Elevated Preoperative Serum Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) Ratio Is Associated with Better Prognosis in Patients Undergoing Curative Treatment for Gastric Adenocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Patients
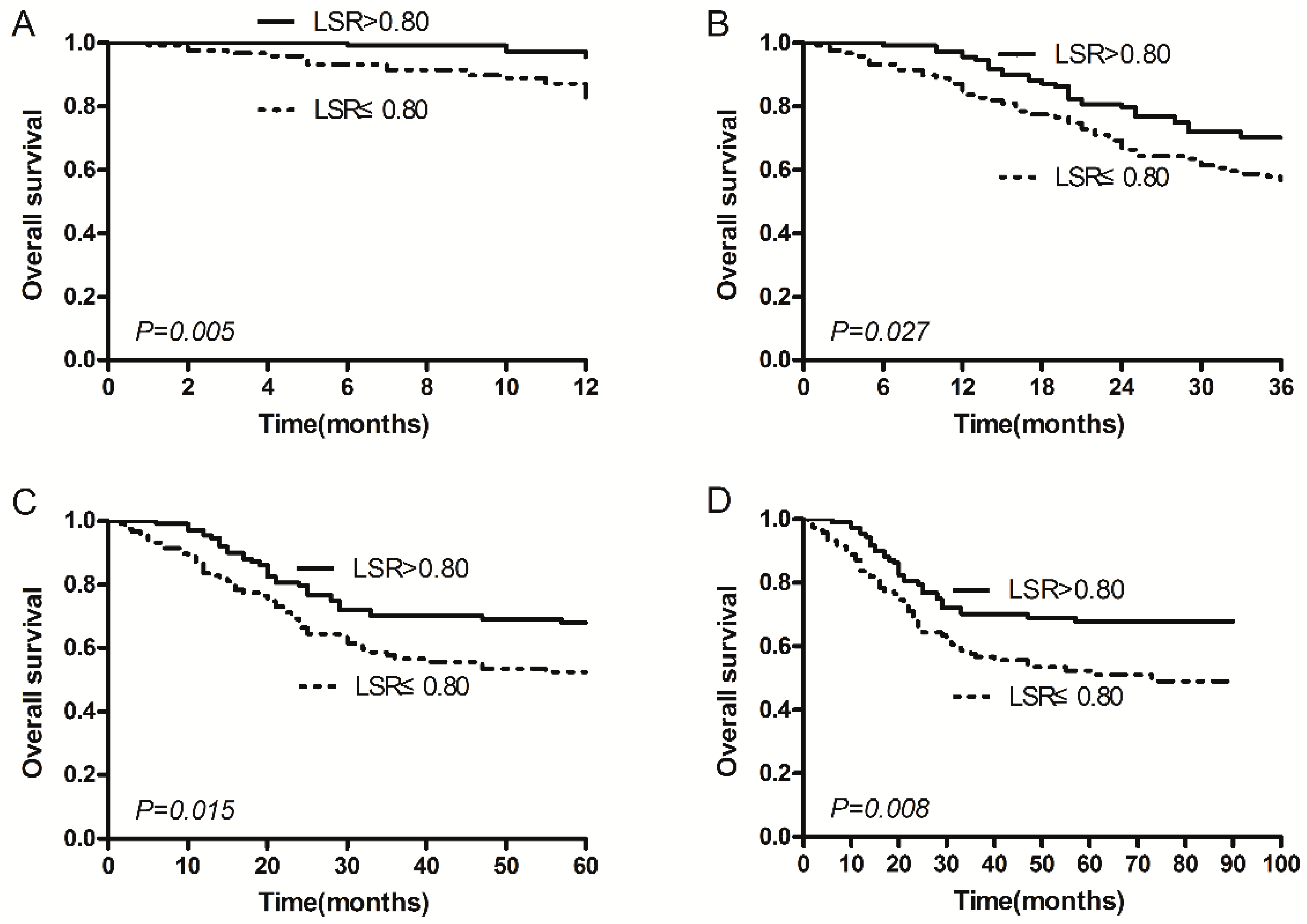
2.2. Serum ALT/AST Ratio (LSR) and Survival
2.3. The Relationship between the ALT/AST Ratio and Clinicopathologic Characteristics in GA Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Laboratory Measurements
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern. Med. (Tokyo Jpn.) 2008, 47, 1077–1083. [Google Scholar] [CrossRef]
- Thrumurthy, S.G.; Chaudry, M.A.; Hochhauser, D.; Mughal, M. The diagnosis and management of gastric cancer. BMJ 2013, 347. [Google Scholar] [CrossRef] [PubMed]
- Karmen, A.; Wroblewski, F.; Ladue, J.S. Transaminase activity in human blood. J. Clin. Investig. 1955, 34, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Jamali, R.; Pourshams, A.; Amini, S.; Deyhim, M.-R.; Rezvan, H.; Malekzadeh, R. The upper normal limit of serum alanine aminotransferase in Golestan province, northeast Iran. Arch. Iran. Med. 2008, 11, 602–607. [Google Scholar] [PubMed]
- Lin, M.-S.; Lin, H.-S.; Chung, C.-M.; Lin, Y.-S.; Chen, M.-Y.; Chen, P.-H.; Hu, J.-H.; Chou, W.-N.; Huang, J.-C.; Huang, T.-J. Serum aminotransferase ratio is independently correlated with hepatosteatosis in patients with HCV: A cross-sectional observational study. BMJ Open 2015, 5, e008797. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.-P.; Wang, H.-B.; Lin, Y.-H.; Xu, J.; Wang, J.; Wang, K.; Liu, W.-L. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl. Oncol. 2015, 8, 497–503. [Google Scholar]
- Onate-Ocana, L.F.; Aiello-Crocifoglio, V.; Gallardo-Rincon, D.; Herrera-Goepfert, R.; Brom-Valladares, R.; Carrillo, J.F.; Cervera, E.; Mohar-Betancourt, A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann. Surg. Oncol. 2007, 14, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Liu, J.; Kong, P.; Chen, S.; Zhan, Y.; Xu, D. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl. Oncol. 2015, 8, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Xu, Y.; Zhu, H.-Y.; Shi, Y.-Q. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumor Biol. 2015, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Moore, K.; Phillips, L.; Boyle, F.M.; Marsh, D.J.; Baxter, R.C. Novel serum protein biomarker panel revealed by mass spectrometry and its prognostic value in breast cancer. Breast Cancer Res. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Shakoori, A.; Butt, U.; Riffat, R.; Aziz, F. Haematological and biochemical effects of danitol administered for two months in the blood and liver of rabbits. Z. Feur Angew. Zool. 1994, 80, 165–180. [Google Scholar]
- Onyesom, I.; Anosike, E. Changes in rabbit liver function markers after chronic exposure to ethanol. Asian J. Biochem. 2007, 2, 334–342. [Google Scholar]
- Navarro, M.C.; Montialla, M.; Martín, A.; Jiménez, J.; Utrilla, M.P. Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med. 1993, 59, 312–314. [Google Scholar] [CrossRef]
- Toiyama, Y.; Inoue, Y.; Saigusa, S.; Kawamura, M.; Kawamoto, A.; Okugawa, Y.; Hiro, J.; Tanaka, K.; Mohri, Y.; Kusunoki, M. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2013, 33, 5065–5074. [Google Scholar] [PubMed]
- Park, J.H.; Watt, D.G.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C. Colorectal cancer, systemic inflammation, and outcome: Staging the tumor and staging the host. Ann. Surg. 2016, 263, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Uprak, T.K.; Attaallah, W.; Çelikel, Ç.A.; Ayrancı, G.; Yeğen, C. HER-2 incidence in gastric cancer, its association with prognosis and clinicopathological parameters. Turk. J. Surg. Ulus. Cerrahi. Derg. 2015, 31, 207–213. [Google Scholar]
- Sun, Z.; Zhang, N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J. Surg. Oncol. 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Gupta, N.; Zhang, H.; Alshareef, A.; Alqahtani, H.; Bigras, G.; Lewis, J.; Douglas, D.; Kneteman, N.; Lavasanifar, A. Oxidative stress induces the acquisition of cancer stem-like phenotype in breast cancer detectable by using a Sox2 regulatory region-2 (SRR2) reporter. Oncotarget 2015, 7, 3111–3127. [Google Scholar]
- Falfushynska, H.; Gnatyshyna, L.; Deneha, H.; Osadchuk, O.; Stoliar, O. Manifestations of oxidative stress and molecular damages in ovarian cancer tissue. Ukr. Biochem. J. 2015, 87, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Pichler, M.; Absenger, G.; Stotz, M.; Arminger, F.; Weissmueller, M.; Schaberl-Moser, R.; Samonigg, H.; Kornprat, P.; Stojakovic, T. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am. J. Surg. 2014, 208, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.-P.; Fan, S.-T.; Lo, C.-M.; Liu, C.-L.; Ng, I.O.-L.; Wong, J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J. Clin. Oncol. 2000, 18, 1094–1101. [Google Scholar] [PubMed]
- Shen, S.-L.; Fu, S.-J.; Chen, B.; Kuang, M.; Li, S.-Q.; Hua, Y.-P.; Liang, L.-J.; Guo, P.; Hao, Y.; Peng, B.-G. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann. Surg. Oncol. 2014, 21, 3802–3809. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sudderth, J.; Dang, T.; Bachoo, R.G.; McDonald, J.G.; de Berardinis, R.J. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009, 69, 7986–7993. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H. Anticancer effect of lycopene in gastric carcinogenesis. J. Cancer Prev. 2015, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.; McMillan, D.; McArdle, C.; Angerson, W.; Dunlop, D. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.; Napier, S.; van der Waal, I.; Snijders, P.; Walboomers, J.; Lamey, P.; Cowan, C.; Gregg, T.; Maxwell, P.; Meijer, C. Suprabasal p53 immunoexpression is strongly associated with high grade dysplasia and risk for malignant transformation in potentially malignant oral lesions from Northern Ireland. J. Clin. Pathol. 2002, 55, 98–104. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | No. of Patients (%) | OS (Months) Mean (95% CI) | p-Value Log-Rank |
|---|---|---|---|
| Sex | |||
| Male | 160 (69.3%) | 62.5 (57.0–67.9) | 0.478 |
| Female | 71 (30.7%) | 59.7 (51.4–68.1) | |
| Age (years) | |||
| ≤56 | 118 (51.1%) | 64.4 (58.2–70.6) | 0.183 |
| >56 | 113 (48.9%) | 58.8 (52.1–65.6) | |
| Smoking Behaviour | |||
| Yes | 64 (27.7%) | 65.5 (57.1–73.9) | 0.308 |
| No | 167 (72.3) | 60.4 (54.9–65.8) | |
| Family History | |||
| Yes | 23 (10.0%) | 63.3 (50.3–76.3) | 0.563 |
| No | 208 (90.0%) | 61.5 (56.4–66.4) | |
| Pathological Stage a | |||
| I | 29 (12.6%) | 86.8 (84.4–89.1) | <0.001 |
| II | 52 (22.5%) | 85.9 (82.1–89.8) | |
| III | 94 (40.7%) | 57.9 (50.9–64.9) | |
| IV | 56 (24.2%) | 29.6 (21.7–37.5) | |
| Lymph Node Metastasis (N) | |||
| N0 | 17 (7.3%) | 74.9 (62.2–87.6) | <0.001 |
| N1 | 38 (16.5%) | 70.8 (60.1–80.5) | |
| N2 | 156 (67.5%) | 60.3 (54.9–65.8) | |
| N3 | 20 (8.7%) | 35.4 (19.7–51.1) | |
| Distant Metastases | |||
| Yes | 39 (16.9%) | 25.4 (17.7–33.2) | <0.001 |
| No | 192 (83.1%) | 68.7 (64.1–73.3) | |
| Tumor Location a | |||
| Upper | 49 (21.2%) | 51.3 (41.7–60.8) | 0.062 |
| Middle | 61 (26.4%) | 58.4 (49.9–66.9) | |
| Lower | 121 (52.4%) | 66.7 (60.5–72.9) | |
| Maximun Tumor Diameter (cm) | |||
| ≤5 | 144 (62.3%) | 70.1 (64.9–75.4) | <0.001 |
| >5 | 87 (37.7%) | 48.1 (40.4–55.7) | |
| Serous Infiltration | |||
| S0 | 44 (19.1%) | 86.0 (81.8–90.1) | <0.001 |
| S1 | 37 (16.0%) | 63.9 (52.2–75.5) | |
| S2 | 100 (43.3%) | 58.3 (51.5–65.2) | |
| S3 | 50 (21.6%) | 43.0 (33.3–52.7) | |
| ALB (g/L) | |||
| ≤35 | 21 (9.1) | 41.7 (26.9–56.4) | 0.017 |
| >35 | 210 (90.9%) | 63.7 (58.9–68.4) | |
| ALT (U/L) | |||
| ≤14.6 | 114 (49.4%) | 59.3 (52.9–65.7) | 0.334 |
| >14.6 | 117 (50.6%) | 64.1 (57.6–70.6) | |
| AST (U/L) | |||
| ≤18.5 | 116 (50.2%) | 61.1 (54.7–67.5) | 0.868 |
| >18.5 | 115 (49.8%) | 62.4 (55.8–68.9) | |
| LSR (ALT/AST) | |||
| ≤0.80 | 117 (50.6%) | 55.5 (48.9–62.1) | 0.008 |
| >0.80 | 114 (49.4%) | 68.2 (62.1–74.3) | |
| CRP (mg/L) | |||
| ≤1.56 | 116 (50.2%) | 65.4 (59.2–71.6) | 0.156 |
| >1.56 | 115 (49.8%) | 58.0 (51.4–64.7) | |
| CA19-9 (U/mL) | |||
| ≤10.97 | 117 (50.6%) | 66.2 (59.8–72.6) | 0.081 |
| >10.97 | 114 (49.4%) | 57.5 (51.1–64.0) | |
| CA72-4 (U/mL) | |||
| ≤1.8 | 116 (50.2%) | 69.7 (63.7–75.5) | <0.001 |
| >1.8 | 115 (49.8%) | 53.9 (47.2–60.5) | |
| CEA (ng/mL) | |||
| ≤2.17 | 117 (50.6%) | 66.2 (60.0–72.6) | 0.057 |
| >2.17 | 114 (49.4%) | 57.2 (50.7–63.7) | |
| Fbg (g/L) | |||
| ≤2.82 | 116 (50.2%) | 66.6 (60.4–72.8) | 0.046 |
| >2.82 | 115 (49.8%) | 56.8 (50.2–63.4) | |
| GPS | |||
| 0 | 193 (83.6%) | 64.3 (59.4–69.1) | 0.066 |
| 1 | 34 (14.7%) | 50.1 (37.0–63.1) | |
| 2 | 4 (1.7%) | 43.5 (13.8–73.2) | |
| p53 | |||
| 0 | 56 (24.2%) | 59.7 (50.2–69.3) | 0.018 |
| +1 | 75 (32.5%) | 70.3 (63.4–77.2) | |
| +2 | 34 (14.7%) | 59.0 (47.2–70.7) | |
| +3 | 66 (28.6%) | 52.9 (44.1–61.6) | |
| Blood Type | |||
| A | 63 (27.3%) | 57.2 (48.3–66.1) | 0.412 |
| B | 16 (6.9%) | 51.9 (37.5–66.2) | |
| AB | 57 (24.7%) | 59.2 (50.2–68.2) | |
| O | 95 (41.1%) | 66.3 (59.3–73.2) | |
| Patient Characteristics | Univariate Analysis HR (95% CI) | p-Value | Multivariate Analysis HR (95% CI) | p-Value |
|---|---|---|---|---|
| Sex | 0.853 (0.549–1.326) | 0.481 | - | - |
| Age (years) | 1.326 (0.872–2.018) | 0.187 | - | - |
| Smoking Behaviour | 0.777 (0.476–1.268) | 0.313 | - | - |
| Family History | 0.808 (0.391–1.672) | 0.566 | - | - |
| Pathological Stage | 3.666 (2.694–4.988) | <0.001 | 3.118 (2.044–4.756) | <0.001 |
| Lymph Node Metastasis (N) | 1.988 (1.375–2.874) | <0.001 | 0.831 (0.554–1.247) | 0.371 |
| Distant Metastases | 5.286 (3.360–8.316) | <0.001 | 1.957 (1.119–3.422) | 0.019 |
| Tumor Location | 0.746 (0.581–0.957) | 0.021 | 0.852 (0.646–1.123) | 0.255 |
| Maximun Tumor Diameter (cm) | 2.528 (1.660–3.848) | <0.001 | 1.180 (0.752–1.852) | 0.472 |
| Serous Infiltration | 2.038 (1.587–2.618) | <0.001 | 0.972 (0.708–1.334) | 0.860 |
| ALB (g/L) | 0.485 (0.264–0.892) | 0.020 | 0.776 (0.247–2.438) | 0.664 |
| ALT (U/L) | 0.814 (0.535–1.239) | 0.337 | - | - |
| AST (U/L) | 0.965 (0.636–1.466) | 0.869 | - | - |
| LSR (ALT/AST) | 0.565 (0.368–0.868) | 0.009 | 0.610 (0.388–0.958) | 0.032 |
| CRP (mg/L) | 1.351 (0.888–2.057) | 0.160 | - | - |
| CA19-9 (U/mL) | 1.454 (0.950–2.224) | 0.085 | 0.710 (0.442–1.139) | 0.155 |
| CA72-4 (U/mL) | 2.035 (1.318–3.144) | 0.001 | 1.479 (0.941–2.325) | 0.090 |
| CEA (ng/mL) | 1.501 (0.983–2.293) | 0.060 | 1.331 (0.811–2.184) | 0.258 |
| Fbg (g/L) | 1.530 (1.002–2.338) | 0.049 | 1.070 (0.669–1.713) | 0.778 |
| GPS | 1.565 (1.033–2.373) | 0.035 | 0.929 (0.431–2.004) | 0.851 |
| p53 | 1.212 (1.005–1.462) | 0.045 | 1.128 (0.917–1.387) | 0.255 |
| Blood Type | 0.875 (0.741–1.033) | 0.115 | - | - |
| Patient Characteristics | LSR ≤ 0.80 (n = 117) | LSR > 0.80 (n = 114) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 73 | 87 | 0.023 |
| Female | 44 | 27 | |
| Age (years) | |||
| ≤56 | 54 | 64 | 0.148 |
| >56 | 63 | 50 | |
| Smoking Behavior | |||
| Yes | 90 | 77 | 0.141 |
| No | 27 | 37 | |
| Family History | |||
| Yes | 107 | 101 | 0.515 |
| No | 10 | 13 | |
| Pathological Stage | |||
| I | 13 | 16 | 0.425 |
| II | 23 | 29 | |
| III | 48 | 46 | |
| IV | 33 | 23 | |
| Lymph Node Metastasis (N) | |||
| N0 | 7 | 10 | 0.168 |
| N1 | 22 | 16 | |
| N2 | 74 | 82 | |
| N3 | 14 | 6 | |
| Distant Metastases | |||
| Yes | 94 | 98 | 0.294 |
| No | 23 | 16 | |
| Tumor Location | |||
| Upper | 27 | 22 | 0.247 |
| Middle | 35 | 26 | |
| Lower | 55 | 66 | |
| Maximum Tumor Diameter (cm) | |||
| ≤5 | 65 | 79 | 0.041 |
| >5 | 52 | 35 | |
| Serous Infiltration | |||
| S0 | 16 | 28 | 0.055 |
| S1 | 23 | 14 | |
| S2 | 48 | 52 | |
| S3 | 30 | 20 | |
| ALB (g/L) | |||
| ≤35 | 16 | 5 | 0.020 |
| >35 | 101 | 109 | |
| ALT (U/L) | |||
| ≤14.6 | 94 | 20 | <0.001 |
| >14.6 | 23 | 94 | |
| AST (U/L) | |||
| ≤18.5 | 70 | 46 | 0.004 |
| >18.5 | 47 | 68 | |
| CRP (mg/L) | |||
| ≤1.56 | 50 | 66 | 0.025 |
| >1.56 | 67 | 48 | |
| CA19-9 (U/mL) | |||
| ≤10.97 | 49 | 68 | 0.008 |
| >10.97 | 68 | 46 | |
| CA72-4 (U/mL) | |||
| ≤1.8 | 61 | 55 | 0.600 |
| >1.8 | 56 | 59 | |
| CEA (ng/mL) | |||
| ≤2.17 | 58 | 59 | 0.793 |
| >2.17 | 59 | 55 | |
| Fbg (g/L) | |||
| ≤2.82 | 57 | 59 | 0.694 |
| >2.82 | 60 | 55 | |
| GPS | |||
| 0 | 91 | 102 | 0.054 |
| 1 | 23 | 11 | |
| 2 | 3 | 1 | |
| p53 | |||
| 0 | 23 | 33 | 0.075 |
| +1 | 42 | 33 | |
| +2 | 13 | 21 | |
| +3 | 39 | 27 | |
| Blood type | |||
| A | 35 | 28 | 0.736 |
| B | 28 | 29 | |
| AB | 9 | 7 | |
| O | 45 | 50 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Li, J.-P.; Li, L.-F.; Zeng, T.; He, X. Elevated Preoperative Serum Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) Ratio Is Associated with Better Prognosis in Patients Undergoing Curative Treatment for Gastric Adenocarcinoma. Int. J. Mol. Sci. 2016, 17, 911. https://doi.org/10.3390/ijms17060911
Chen S-L, Li J-P, Li L-F, Zeng T, He X. Elevated Preoperative Serum Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) Ratio Is Associated with Better Prognosis in Patients Undergoing Curative Treatment for Gastric Adenocarcinoma. International Journal of Molecular Sciences. 2016; 17(6):911. https://doi.org/10.3390/ijms17060911
Chicago/Turabian StyleChen, Shu-Lin, Jian-Pei Li, Lin-Fang Li, Tao Zeng, and Xia He. 2016. "Elevated Preoperative Serum Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) Ratio Is Associated with Better Prognosis in Patients Undergoing Curative Treatment for Gastric Adenocarcinoma" International Journal of Molecular Sciences 17, no. 6: 911. https://doi.org/10.3390/ijms17060911