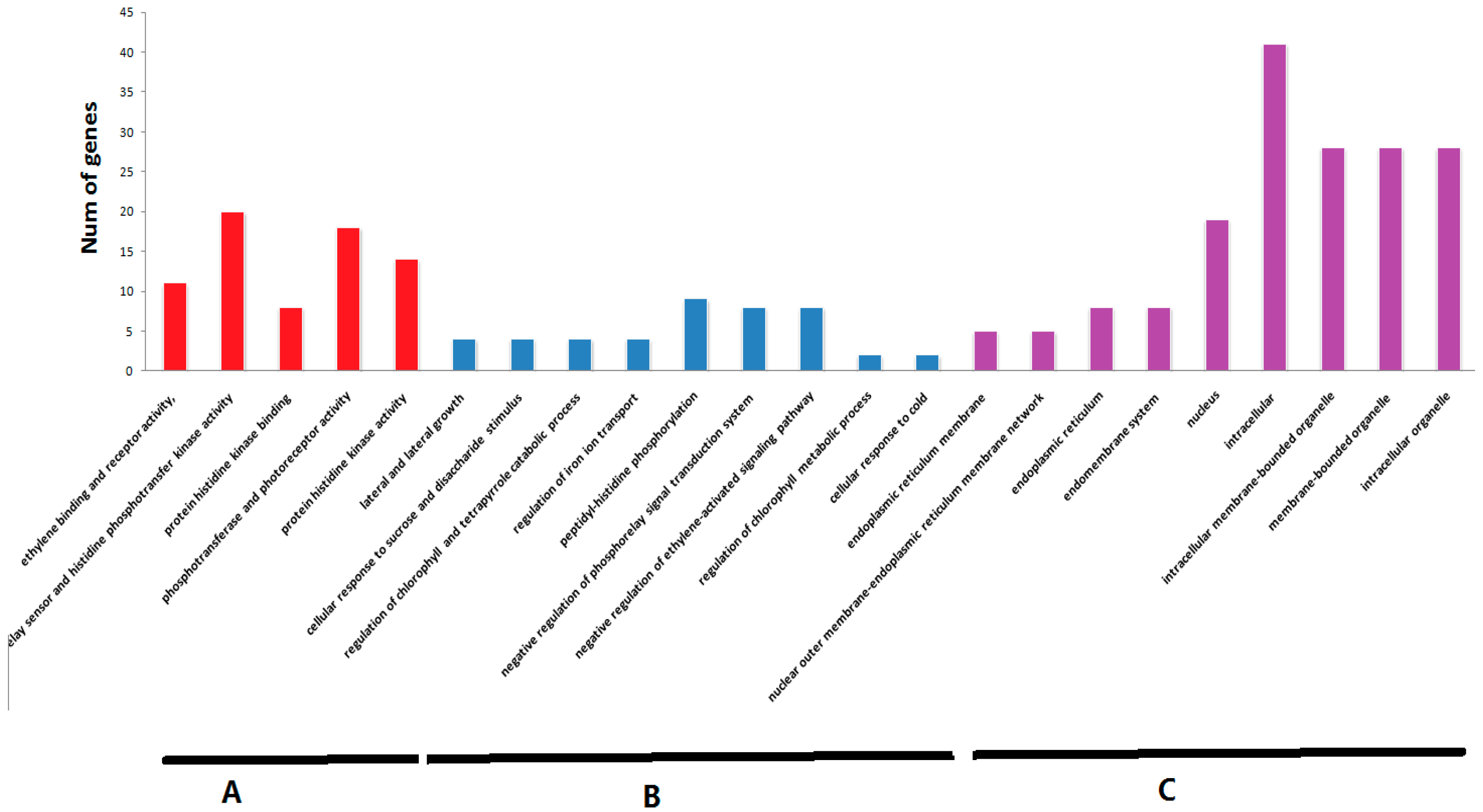
2.7. Analysis of Cis-Elements in Putative Promoter Regions of TCS Genes in Tomato
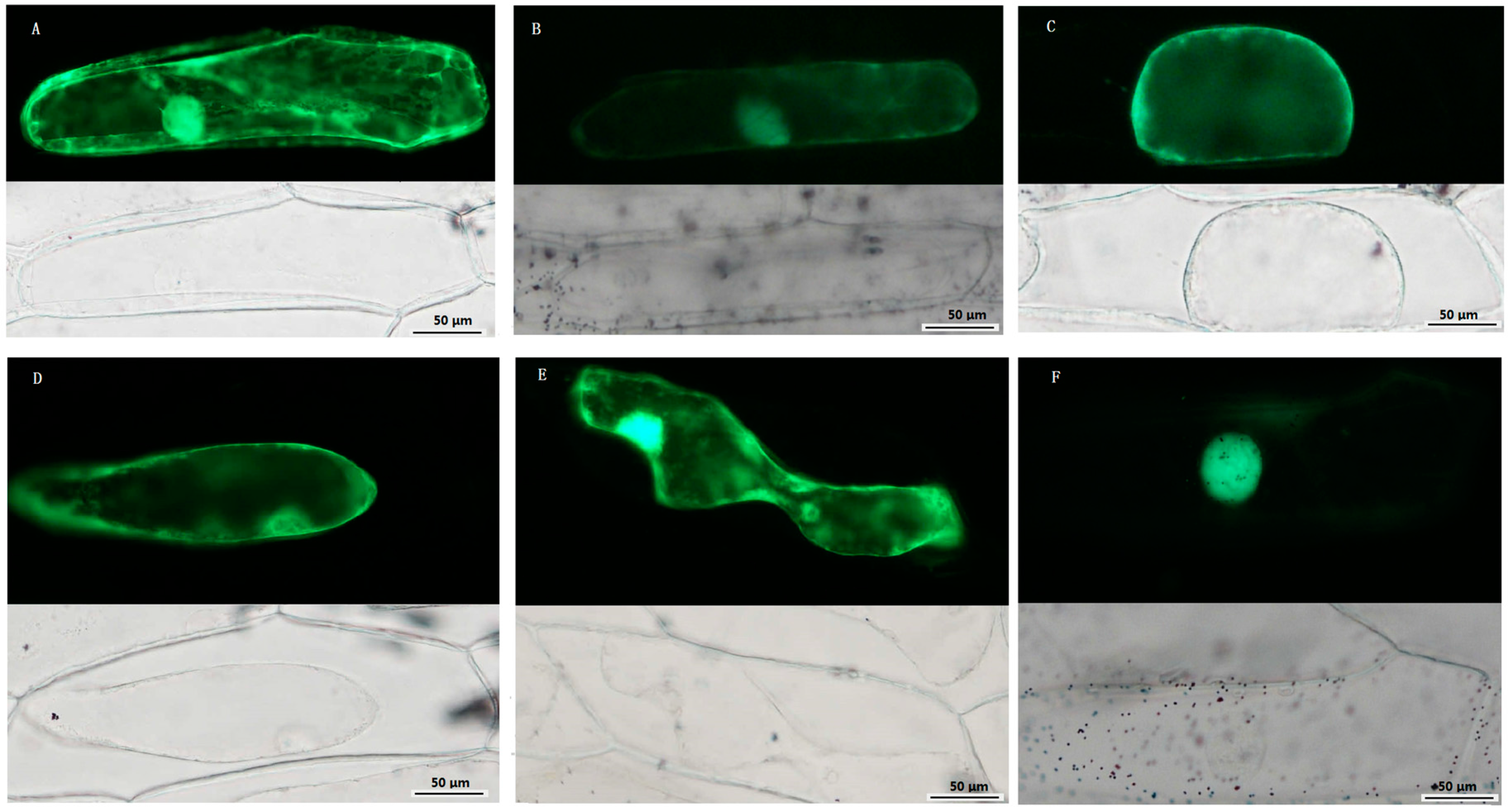
2.8. Subcellular Localization of TCS Proteins from Tomato
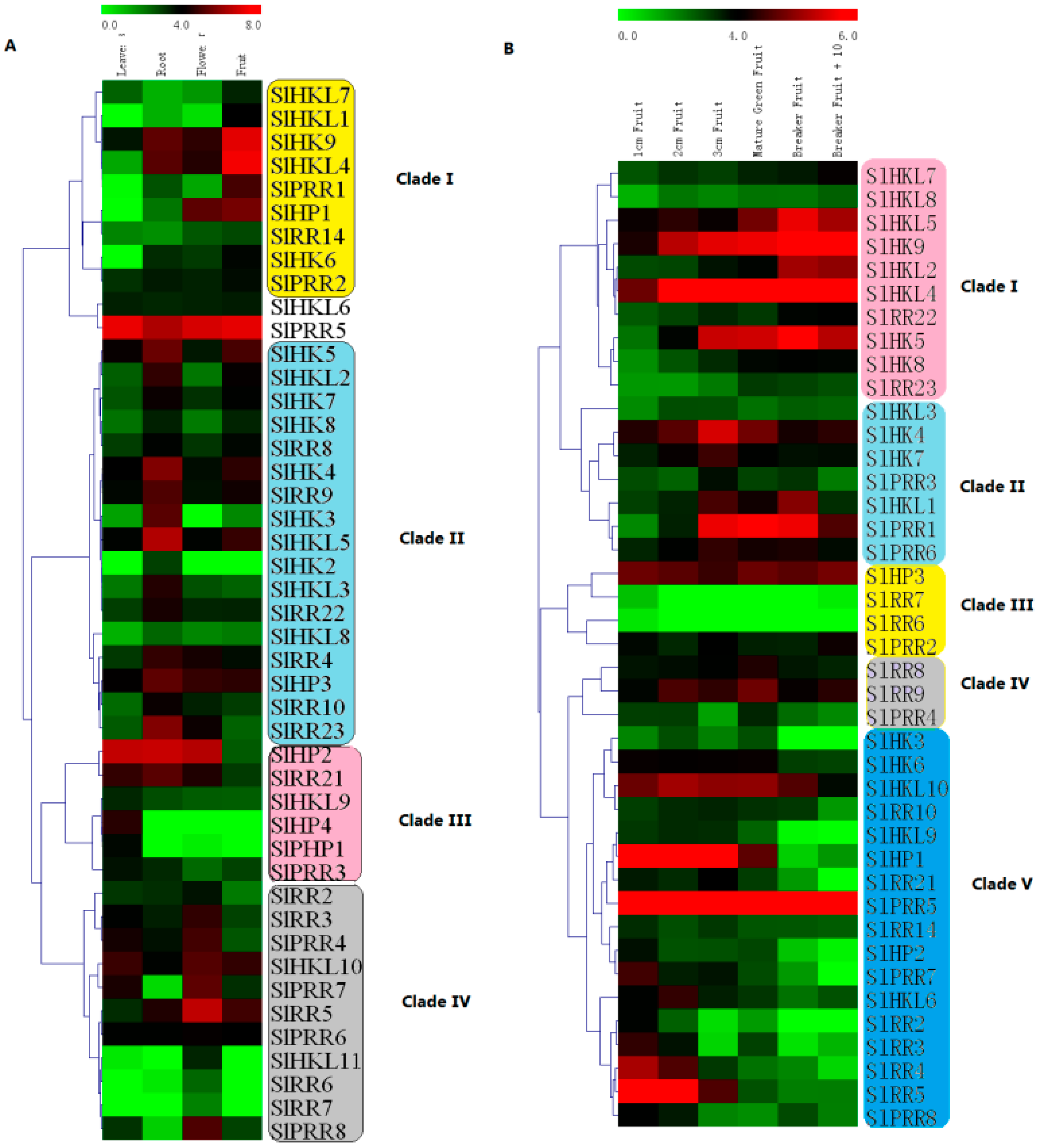
2.9. Expression Profiles of Tomato TCS Genes in Various Organs
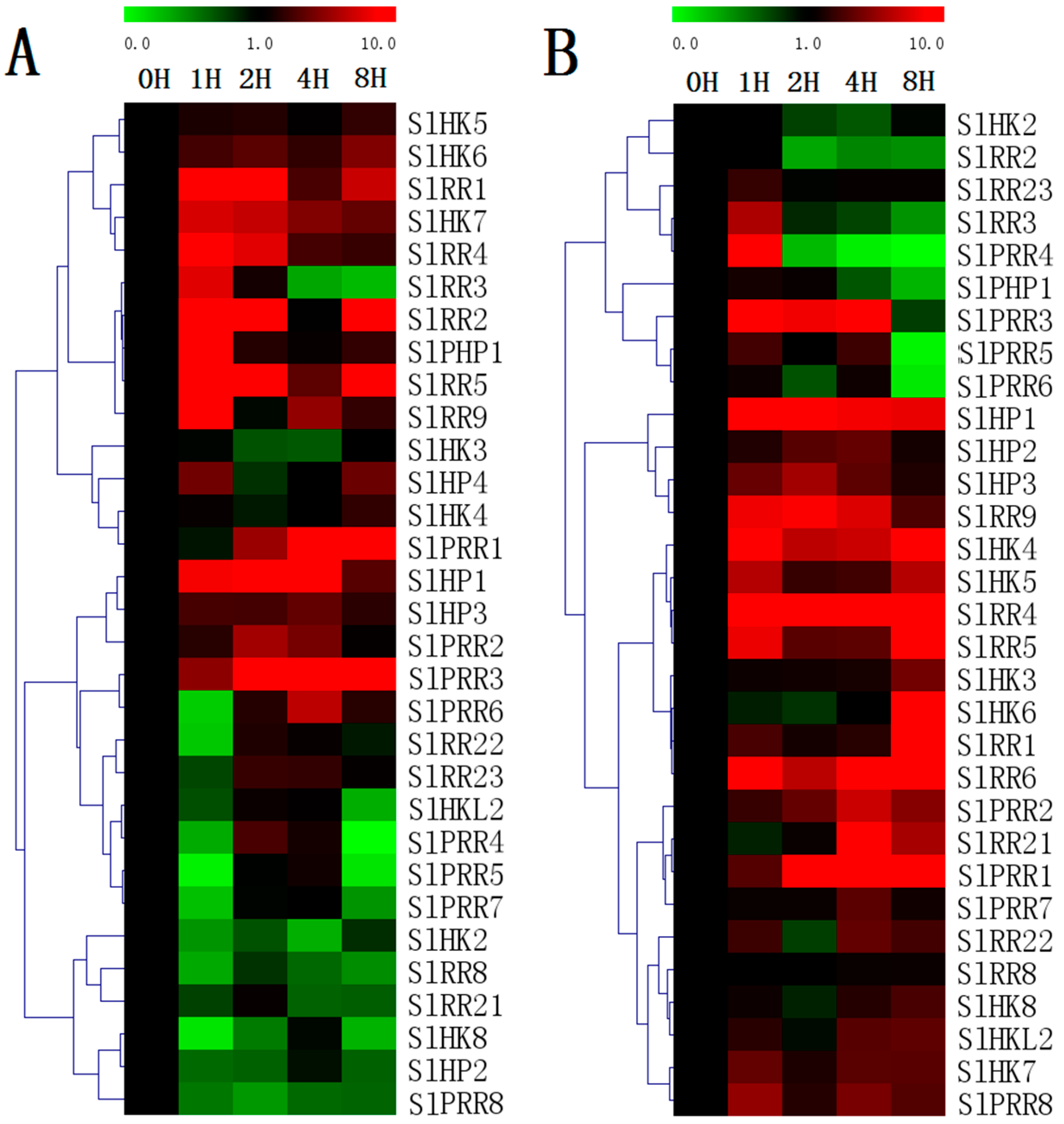
2.10. Expression Profiles of TCS Genes in Response to Exogenous Hormones and Abiotic Stresses
3. Discussion
4. Materials and Methods
4.1. Identification of TCS Genes in Tomato
4.2. Gene Structure Construction, Motif Analysis, and Phylogenetic Analysis
4.3. Chromosomal Localization and Evolutionary Analysis of TCS Genes
4.4. Analysis of Putative Promoter Regions TCS Genes in Tomato
4.5. Subcellular Localization
4.6. Tomato Plant Growth and Treatments
4.7. Expression Analysis of TCS Genes in Growth and Development Gene
4.8. RNA Isolation and qRT–PCR
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mizuno, T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997, 4, 16–18. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Urao, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two-component systems in plant signal transduction. Trends Plant Sci. 2000, 5, 67–74. [Google Scholar] [CrossRef]
- Hwang, I.; Chen, H.C.; Sheen, J. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 2002, 129, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Kieber, J.J.; Shiu, S.H. Two-component signaling elements and histidyl-aspartyl phosphorelays. Arabidopsis Book 2008, e0112. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Harter, K. Plant two-component systems: Principles, functions, complexity and cross talk. Planta 2004, 219, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Singh, A.; Kumar, M.; Kushwaha, H.R.; Lynn, A.M.; Singla-Pareek, S.L. Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol. 2006, 142, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Ma, Q.; Lin, Y.; Tang, X.; Zhou, Y.; Zhu, S.; Fan, J.; Cheng, B. Genome-wide identification, classification, and analysis of two-component signal system genes in maize. Genet. Mol. Res. 2011, 10, 3316–3330. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Yoshida, T.; Sakurai, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Genome-wide analysis of two-component systems and prediction of stress-responsive two-component system members in soybean. DNA Res. 2010, 17, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Kong, L.; Lv, Y.; Zou, M.; Lu, G.; Cao, J.; Yu, X. Genome-wide identification, phylogeny, duplication, and expression analyses of two-component system genes in Chinese cabbage (Brassica rapa ssp. pekinensis). DNA Res. 2014, 21, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef] [PubMed]
- Wohlbach, D.J.; Quirino, B.F.; Sussman, M.R. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell 2008, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal. Behav. 2010, 5, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Liu, J.; Bennett, M.H.; Mansfield, J.W.; Desikan, R. Arabidopsis histidine kinase 5 regulates salt sensitivity and resistance against bacterial and fungal infection. New Phytol. 2012, 194, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Jane, W.N.; Verslues, P.E. Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 2013, 161, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J. Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiol. 2013, 161, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Van Ha, C.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M.; et al. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Van Ha, C.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; et al. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kusano, M.; Fukushima, A.; Kita, M.; Ito, S.; Yamashino, T.; Saito, K.; Sakakibara, H.; Mizuno, T. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol. 2009, 50, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Urao, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of genes for two-component phosphorelay mediators with a single HPt domain in Arabidopsis thaliana. FEBS Lett. 1998, 437, 11–14. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Q.; Wu, J.; Zhang, L.; Jiao, X.; Zhang, S.; Zhang, Z.; Sun, D.; Lu, T.; Sun, Y. Two rice authentic histidine phosphotransfer proteins, OsAHP1 and OsAHP2, mediate cytokinin signaling and stress responses in rice. Plant Physiol. 2014, 165, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Qin, T.; Yao, W.; Wen, D.; Zhang, A.; Tan, M.; Jiang, M. OsHK3 is a crucial regulator of abscisic acid signaling involved in antioxidant defense in rice. J. Integr. Plant Biol. 2015, 57, 213–228. [Google Scholar]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Genome-wide expression profiling of soybean two-component system genes in soybean root and shoot tissues under dehydration stress. DNA Res. 2011, 8, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Pressman, E.; Meir, S.; Khoury, R.; Altahan, L. Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat-stress conditions. AOB Plants 2012, 2012, pls024. [Google Scholar] [CrossRef] [PubMed]
- D’Amico-Damiao, V.; Cruz, F.J.R.; Gavassi, M.A.; Santos, D.M.M.; Melo, H.C.; Carvalho, R.F. Photomorphogenic modulation of water stress in tomato (Solanum lycopersicum L.): The role of phytochromes A, B1, and B2. J. Hortic. Sci. Biotechnol. 2015, 90, 25–30. [Google Scholar]
- Harry, K.; Denise, T. The tomato ethylene receptor gene family: Form and function. Physiol. Plant. 2002, 115, 336–341. [Google Scholar]
- Pratt, L.H.; Cordonnier-Pratt, M.M.; Kelmenson, P.M.; Lazarova, G.I.; Kubota, T.; Alba, R.M. The phytochrome gene family in tomato (Solanum lycopersicum L.). Plant Cell Environ. 1997, 20, 672–677. [Google Scholar] [CrossRef]
- Tieman, D.M.; Taylor, M.G.; Ciardi, J.A.; Klee, H.J. The Tomato Ethylene Receptors NR and LeETR4 Are Negative Regulators of Ethylene Response and Exhibit Functional Compensation within a Multigene Family. Proc. Natl. Acad. Sci. USA 2000, 97, 5663–5668. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sharma, S.; Santisree, P.; Kilambi, H.V.; Appenroth, K.; Sreelakshmi, Y.; Sharma, R. Complex and shifting interactions of phytochromes regulate fruit development in tomato. Plant Cell Environ. 2014, 37, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Gahlaut, V.; Mathur, S.; Dhariwal, R.; Khurana, J.P.; Tyagi, A.K.; Balyan, H.S.; Gupta, P.K. A multi-step phosphorelay two-component system impacts on tolerance against dehydration stress in common wheat. Funct. Integr. Genom. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Niwa, Y.; Yamashino, T.; Mizuno, T. A genome-wide compilation of the two-component systems in Lotus japonicus. DNA Res. 2009, 16, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamashino, T.; Nakanishi, H.; Mizuno, T. Classification of the genes involved in the two-component system of the moss Physcomitrella patens. Biosci. Biotechnol. Biochem. 2010, 74, 2542–2545. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, N.C.; Su, Y.S.; Lagarias, J.C. Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Hayama, R.; Coupland, G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr. Opin. Plant Biol. 2003, 6, 13–19. [Google Scholar] [CrossRef]
- Pan, Y.; Bradley, G.; Pyke, K.; Ball, G.; Lu, C.; Fray, R.; Marshall, A.; Jayasuta, S.; Baxter, C.; van Wijk, R. Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol. 2013, 161, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Pils, B.; Heyl, A. Unraveling the Evolution of Cytokinin Signaling. Plant Physiol. 2009, 151, 782–791. [Google Scholar] [CrossRef] [PubMed]
- SubLoc. Avaliable online: http://www.bioinfo.tsinghua.edu.cn/SubLoc/eu_predict.htm (accessed on 3 July 2016).
- Dortay, H.; Gruhn, N.; Pfeifer, A.; Schwerdtner, M.; Schmülling, T.; Heyl, A. Toward an interaction map of the two-component signaling pathway of Arabidopsis thaliana. J. Proteom. Res. 2008, 7, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.T.G. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [Green Version]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.H. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, J.A.; Tieman, D.M.; Jones, J.B.; Klee, H.J. Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol. Plant Microb. Interact. 2001, 14, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Kovács, J.; Borbély, P.; Takács, Z.; Szepesi, Á.; Tari, I. Salt stress-induced production of reactive oxygen-and nitrogen species and cell death in the ethylene receptor mutant Never ripe and wild type tomato roots. Plant Physiol. Biochem. 2015, 97, 313–322. [Google Scholar]
- Phytozome. Avaliable online: http://phytozome.jgi.doe.gov/pz/portal.html (accessed on 3 July 2016).
- ExPASy. Avaliable online: http://web.expasy.org/compute_pi/ (accessed on 3 July 2016).
- Gene Structure Display Server. Avaliable online: http://gsds.cbi.pku.edu.cn (accessed on 3 July 2016).
- MEME. Avaliable online: http://meme-suite.org/tools/meme (accessed on 3 July 2016).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, S. Genome-wide analysis of the MADS-box gene family in cucumber. Genome 2012, 55, 245–256. [Google Scholar] [CrossRef] [PubMed]
- PGDD. Avaliable online: http://chibba.agtec.uga.edu/duplication/ (accessed on 3 July 2016).
- ClustalW. Avaliable online: http://www.genome.jp/tools/clustalw/ (accessed on 3 July 2016).
- PAML. Avaliable online: http://www.bork.embl.de/pal2nal/ (accessed on 3 July 2016).
- Yuan, S.; Xu, B.; Jing, Z.; Zheni, X.; Qiang, C.; Zhimin, Y.; Qingsheng, C.; Bingru, H. Comprehensive analysis of CCCH-type zinc finger family genes facilitates functional gene discovery and reflects recent allopolyploidization event in tetraploid switchgrass. BMC Genom. 2015, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- PlantCARE. Avaliable online: http://bioinformatics.psb.ugent.be/webtools/plantcare/html/search_CARE.html (accessed on 3 July 2016).
- Gan, C. Gene gun accelerates DNA-coated particles to transform intact-cells. Scientist 1989, 3, 25. [Google Scholar]
- Tomato eFP Browser. Avaliable online: http://bar.utoronto.ca/efp_tomato/cgi-bin/efpWeb.cgi (accessed on 3 July 2016).
- Saeed, A.I.; Bhagabati, N.K.; Braisted, J.C.; Wei, L.; Sharov, V.; Howe, E.A.; Li, J.; Thiagarajan, M.; White, J.A.; Quackenbush, J. TM4 Microarray Software Suite. Method Enzymol. 2006, 411, 134–193. [Google Scholar]
- KEGG. Avaliable online: http://www.genome.jp/kaas-bin/kaas_main (accessed on 3 July 2016).
- Wu, J.; Liu, S.; He, Y.; Guan, X.; Zhu, X.; Cheng, L.; Wang, J.; Lu, G. Genome-wide analysis of SAUR gene family in Solanaceae species. Gene 2012, 509, 38–50. [Google Scholar] [CrossRef] [PubMed]



| Species | HK(L) | HP (Pseudo-HP) | Type-A RR | Type-B RR | Type-C RR | Pseudo RR | Total | Reference |
|---|---|---|---|---|---|---|---|---|
| Arabidopsis thaliana | 17 (9) | 6 (1) | 10 | 12 | 2 | 9 | 56 | [4] |
| Oryza sativa | 11 (3) | 5 (3) | 13 | 13 | 2 | 8 | 52 | [7] |
| Lotus japonicus | 14 | 7 | 7 | 11 | 1 | 5 | 40 | [31] |
| Glycine max | 36 (15) | 13 | 18 | 15 | 3 | 13 | 98 | [9] |
| Zea mays | 11 (3) | 9 (2) | 16 | 9 | 3 | 11 | 59 | [8] |
| Physcomitrella patens | 18 | 3 | 7 | 5 | 2 | 4 | 39 | [32] |
| Triticum aestivum | 7 | 10 | 41 | 2 | 0 | 2 | 45 | [30] |
| Brassica rapa | 20 (9) | 8 (1) | 21 | 17 | 4 | 15 | 85 | [10] |
| Solanum lycopersicum | 20 (11) | 6 (2) | 7 | 23 | 1 | 8 | 65 | – |
| Duplicated Gene Pairs | Ks | Ka | Ka/Ks | Duplicated Type | Purify Selection | Time (Mya *) |
|---|---|---|---|---|---|---|
| SlHKL1/SlHKL4 | 0.68 | 0.23 | 0.34 | Segmental | Yes | 52.31 |
| SlRR9/SlRR13 | 0.79 | 0.38 | 0.48 | Segmental | Yes | 60.77 |
| SlRR22/SlRR23 | 0.74 | 0.17 | 0.23 | Segmental | Yes | 56.92 |
| SlPRR1/SlPRR2 | 0.60 | 0.21 | 0.35 | Segmental | Yes | 46.15 |
| SlRR16/SlRR18 | 0.34 | 0.29 | 0.84 | Tandem | Yes | 26.15 |
| SlRR16/SlRR27 | 0.32 | 0.23 | 0.71 | Tandem | Yes | 24.98 |
| SlRR16/SlRR28 | 0.24 | 0.20 | 0.85 | Tandem | Yes | 18.48 |
| SlRR16/SlRR26 | 0.20 | 0.19 | 0.99 | Tandem | Yes | 15.07 |
| SlRR18/SlRR27 | 0.20 | 0.12 | 0.59 | Tandem | Yes | 15.08 |
| SlRR18/SlRR28 | 0.31 | 0.26 | 0.84 | Tandem | Yes | 23.90 |
| SlRR18/SlRR26 | 0.19 | 0.20 | 1.06 | Tandem | No | 14.80 |
| SlRR18/SlRR25 | 0.21 | 0.18 | 0.84 | Tandem | Yes | 16.10 |
| SlRR25/SlRR27 | 0.17 | 0.10 | 0.59 | Tandem | Yes | 13.45 |
| SlRR25/SlRR28 | 0.08 | 0.06 | 0.79 | Tandem | Yes | 5.97 |
| SlRR25/SlRR26 | 0.11 | 0.10 | 0.91 | Tandem | Yes | 8.28 |
| SlRR26/SlRR27 | 0.35 | 0.33 | 0.96 | Tandem | Yes | 26.55 |
| SlRR26/SlRR28 | 0.16 | 0.15 | 0.94 | Tandem | Yes | 12.34 |
| SlRR27/SlRR28 | 0.11 | 0.06 | 0.58 | Tandem | Yes | 8.41 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, X.; Ye, L.; Pan, C.; Chen, L.; Zou, T.; Lu, G. Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Tomato. Int. J. Mol. Sci. 2016, 17, 1204. https://doi.org/10.3390/ijms17081204
He Y, Liu X, Ye L, Pan C, Chen L, Zou T, Lu G. Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Tomato. International Journal of Molecular Sciences. 2016; 17(8):1204. https://doi.org/10.3390/ijms17081204
Chicago/Turabian StyleHe, Yanjun, Xue Liu, Lei Ye, Changtian Pan, Lifei Chen, Tao Zou, and Gang Lu. 2016. "Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Tomato" International Journal of Molecular Sciences 17, no. 8: 1204. https://doi.org/10.3390/ijms17081204








