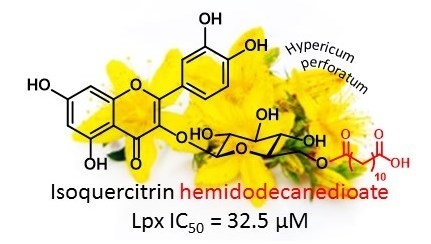
Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties
Abstract
:1. Introduction
2. Results and Discussion
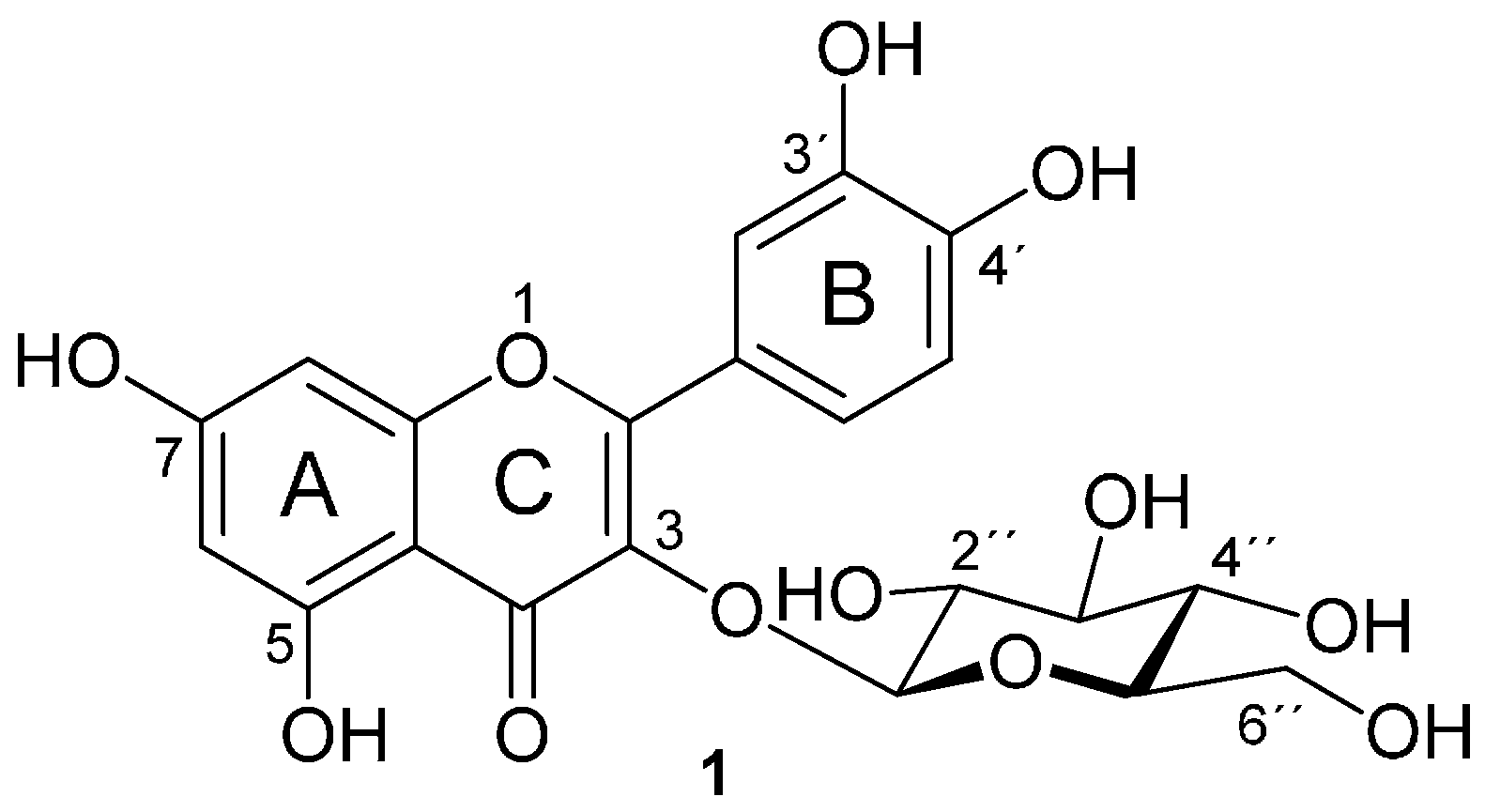
2.1. The Preparation of Isoquercitrin Esters
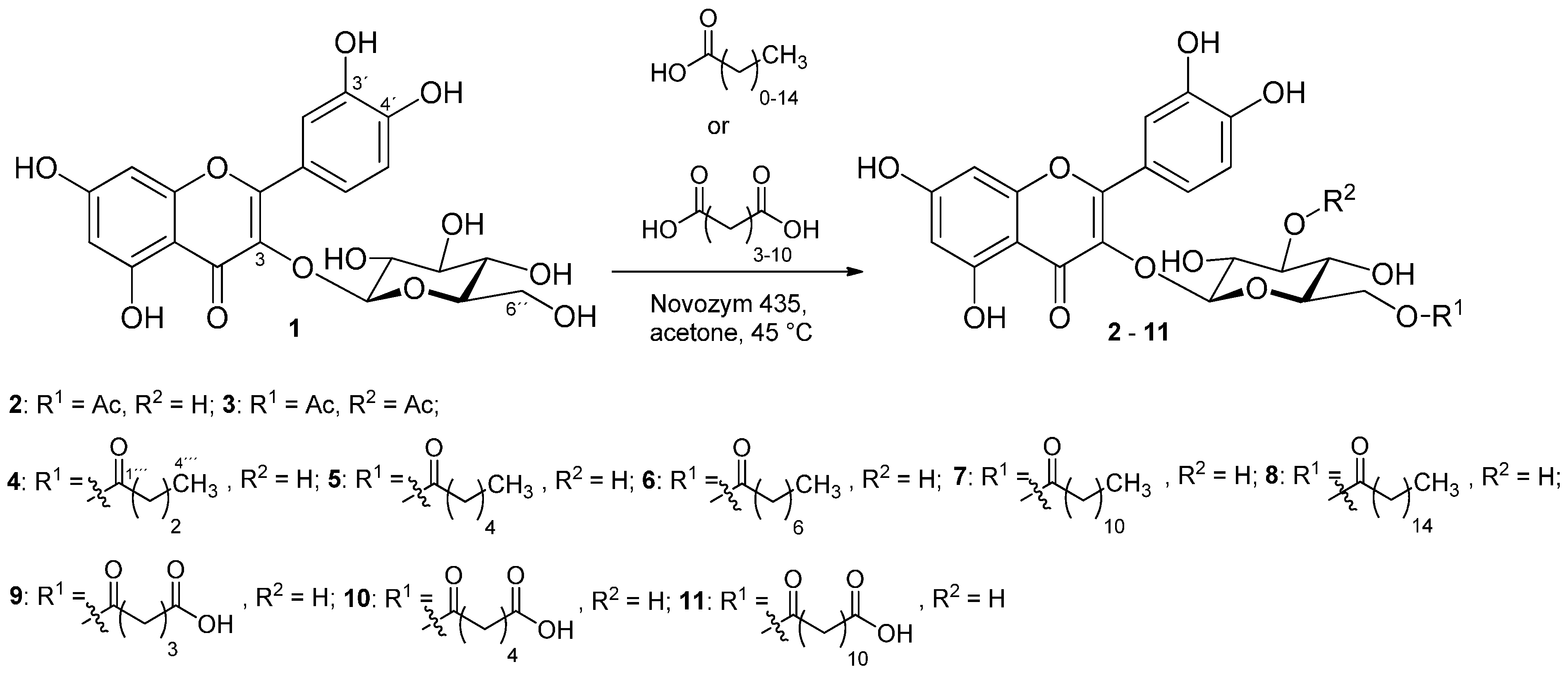
2.1.1. Synthesis of Esters of Isoquercitrin and Monocarboxylic Aliphatic Acids (2–8)
2.1.2. Synthesis of Esters of Isoquercitrin with Aliphatic Dicarboxylic Acids (9–11)
2.2. Partition Coefficient
2.3. Antioxidant Activity of Isoquercitrin Derivatives
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS) Methods
3.3. HPLC Analysis
3.4. Measurement of Log P
3.5. Chemistry
3.5.1. Synthesis of Isoquercitrin Esters 2–8
3.5.2. General Procedure–Synthesis of Isoquercitrin Esters 9–11
3.6. Antioxidant Activity Measurement
3.6.1. Folin–Ciocalteau Reduction (FCR) Assay
3.6.2. DPPH Assay
3.6.3. ABTS· Scavenging
3.6.4. Inhibition of Microsomal Lipid Peroxidation
3.6.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| CAL-B | Lipase B from Candida antarctica |
| COSY | Correlation spectroscopy |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical |
| FCR | Folin–Ciocalteau reduction assay |
| GAE | Gallic acid equivalents |
| HMBC | Heteronuclear multiple-bond correlation spectroscopy |
| HSQC | Heteronuclear single-quantum correlation spectroscopy |
| IC50 | The concentration of the tested compound that inhibited the reaction by 50% |
| IQ | Isoquercitrin |
| LDL | Low-density lipoprotein |
| Lpx | Lipid peroxidation |
| PBS | Phosphate buffer saline |
| PDA | Photodiode array |
| TBARS | Thiobarbituric acid reactive substances |
| TE | Trolox-equivalent |
References
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; van Trijp, J.M.P.; Buysman, M.N.C.P.; Van der Gaag, M.S.; Mengelers, M.J.B.; de Vries, J.H.M.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcino genic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drug 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Gerstorferová, D.; Fliedrová, B.; Halada, P.; Marhol, P.; Křen, V.; Weignerová, L. Recombinant α-l-rhamnosidase from Aspergillus terreus in selective trimming of rutin. Process Biochem. 2012, 47, 828–835. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.M.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Chebil, L.; Bouroukba, M.; Gaiani, C.; Charbonel, C.; Khaldi, M.; Engasser, J.M.; Ghoul, M. Elucidation of the kinetic behavior of quercetin, isoquercitrin, and rutin solubility by physicochemical and thermodynamic investigation. Ind. Eng. Chem. Res. 2013, 52, 1464–1470. [Google Scholar] [CrossRef]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Danieli, B.; Bertario, A. Chemoenzymatic synthesis of 6’’-O-(3-arylprop-2-enoyl) derivatives of the flavonol glucoside isoquercitrin. Helv. Chim. Acta 1993, 76, 2981–2991. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Ardhaoui, M.; Falcimaigne, A.; Engasser, J.M.; Moussou, P.; Pauly, G.; Ghoul, M. Acylation of natural flavonoids using lipase of Candida antarctica as biocatalyst. J. Mol. Catal. B-Enzym. 2004, 29, 63–67. [Google Scholar] [CrossRef]
- Chebil, L.; Anthoni, J.; Humeau, C.; Gerardin, C.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids: Effect of the nature of the substrate, origin of lipase and operating conditions on conversion yield and regioselectivity. J. Agric. Food Chem. 2007, 55, 9496–9502. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.H.; Humeau, C.; Chevalot, I.; Harscoat-Schiavo, C.; Vanderesse, R.; Blanchard, F.; Fick, M. Effect of acyl donor chain length on isoquercitrin acylation and biological activities of corresponding esters. Process Biochem. 2010, 45, 382–389. [Google Scholar] [CrossRef]
- Fabre, J.; Betbeder, D.; Paul, F.; Monsan, P.; Perie, J. Versatile enzymatic diacid ester: Synthesis of butyl delta-d-glucopyranoside. Tetrahedron 1993, 49, 10877–10882. [Google Scholar] [CrossRef]
- McCabe, R.W.; Taylor, A. An investigation of the acyl-binding site of Candida antarctica lipase B. Enzyme Microb. Technol. 2004, 35, 393–398. [Google Scholar] [CrossRef]
- Ottolina, G.; Carrea, G.; Riva, S. Regioselective enzymatic preparation of hemisuccinates of polyhydroxylated steroids. Biocatalysis 1991, 5, 131–136. [Google Scholar] [CrossRef]
- Bassanini, I.; Hult, K.; Riva, S. Dicarboxylic esters: Useful tools for the biocatalyzed synthesis of hybrid compounds and polymers. Beilstein J. Org. Chem. 2015, 11, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Magrone, P.; Cavallo, F.; Panzeri, W.; Passarella, D.; Riva, S. Exploiting enzymatic regioselectivity: A facile methodology for the synthesis of polyhydroxylated hybrid compounds. Org. Biomol. Chem. 2010, 8, 5583–5590. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, E.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Biocatalytic synthesis and antitumor activities of novel silybin acylated derivatives with dicarboxylic acids. New Biotechnol. 2011, 28, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Matsuda, N.; Kashino, Y.; Fujikura, Y.; Nakamura, T.; Kato, Y.; Shimizu, R.; Okuyama, S.; Tanaka, H.; Koda, T.; et al. α-Oligoglucosylation of a sugar moiety enhances the biovailability of quercetin glucosides in humans. Arch. Biochem. Biophys. 2010, 501, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Ziaullah; Bhullar, K.S.; Warnakulasuriya, S.N.; Rupasinghe, H.P.V. Biocatalytic synthesis, structural elucidation, antioxidant capacity and tyrosinase inhibition activity of long chain fatty acid acylated derivatives of phloridzin and isoquercitrin. Bioorg. Med. Chem. 2013, 21, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: Implication for their lipid-peroxidation inhibition. J. Phys. Chem. B 2011, 116, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Mellou, F.; Lazari, D.; Skaltsa, H.; Tselepis, A.D.; Kolisis, E.; Stamatis, H. Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. J. Biotechnol. 2005, 116, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Weignerová, L.; Marhol, P.; Gerstorferová, D.; Křen, V. Preparatory production of quercetin-3-beta-d-glucopyranoside using alkali-tolerant thermostable α-l-rhamnosidase from Aspergillus terreus. Biores. Technol. 2012, 115, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Deby, C.; Magotteaux, G. Essential fatty acids and antioxidizing substances in tissues of mouse. C. R. Séances Soc. Biol. Fil. 1970, 164, 2675–2681. [Google Scholar] [PubMed]
- Joyeux, M.; Mortier, F.; Fleurentin, J. Screening of antiradical, antilipoperoxidant and hepatoprotective effects of 9 plant-extracts used in caribbean folk medicine. Phytother. Res. 1995, 9, 228–230. [Google Scholar] [CrossRef]
| Compounds | Partition Coefficient | log P | FCR (GAE) | DPPH (IC50; µM) | ABTS (TE) | Lpx (IC50; µM) | |
|---|---|---|---|---|---|---|---|
| 1 | Isoquercitrin | 1.52 ± 0.01 | 0.18 | 1.84 ± 0.04 a | 1.40 ± 0.06 d | 1.97 ± 0.06 i | 972 ± 11 |
| 2 | IQ acetate | 13.76 ± 0.01 | 1.14 | 1.63 ± 0.07 b | 2.51 ± 0.10 e | 2.04 ± 0.07 i | 42.0 ± 2.1 k |
| 3 | IQ diacetate | 38.99± 0.01 | 1.59 | 1.67 ± 0.11 b | 2.15 ± 0.04 f | 1.56 ± 0.04 | 433 ± 19 |
| 4 | IQ butyrate | 90.64 ± 0.03 | 1.96 | 1.72 ± 0.03 a | 2.51 ± 0.17 e | 2.16 ± 0.08 i | 24.1 ± 0.8 l |
| 5 | IQ hexanoate | 126.43 ± 0.01 | 2.10 | 1.48 ± 0.05 b | 3.07 ± 0.10 g | 2.11 ± 0.09 i | 19.7 ± 0.2 l |
| 6 | IQ octanoate | n.d. | n.d. | 1.47 ± 0.10 b | 2.23 ± 0.08 e,f | 1.28 ± 0.07 | 186 ± 3 m |
| 7 | IQ dodecanoate | n.d. | n.d. | 0.63 ± 0.07 c | 1.58 ± 0.05 d | 1.06 ± 0.09 j | 432 ± 24 |
| 8 | IQ palmitate | n.d. | n.d. | 0.76 ± 0.03 c | 2.23 ± 0.03 e,f | 1.00 ± 0.06 j | 1091 ± 36 |
| 9 | IQ hemiglutarate | 0.07 ± 0.01 | −1.15 | 1.84 ± 0.05 a | 1.77 ± 0.06 d | 2.13 ± 0.08 i | 1341 ± 54 |
| 10 | IQ hemiadipate | 0.10 ± 0.01 | −1.00 | 1.46 ± 0.14 b | 2.19 ± 0.12 e,f | 2.12 ± 0.10 i | 250 ± 19 |
| 11 | IQ hemidodecanedioate | 3.00 ± 0.03 | 0.48 | 1.58 ± 0.05 b | 2.97 ± 0.22 g | 2.16 ± 0.08 i | 32.5 ± 1.6 k |
| Quercetin | 86.27 ± 0.02 | 1.94 | 2.80 ± 0.16 | 3.78 ± 0.15 h | 2.14 ± 0.10 i | 29.8 ± 0.6 k | |
| Rutin | 0.41 ± 0.01 | −0.39 | 2.19 ± 0.10 | 3.66 ± 0.39 h | 1.79 ± 0.09 | 202 ± 8 m | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vavříková, E.; Langschwager, F.; Jezova-Kalachova, L.; Křenková, A.; Mikulová, B.; Kuzma, M.; Křen, V.; Valentová, K. Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties. Int. J. Mol. Sci. 2016, 17, 899. https://doi.org/10.3390/ijms17060899
Vavříková E, Langschwager F, Jezova-Kalachova L, Křenková A, Mikulová B, Kuzma M, Křen V, Valentová K. Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties. International Journal of Molecular Sciences. 2016; 17(6):899. https://doi.org/10.3390/ijms17060899
Chicago/Turabian StyleVavříková, Eva, Fanny Langschwager, Lubica Jezova-Kalachova, Alena Křenková, Barbora Mikulová, Marek Kuzma, Vladimír Křen, and Kateřina Valentová. 2016. "Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties" International Journal of Molecular Sciences 17, no. 6: 899. https://doi.org/10.3390/ijms17060899
APA StyleVavříková, E., Langschwager, F., Jezova-Kalachova, L., Křenková, A., Mikulová, B., Kuzma, M., Křen, V., & Valentová, K. (2016). Isoquercitrin Esters with Mono- or Dicarboxylic Acids: Enzymatic Preparation and Properties. International Journal of Molecular Sciences, 17(6), 899. https://doi.org/10.3390/ijms17060899