Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs
Abstract
:1. Introduction
2. Developments of in Vitro Methods
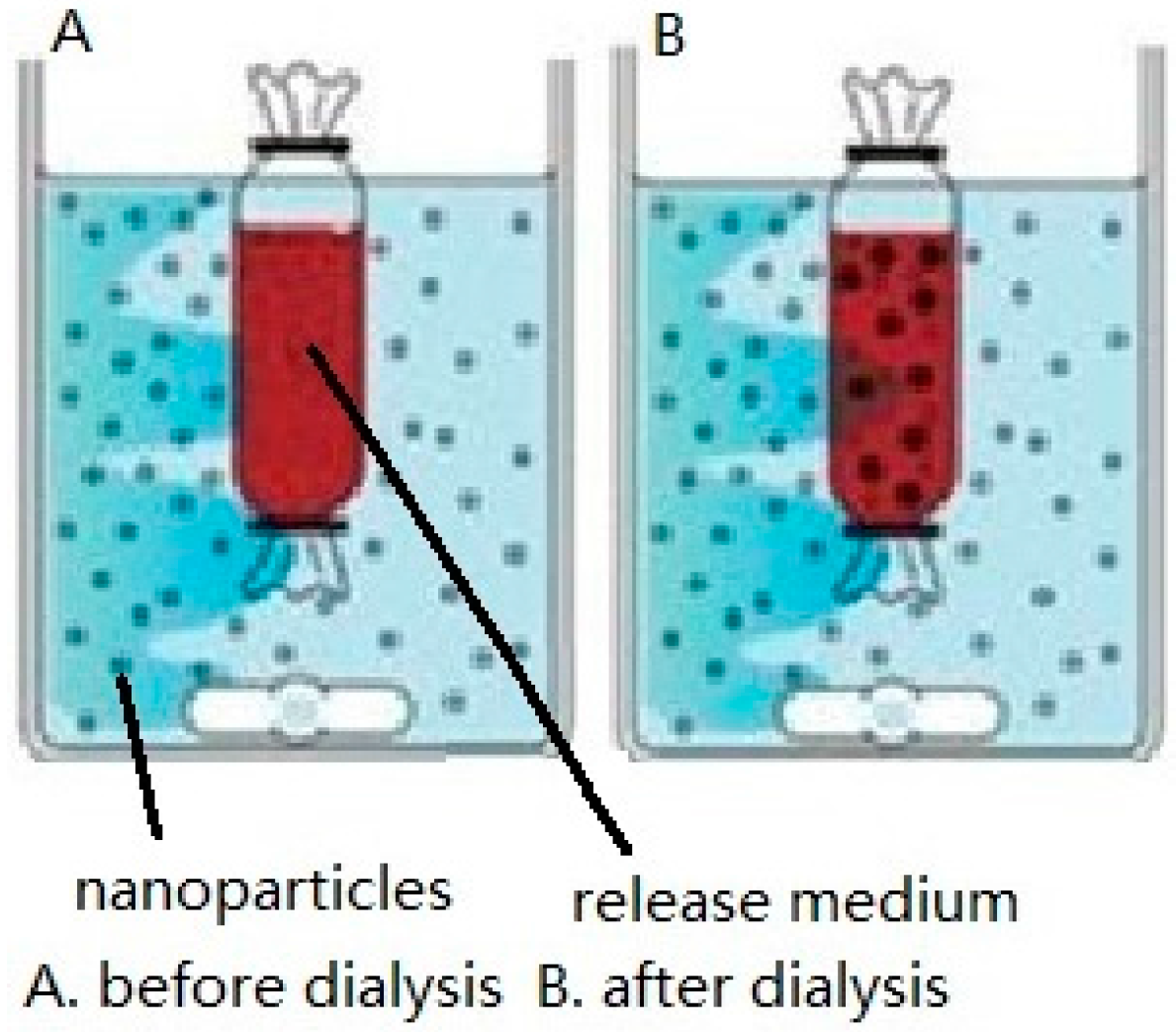
2.1. Dialysis Bag
2.2. Rat Gut Sac
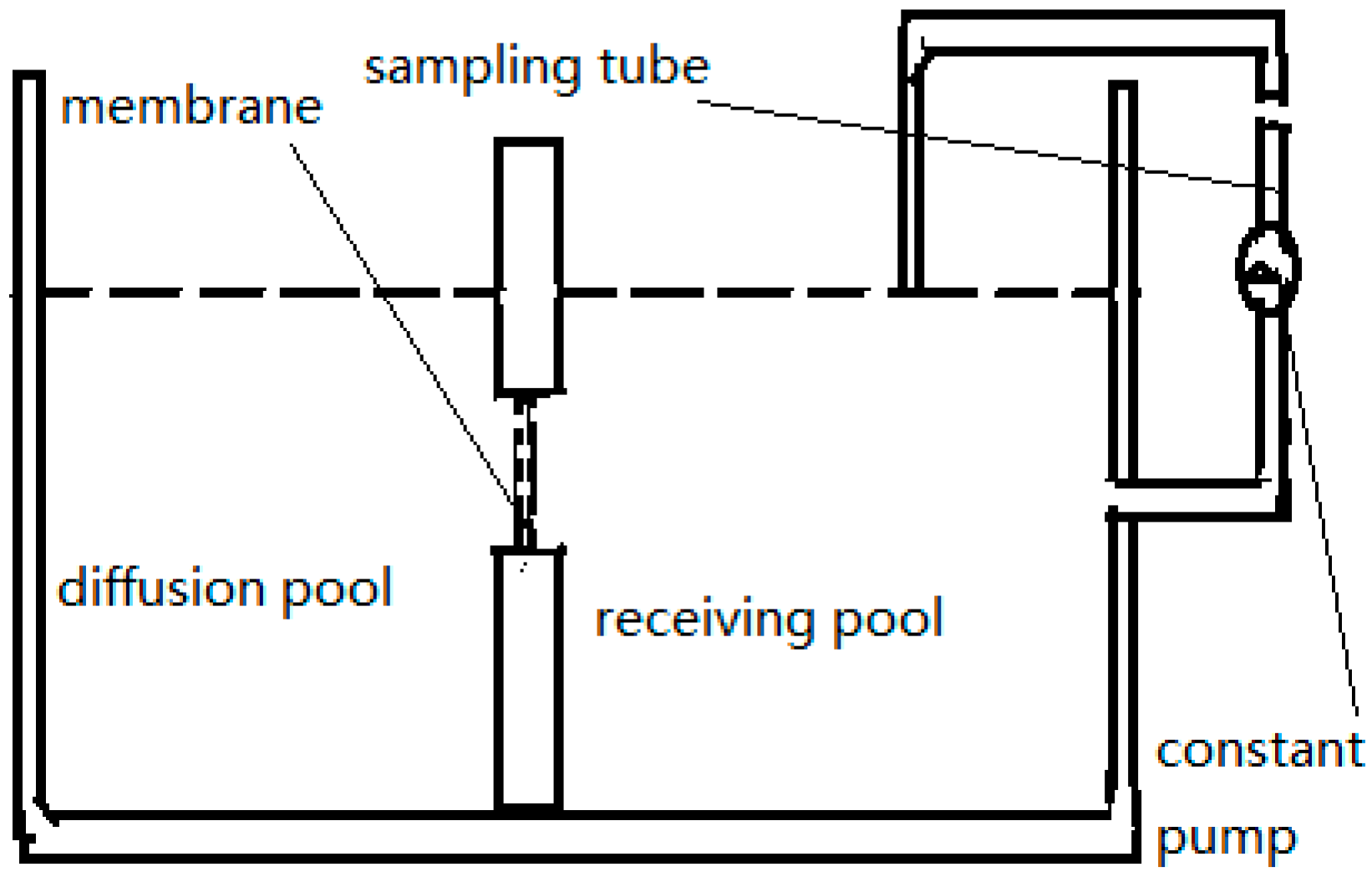
2.3. Ussing Chamber
2.4. Cell Culture Model
2.4.1. Caco-2 Monolayer
2.4.2. Co-Cultures of Caco-2 and HT29-MTX
3. Developments of in Situ Methods
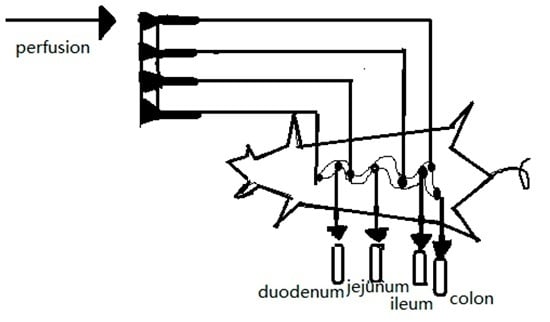
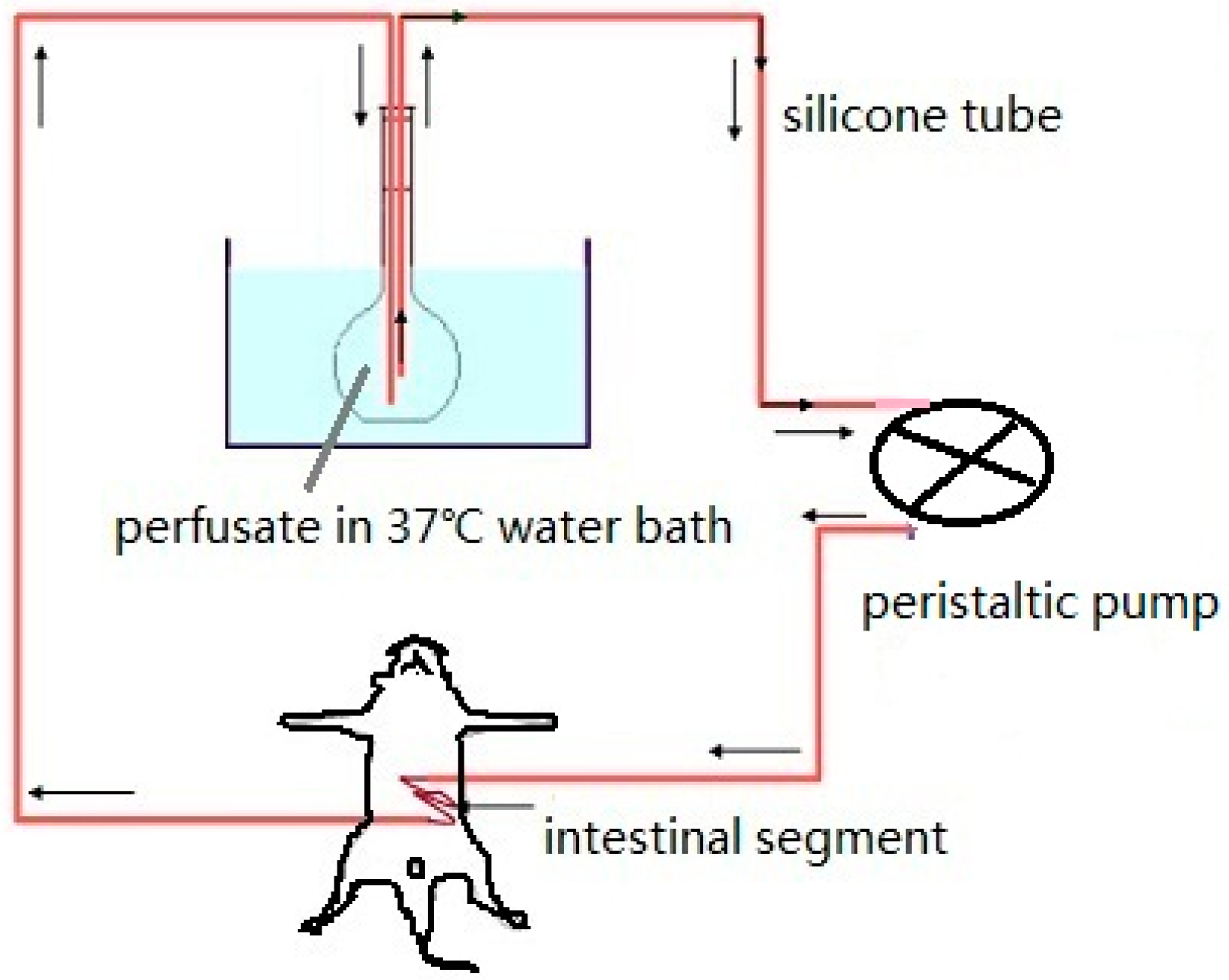
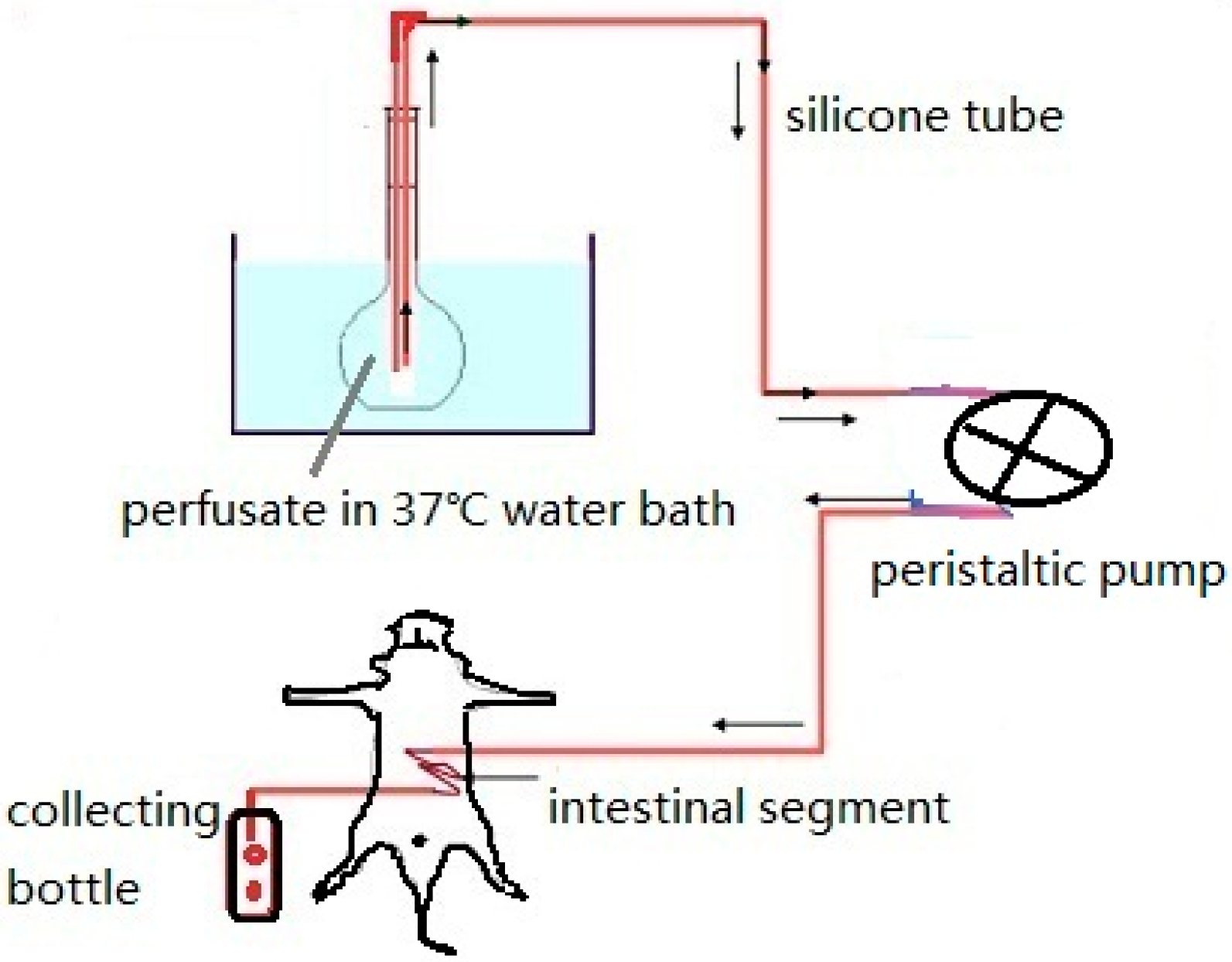
3.1. Intestinal Perfusion
3.1.1. Circular Perfusion Method
3.1.2. Single-Pass Perfusion
3.2. Intestinal Loop Method
3.3. Intestinal Vascular Cannulation
4. Development of in Vivo Methods
5. Conclusions
| Model | Advantages | Disadvantages | ||
|---|---|---|---|---|
| In vitro | Dialysis bag | Positive dialysis | It promotes the exchange of substances internal and external dialysis bags a and avoids the loss of NPs during the sampling. | NP solution suffers reduced mixing with the release medium [20]. |
| Reverse dialysis | NPs mix better with the release medium. | It is difficult to sample. | ||
| Rut gut sac | Everted gut sac | Drugs can contact well with the intestinal mucosa. | There will be morphological damages to intestinal tissue [31]. | |
| Non-everted gut sac | The procedure is simple. There are minor intestinal morphological changes as sampling [31]. | Drugs can not contact well with intestinal mucosa. | ||
| Ussing chamber | It is suitable to study the absorption of different intestine segments. Samples are clean and easy to be analysed. | It lacks blood and nerve supply. The mucosa loses activity easily [10,37]. | ||
| Cell culture | Caco-2 monolayer | It can be used to distinguish different absorption pathways in the intestinal cavity, to determine the way of drug absorption and the kinetic parameters of drug absorption [54,55]. | It lacks mucous layer and some metabolic enzymes. It is impermeable to hydrophilic or paracellular transport [13,56]. | |
| Co-culture of Caco-2 and HT29-MTX | It mimicks well the intestinal cells [37,42]. | It’s hard to co-culture. | ||
| In situ | Intestinal perfusion | Circular perfusion | It has complete blood supply and nerve domination. | Animals may die during the experiment. Samples are complicated to test. |
| Single-pass perfusion | It has complete blood supply and nerve domination. | Animals may die during the experiment. Samples are complicated to test. | ||
| Intestinal loop | It is easier to operate than intestinal perfusion. | It is difficult to analyse the samples. | ||
| Intestinal Vascular cannulation | It simulates well the intestinal absorption of nanoparticles [80]. | It is difficult to sample. | ||
| In vivo | Blood or urine detection | It can truly reflect the NP intestinal absorption in vivo [87,88,89]. | It can not study the absorption mechanism and reflect the partial intestinal absorption [90,91]. | |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, R.A.; Fadl-allah, S.A.; El-Bagoury, N.; El-Rab, S.M.F.G. Improvement of corrosion resistance and antibacterial effect of NiTi orthopedic materials by chitosan and gold nanoparticles. Appl. Surf. Sci. 2014, 292, 390–399. [Google Scholar] [CrossRef]
- Kang, X.; Yang, D.; Ma, P.; Dai, Y.; Shang, M.; Geng, D.; Cheng, Z.; Lin, J. Fabrication of hollow and porous structured GdVO4:Dy3+ nanospheres as anticancer drug carrier and MRI contrast agent. Langmuir ACS J. Surf. Colloids 2013, 29, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.; Peleteiro, M.; Diaz-Freitas, B.; Sanchez, A.; Gonzalez-Fernandez, A.; Alonso, M.J. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: A needle-free vaccination strategy. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Lee, J.S.; Bae, J.W.; Choi, J.H.; Lee, Y.; Son, J.Y.; Park, K.D. Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resistant tumor in mice. Int. J. Pharm. 2015, 495, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Kim, J.; Le, L.V.; Nemeth, C.L.; Chirra, H.D.; Desai, T.A. Micro/nanofabricated platforms for oral drug delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, L.; Liu, Y.; Wang, G.; Zhang, L.; Qiao, Y. Comparison of two approaches of intestinal absorption by puerarin. J. Pharmacol. Toxicol. Methods 2014, 70, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.H.; Liang, R.C.; Sun, F.Y.; Shi, Y.N.; Wang, A.P.; Liu, W.H.; Sun, K.X.; Li, Y.X. The glucose-lowering potential of exenatide delivered orally via goblet cell-targeting nanoparticles. Pharm. Res. 2015, 32, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Costa Lima, S.A.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Santos, H.A. Improving oral absorption via drug-loaded nanocarriers: Absorption mechanisms, intestinal models and rational fabrication. Curr. Drug Metab. 2013, 14, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Mazak, K.; Noszal, B. Drug delivery: A process governed by species-specific lipophilicities. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 62, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.V.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.; Rietjens, I.M.; Bouwmeester, H. Progress and future of in vitro models to study translocation of nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Woitiski, C.B.; Sarmento, B.; Carvalho, R.A.; Neufeld, R.J.; Veiga, F. Facilitated nanoscale delivery of insulin across intestinal membrane models. Int. J. Pharm. 2011, 412, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Saremi, S.; Atyabi, F.; Akhlaghi, S.P.; Ostad, S.N.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128. [Google Scholar]
- Constantinides, P.P.; Lambert, K.J.; Tustian, A.K.; Schneider, B.; Lalji, S.; Ma, W.; Wentzel, B.; Kessler, D.; Worah, D.; Quay, S.C. Formulation development and antitumor activity of a filter-sterilizable emulsion of paclitaxel. Pharm. Res. 2000, 17, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Cao, Y.; Zhu, X.Y.; Cui, J.H.; Cao, Q.R. Optimization of process variables of zanamivir-loaded solid lipid nanoparticles and the prediction of their cellular transport in Caco-2 cell model. Int. J. Pharm. 2015, 478, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Alam, M.A.; Singh, A.T.; Iqbal, Z.; Panda, A.K.; Talegaonkar, S. Biodegradable polymeric nanoparticles for oral delivery of epirubicin: In vitro, ex vivo, and in vivo investigations. Colloids Surfaces B Biointerfaces 2015, 128, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, L.; Zhou, L.; Zhang, Z.H.; Cao, W.; Wu, Q.Q. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int. J. Nanomed. 2012, 7, 4581–4591. [Google Scholar]
- Antunes, F.; Andrade, F.; Araujo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mittal, A.; Jain, A.K.; Mahajan, R.R.; Singh, D. Cyclosporin a loaded plga nanoparticle: preparation, optimization, in-vitro characterization and stability studies. Curr. Nanosci. 2010, 6, 422–431. [Google Scholar] [CrossRef]
- Chellampillai, B.; Pawar, A.P. Andrographolide, a novel bioactive phytoconstituent encapsulated in sustained release biodegradable nanoparticles. Int. J. Nanotechnol. 2011, 8, 764–778. [Google Scholar] [CrossRef]
- Wilson, T.H.; Wiseman, G. The use of sacs of everted small intestine for the sdudy of the transference of substances from the mucosal to the serosal surface. J. Physiol. 1954, 123, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Yang, X.L.; Yang, Z.L.; Kou, J.P.; Li, F. Enhancement of absorption and bioavailability of echinacoside by verapamil or clove oil. Drug Des. Dev. Ther. 2015, 9, 4685–4693. [Google Scholar]
- De Souza, A.L.; Andreani, T.; De Oliveira, R.N.; Kiill, C.P.; dos Santos, F.K.; Allegretti, S.M.; Chaud, M.V.; Souto, E.B.; Silva, A.M.; Gremiao, M.P. In vitro evaluation of permeation, toxicity and effect of praziquantel-loaded solid lipid nanoparticles against Schistosoma mansoni as a strategy to improve efficacy of the schistosomiasis treatment. Int. J. Pharm. 2014, 463, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Kaul, S.; Ritschel, W.A. Studies of the intestinal transfer of coumarin and 7-hydroxycoumarin across guinea pig and rat small intestine. Arzneim. Forschung. 1981, 31, 790–795. [Google Scholar]
- Barthe, L.; Bessouet, M.; Woodley, J.F.; Houin, G. The improved everted gut SAC a simple method to study intestinal P-glycoprotein. Int. J. Pharm. 1998, 173, 255–258. [Google Scholar] [CrossRef]
- Sharma, P.; Chawla, H.P.; Panchagnula, R. LC determination of cephalosporins in in vitro rat intestinal SAC absorption model. J. Pharm. Biomed. Anal. 2002, 27, 39–50. [Google Scholar] [CrossRef]
- Dixit, P.; Jain, D.K.; Dumbwani, J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J. Pharmacol. Toxicol. Methods 2012, 65, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Balimane, P.V.; Chong, S.; Morrison, R.A. Current methodologies used for evaluation of intestinal permeability and absorption. J. Pharmacol. Toxicol. Methods 2000, 44, 301–312. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active Transport of Sodium as the Source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Schimpel, C.; Teubl, B.; Absenger, M.; Meindl, C.; Frohlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Andrade, F.; Ferreira, D.; Nielsen, H.M.; Sarmento, B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug Metab. 2013, 14, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, S.; Bouchemal, K.; Sharma, C.P.; Vauthier, C. Surface-functionalized polymethacrylic acid based hydrogel microparticles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 74, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Hintzen, F.; Shahnaz, G.; Rahmat, D.; Leithner, K.; Bernkop-Schnurch, A. Development and in vitro evaluation of slippery nanoparticles for enhanced diffusion through native mucus. Nanomed. Nanotechnol. Biol. Med. 2014, 9, 387–396. [Google Scholar]
- Westerhout, J.; van de Steeg, E.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.; Verwei, M.; Wortelboer, H.M. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Calleja, P.; Espuelas, S.; Vauthier, C.; Ponchel, G.; Irache, J.M. Controlled release, intestinal transport, and oral bioavailablity of paclitaxel can be considerably increased using suitably tailored pegylated poly(anhydride) nanoparticles. J. Pharm. Sci. 2015, 104, 2877–2886. [Google Scholar] [CrossRef] [PubMed]
- Kollner, S.; Dunnhaupt, S.; Waldner, C.; Hauptstein, S.; Pereira de Sousa, I.; Bernkop-Schnurch, A. Mucus permeating thiomer nanoparticles. Eur. J. Pharm. Biopharm. 2015, 97, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sharma, C.P. Simultaneous effect of thiolation and carboxylation of chitosan particles towards mucoadhesive oral insulin delivery applications: an in vitro and in vivo evaluation. J. Biomed. Nanotechnol. 2015, 11, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.S.; Thakker, D.R. In vitro models for selection of development candidatesapplications of the Caco-2 model in the design and development of orally active drugs: Elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv. Drug Deliv. Rev. 1997, 23, 77–98. [Google Scholar] [CrossRef]
- Beduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [PubMed]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Yucel, C.; Degim, Z.; Yilmaz, S. Nanoparticle and liposome formulations of doxycycline: Transport properties through Caco-2 cell line and effects on matrix metalloproteinase secretion. Biomed. Pharmacother. 2013, 67, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Aoyama, Y.; Khafagy el, S.; Henmi, M.; Takeda-Morishita, M. Effect of different intestinal conditions on the intermolecular interaction between insulin and cell-penetrating peptide penetratin and on its contribution to stimulation of permeation through intestinal epithelium. Eur. J. Pharm. Biopharm. 2015, 94, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Janich, S.; Roessler, B. J.; Hilfinger, J.M.; Amidon, G.L. HT29-MTX/Caco-2 Cocultures as an in vitro model for the intestinal epithelium: In vitrol-in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996, 85, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Ling, L.Q.; Guo, L.; Gong, T.; Sun, X.; Zhang, Z.R. Intestinal absorption characteristics of imperialine: In vitro and in situ assessments. Acta Pharmacol. Sin. 2015, 36, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Zhang, W.; Xie, C.; Wei, G.; Lu, W. Oligoarginine-modified biodegradable nanoparticles improve the intestinal absorption of insulin. Int. J. Pharm. 2013, 448, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Cheng, W.P.; Gadad, P.; Skene, K.; Smith, M.; Smith, G.; McKinnon, A.; Knott, R. Uptake and transport of novel amphiphilic polyelectrolyte-insulin nanocomplexes by Caco-2 cells-towards oral insulin. Pharm. Res. 2011, 28, 886–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, S.; Devocelle, M.; Ryan, S.; McClean, S.; Brayden, D.J. Impact of amino acid replacements on in vitro permeation enhancement and cytotoxicity of the intestinal absorption promoter, melittin. Int. J. Pharm. 2010, 387, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Kanzer, J.; Hummel, J.; Fricker, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 36, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Stenberg, P.; Artursson, P.; Kissel, T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm. Res. 2001, 18, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Shahbazi, M.A.; Araujo, F.; Zhang, H.; Makila, E.M.; Kauppila, J.; Sarmento, B.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Chitosan-modified porous silicon microparticles for enhanced permeability of insulin across intestinal cell monolayers. Biomaterials 2014, 35, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, C.Y.; Chai, G.H.; Du, Y.Z.; Hu, F.Q. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol. Pharm. 2013, 10, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Curran, P.F.; Solomon, A.K. Ion and water fluxes in the ileum of rats. J. Gen. Physiol. 1957, 41, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, U.; Johansson, M.; Lennernas, H. Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 1996, 13, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Dai, J.D.; Zhang, H.; Zhang, X.; Wang, J.C.; Zhang, Q. Absorption mechanism of cyclosporine A loaded pH-sensitive nanoparticles in rats. J. Nanosci. Nanotechnol. 2008, 8, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Holenarsipur, V.K.; Gaud, N.; Sinha, J.; Sivaprasad, S.; Bhutani, P.; Subramanian, M.; Singh, S.P.; Arla, R.; Paruchury, S.; Sharma, T.; et al. Absorption and cleavage of enalapril, a carboxyl ester prodrug, in the rat intestine: In vitro, in situ intestinal perfusion and portal vein cannulation models. Biopharm. Drug Dispos. 2015, 36, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L. New perspectives on the theory of permeability and resistance in the study of drug transport and absorption. J. Pharm. Biopharm. 1996, 24, 433–442. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, L.; Guo, C.; Cao, F.; Chen, H.; Zhao, L.; Tan, Q.; Zhu, X.; Zhu, F.; Ding, T. Evaluation of an oral carrier system in rats: Bioavailability and gastrointestinal absorption properties of curcumin encapsulated PBCA nanoparticles. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Feng, N.; Zhang, X.; Wu, S.; Zhao, J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2009, 365, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bu, H.; Gao, Z.; Huang, Y.; Gao, F.; Li, Y. The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int. J. Pharm. 2010, 394, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, Z.R.; Sun, X.; Zuo, J.; Zhao, D.; Gong, T. Mechanisms of phospholipid complex loaded nanoparticles enhancing the oral bioavailability. Mol. Pharm. 2010, 7, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhai, X.; Xue, K.; Hu, L.; Yang, X.; Li, G.; Si, L. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: Linear correlation with oral bioavailabilities in rats. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 43, 132–140. [Google Scholar]
- Zakeri-Milani, P.; Islambulchilar, Z.; Majidpour, F.; Jannatabadi, E.; Lotfipour, F.; Valizadeh, H. A study on enhanced intestinal permeability of clarithromycin nanoparticles. Braz. J. Pharm. Sci. 2014, 50, 121–129. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Z.H.; Li, S.L.; Sun, E.; Tan, X.B.; Song, J.; Jia, X.B. A nanostructured liquid crystalline formulation of 20(S)-protopanaxadiol with improved oral absorption. Fitoterapia 2013, 84, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zakeri-Milani, P.; Valizadeh, H.; Tajerzadeh, H.; Islambulchilar, Z. The utility of rat jejunal permeability for biopharmaceutics classification system. Drug Dev. Ind. Pharm. 2009, 35, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Punyashthiti, K.; Finkelstein, R.A. Enteropathogencity of Escherichia coli. Infect. Immun. 1971, 4, 473–478. [Google Scholar] [PubMed]
- MaClean, S.; Prosser, E.; Meehan, E.; Malley, D.O.; Clarke, N.; Ramtoola, Z.; Brayden, D. Binding and uptake of biodegradable poly-DL-lactide micro- and nanoparticles in intestinal epithelia. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 1998, 6, 153–163. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, J.W.; Yoon, I.S.; Kim, D.D. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: Enhanced intestinal absorption and lymphatic uptake. Int. J. Nanomed. 2014, 9, 495–504. [Google Scholar]
- Pappo, J.; Ermak, T.H. Uptake and translocation of fluorescent latex particles by rabbit Peyer′s patch follicle epithelium: A quantitative model for M cell uptake. Clin. Exp. Immunol. 1989, 76, 144–148. [Google Scholar] [PubMed]
- Hamid, K.A.; Lin, Y.; Gao, Y.; Katsumi, H.; Sakane, T.; Yamamoto, A. The effect of wellsolve, a novel solubilizing agent, on the intestinal barrier function and intestinal absorption of griseofulvin in rats. Biol. Pharm. Bull. 2009, 32, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Konno, Y.; Satsukawa, M.; Kobayashi, T.; Takimoto, Y.; Morisaki, K.; Yamashita, S. Assessment of intestinal availability of various drugs in the oral absorption process using portal vein-cannulated rats. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Usansky, H.H.; Hu, P.; Sinko, P.J. Differential roles of P-glycoprotein, multidrug resistance-associated protein 2, and CYP3A on saquinavir oral absorption in Sprague-Dawley rats. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Shen, Q.; Katsumi, H.; Okada, K.; Fujita, T.; Jiang, X.H.; Yamamoto, A. Effects of Labrasol and other pharmaceutical excipients on the intestinal transport and absorption of rhodamine123, a P-glycoprotein substrate, in rats. Biol. Pharm. Bull. 2007, 30, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, Y.H.; Juang, J.H.; Wey, S.P.; Chen, C.T.; Sung, H.W. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Song, Y.; Nann, T.; Desai, T.A. Intestinal absorption of fluorescently labeled nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Reineke, J.J.; Cho, D.Y.; Dingle, Y.T.; Morello, A.P.; Jacob, J.; Thanos, C.G.; Mathiowitz, E. Unique insights into the intestinal absorption, transit, and subsequent biodistribution of polymer-derived microspheres. Proc. Natl. Acad. Sci. USA 2013, 110, 13803–13808. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Carswell, H.; Brett, R.; Currie, S.; Kumar, M.N. Development and evaluation of polymer nanoparticles for oral delivery of estradiol to rat brain in a model of Alzheimer’s pathology. J. Control. Release Off. J. Control. Release Soc. 2011, 150, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Jeong, H.J.; Yun, K.N.; Kim, D.W.; Sohn, M.H.; Lee, J.K.; Jeong, J.; Lim, S.T. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int. J. Nanomed. 2012, 7, 3203–3209. [Google Scholar]
- Zabaleta, V.; Ponchel, G.; Salman, H.; Agueros, M.; Vauthier, C.; Irache, J.M. Oral administration of paclitaxel with pegylated poly(anhydride) nanoparticles: Permeability and pharmacokinetic study. Eur. J. Pharm. Biopharm. 2012, 81, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, X.L.; Cheng, X.L.; Liang, Z.G.; Liao, C.L.; Wang, Y.C.; Cao, L.J. Characterization, intestinal absorption and pharmacokinetics of long-circulating nanoparticles loaded with panaxnotoginseng saponins. Adv. Mater. Res. 2013, 662, 227–232. [Google Scholar] [CrossRef]
- Kurti, L.; Gaspar, R.; Marki, A.; Kapolna, E.; Bocsik, A.; Veszelka, S.; Bartos, C.; Ambrus, R.; Vastag, M.; Deli, M.A.; Szabo-Revesz, P. In vitro and in vivo characterization of meloxicam nanoparticles designed for nasal administration. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Lavandera, J.L.; Agueros, M.; Irache, J.M. Cyclodextrin/poly(anhydride) nanoparticles as drug carriers for the oral delivery of atovaquone. Biomed. Microdevices 2011, 13, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, A.; Mudassir, J.; Mohtar, N.; Darwis, Y. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013, 8, 2733–2744. [Google Scholar]
- Bei, Y.Y.; Chen, X.Y.; Liu, Y.; Xu, J.Y.; Wang, W.J.; Gu, Z.L.; Xing, K.L.; Zhu, A.J.; Chen, W.L.; Shi, L.S.; Wang, Q.; Zhang, X.N.; Zhang, Q. Novel norcantharidin-loaded liver targeting chitosan nanoparticles to enhance intestinal absorption. Int. J. Nanomed. 2012, 7, 1819–1827. [Google Scholar]
- Jain, A.K.; Swarnakar, N.K.; Godugu, C.; Singh, R.P.; Jain, S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials 2011, 32, 503–515. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int. J. Mol. Sci. 2016, 17, 1171. https://doi.org/10.3390/ijms17071171
Liu W, Pan H, Zhang C, Zhao L, Zhao R, Zhu Y, Pan W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. International Journal of Molecular Sciences. 2016; 17(7):1171. https://doi.org/10.3390/ijms17071171
Chicago/Turabian StyleLiu, Wei, Hao Pan, Caiyun Zhang, Liling Zhao, Ruixia Zhao, Yongtao Zhu, and Weisan Pan. 2016. "Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs" International Journal of Molecular Sciences 17, no. 7: 1171. https://doi.org/10.3390/ijms17071171