Synthesis of New Hydrated Geranylphenols and in Vitro Antifungal Activity against Botrytis cinerea
Abstract
:1. Introduction
2. Results and Discussion
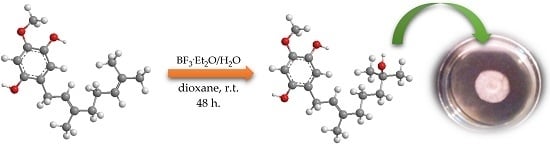
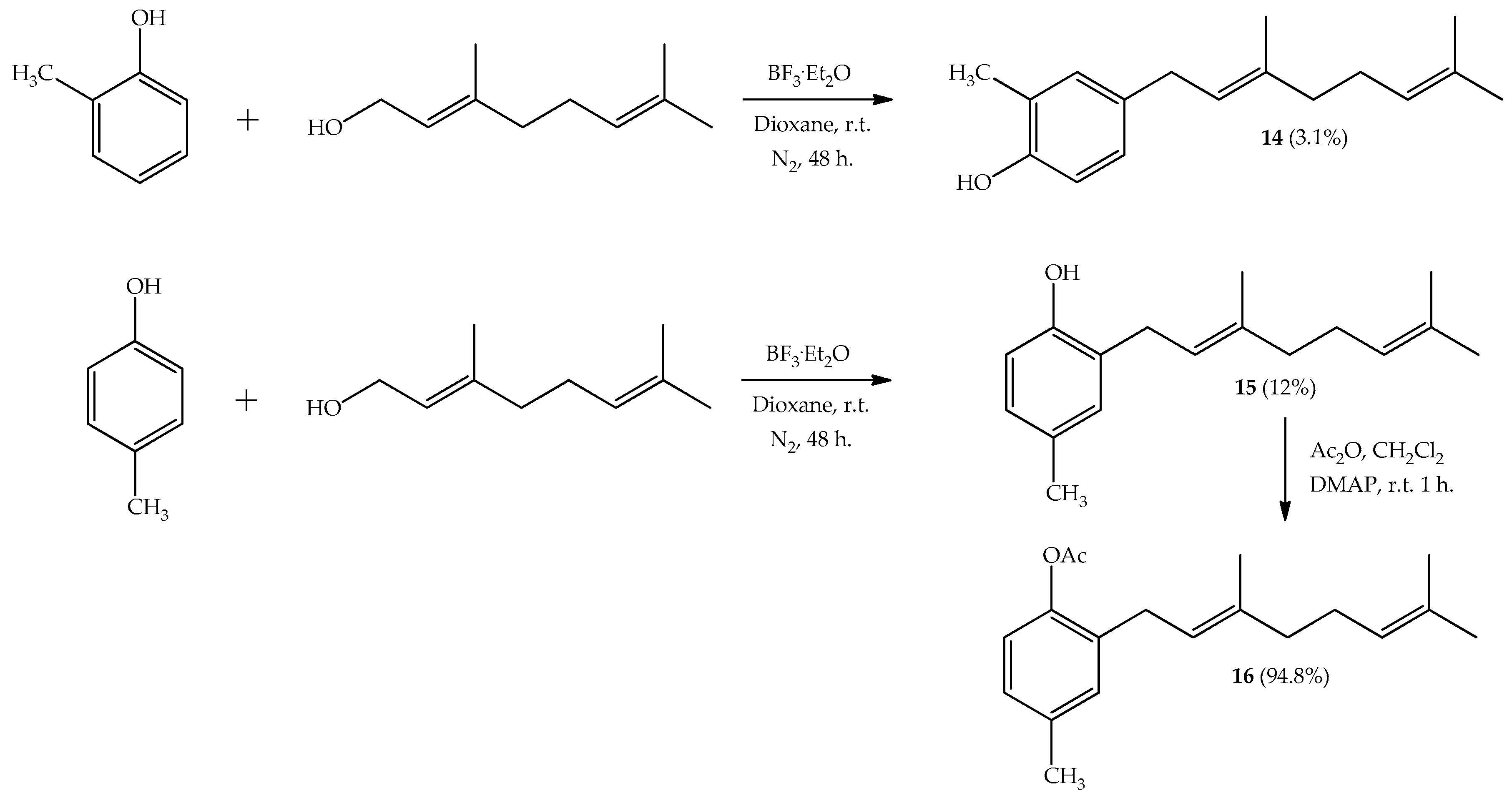
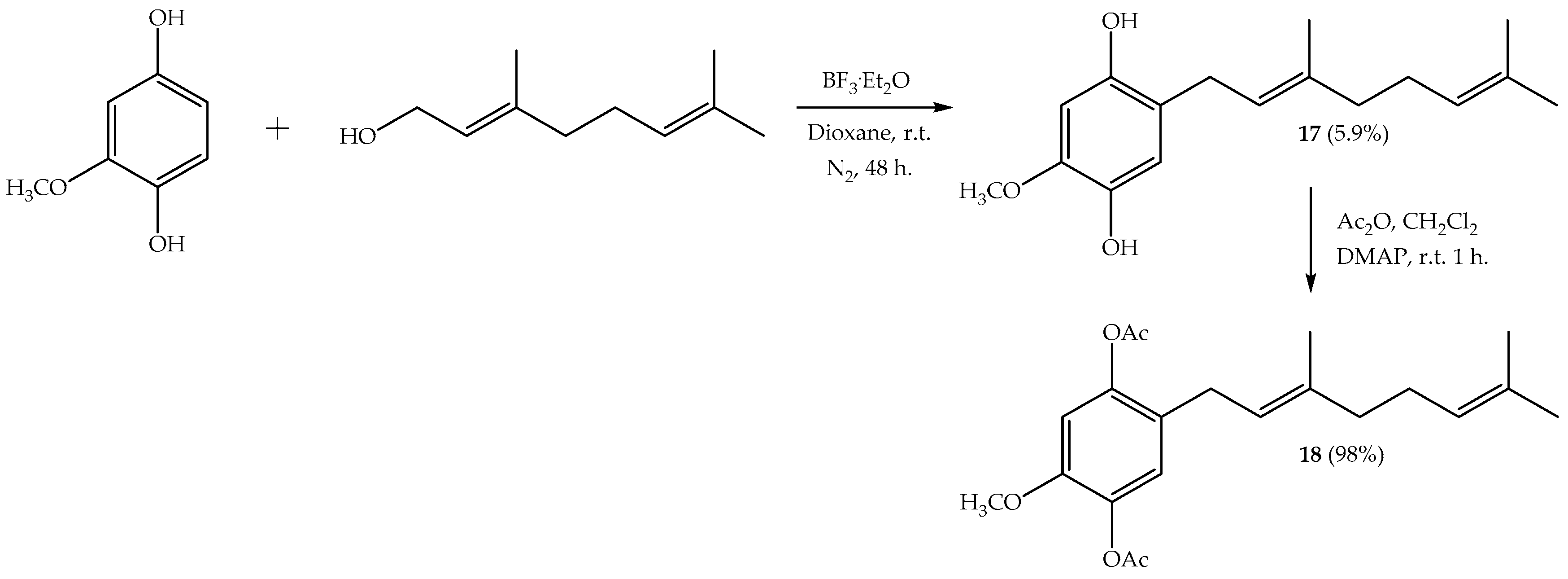
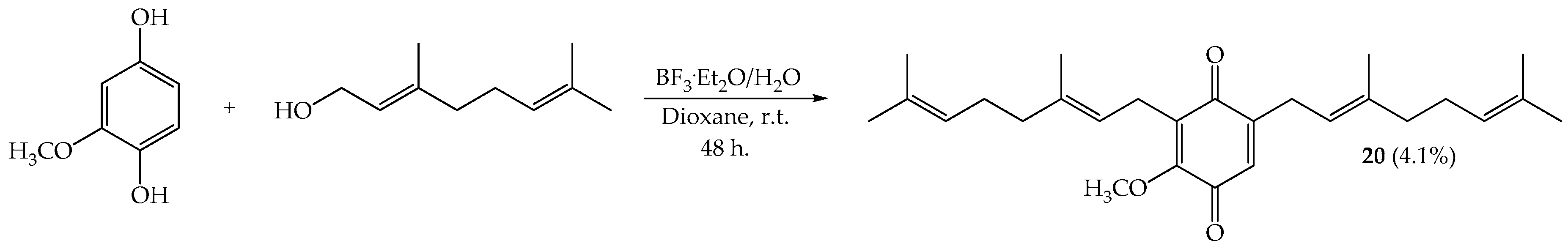
2.1. Synthesis
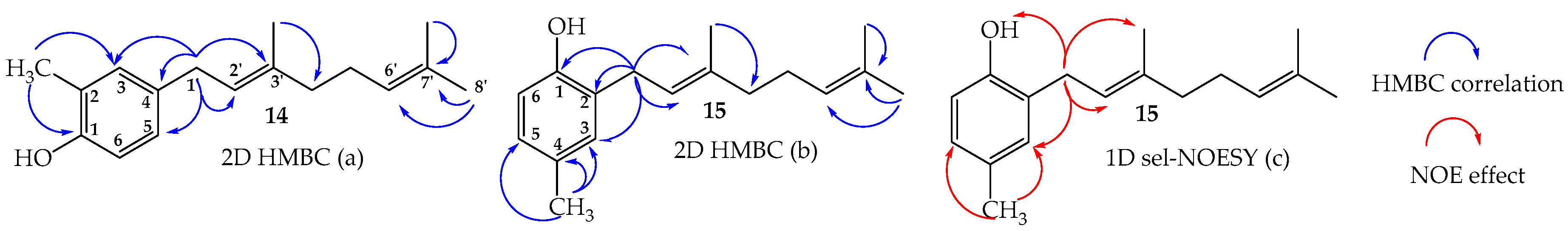
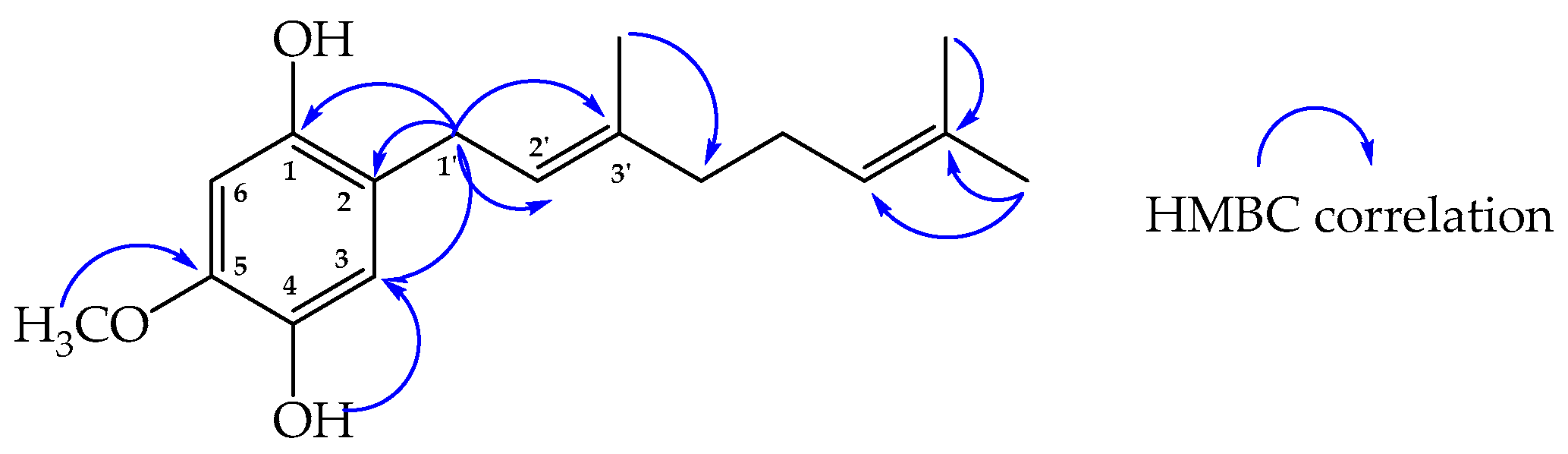
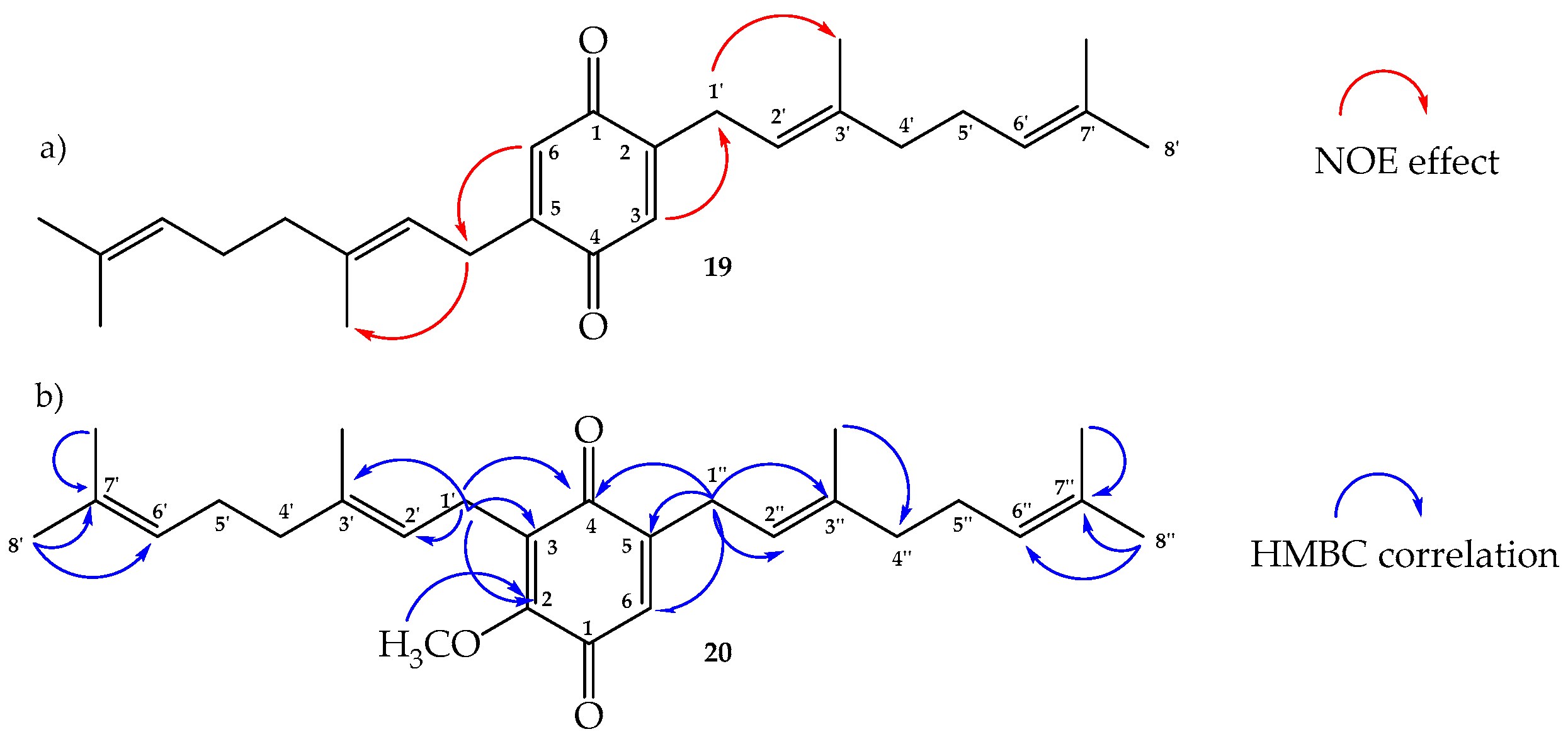
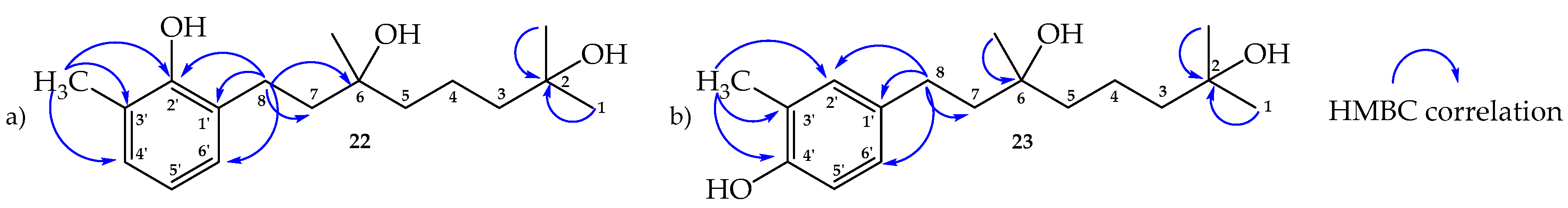
2.2. Structure Determination
2.3. In Vitro Antifungal Activity against B. cinerea.
3. Experimental Section
3.1. General
3.2. Synthesis
3.2.1. Coupling Reaction in Presence of Nitrogen
3.2.2. Coupling Reaction in the Absence of Nitrogen and with Added Water
3.2.3. Acetylation of Geranylated Phenols
3.2.4. Reaction of Geranylated Phenols with BF3·Et2O
3.3. In Vitro Effect of the Compounds on the Mycelial Growth of B. cinerea
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zubia, E.; Ortega, M.J.; Salva, J. Natural products chemistry in marine ascidians of the genus Aplidium. Mini Rev. Org. Chem. 2005, 2, 389–399. [Google Scholar] [CrossRef]
- Marialuisa, M.; Anna, A. Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, O., Eds.; Springer Science + Business Media: New York, NY, USA, 2012. [Google Scholar]
- Reynolds, G.; Rodriguez, E. Geranylhydroquinone: A contact allergen from trichomes of Phacelia crenulata. Phytochemistry 1979, 18, 1567–1568. [Google Scholar] [CrossRef]
- Inouye, H.; Tokura, K.; Tohita, S. Uber die inhaltsstoffe von Pirolaceen, XV zur struktur des pirolatins. Chem. Ber. 1968, 101, 4057–4065. (In German) [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Epstein, W.L.; Terry, D.; Rodriguez, E. A potent contact allergen of Phacelia (Hydrophyllaceae). Contact Dermat. 1980, 6, 272–274. [Google Scholar] [CrossRef]
- Fenical, W. Food-Drugs from the Sea, Proceedings (of the Fourth Food Drugs from the Sea Conference) 1974. In Proceeding of the Food Drugs from the Sea Conference, 17–21 November 1974; Webber, H.H., Ruggieri, G.D., Eds.; Marine Technological Society: Washington, DC, USA.
- Aknin, M.; Dayan, T.L.A.; Rudi, A.; Kashman, Y.; Gaydou, E.M. Hydroquinone antioxidants from the Indian Ocean tunicate Aplidium savignyi. J. Agric. Food Chem. 1999, 47, 4175–4177. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Shindo, T.; Kasanuki, N.; Hasegawa, K. Antioxidant metabolites from the tunicate amaroucium-multiplicatum. J. Nat. Prod. 1989, 52, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Garrido, L.; Zubia, E.; Ortega, M.J.; Salva, J. New meroterpenoids from the ascidian Aplidium conicum. J. Nat. Prod. 2002, 65, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Shubina, L.K.; Fedorov, S.N.; Radchenko, O.S.; Balaneva, N.N.; Kolesnikova, S.A.; Dmitrenok, P.S.; Bode, A.; Dong, Z.; Stonik, V.A. Desmethylubiquinone Q2 from the far-eastern ascidian Aplidium glabrum: Structure and synthesis. Tetrahedron Lett. 2005, 46, 559–562. [Google Scholar] [CrossRef]
- Chan, S.T.; Pearce, A.; Januario, A.H.; Page, M.J.; Kaiser, M.; McLaughlin, R.J.; Harper, J.L.; Webb, V.L.; Barker, D.; Copp, B.R. Anti-inflammatory and antimalarial meroterpenoids from the New Zealand ascidian Aplidium scabellum. J. Org. Chem. 2011, 76, 9151–9156. [Google Scholar] [CrossRef] [PubMed]
- Guella, G.; Mancini, I.; Pietra, F. Verapliquinones—Novel diprenylquinones from an Aplidium Sp. (Ascidiacea) of Ile-Verte waters, Brittany. Helv. Chim. Acta 1987, 70, 621–626. [Google Scholar] [CrossRef]
- Benslimane, A.F.; Pouchus, Y.F.; Leboterff, J.; Verbist, J.F.; Roussakis, C.; Monniot, F. Cytotoxic and antibacterial substances from the ascidian Aplidium antillense. J. Nat. Prod. 1988, 51, 582–583. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; De Giulio, A.; Iodice, C. Biological effects of prenylated hydroquinones: structure-activity relationship studies in antimicrobial, brine shrimp, and fish lethality assays. J. Nat. Prod. 1994, 57, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Rudali, G.; Menetrier, L. Action de la géranyl-hydroquinone surdifférents cancers spontanés et provoqués chez celles souris. Therapie 1967, 22, 895–899. [Google Scholar] [PubMed]
- Rudali, G. Research on the radioprotective action of geranyl-hydroquinone. C. R. Seances Soc. Biol. Ses Fil. 1966, 160, 1365–1369. [Google Scholar]
- Rodriguez, E. Plant Resistance to Insects; Hedin, P., Ed.; American Chemical Society: Washington, DC, USA, 1983. [Google Scholar]
- Rueda, A.; Zubia, E.; Ortega, M.J.; Salva, J. A new cytotoxic prenylhydroquinone from a Mediterranean tunicate of the genus Aplydium. Nat. Prod. Lett. 1998, 11, 127–130. [Google Scholar] [CrossRef]
- Reynolds, G.; Rodriguez, E. Prenylated phenols that cause contact dermatitis from trichomes of Phaceliaixodes. Planta Med. 1981, 43, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z.G. Evaluation of cancer-preventive activity and structure-activity relationships of 3-demethylubiquinone Q2, isolated from the ascidian Aplidium glabrum, and its synthetic analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Agafonova, I.G.; Bode, A.M.; Jin, J.O.; Kwak, J.Y.; Dong, Z.; Stonik, V.A. Anticancer activity of 3-demethylubiquinone Q2. In vivo experiments and probable mechanism of action. Anticancer Res. 2008, 28, 927–932. [Google Scholar] [PubMed]
- Simon-Levert, A.; Arrault, A.; Bontemps-Subielos, N.; Canal, C.; Banaigs, B. Meroterpenes from the ascidian Aplidium aff. densum. J. Nat. Prod. 2005, 68, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Simon-Levert, A.; Menniti, C.; Soulere, L.; Geneviere, A.M.; Barthomeuf, C.; Banaigs, B.; Witczak, A. Marine natural meroterpenes: Synthesis and antiproliferative activity. Mar. Drugs 2010, 8, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from marine invertebrates: Structures, occurrence, and ecological implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertanha, C.S.; Januario, A.H.; Alvarenga, T.A.; Pimenta, L.P.; Andrade e Silva, M.L.; Cunha, W.R.; Pauletti, P.M. Quinone and hydroquinone metabolites from the ascidians of the genus Aplidium. Mar. Drugs 2014, 12, 3608–3633. [Google Scholar] [CrossRef] [PubMed]
- Baeza, E.; Catalan, K.; Pena-Cortes, H.; Espinoza, L.; Villena, J.; Carrasco, H. Synthesis of geranylhydroquinone derivatives with potential cytotoxic activity. Quim. Nova 2012, 35, 523–526. [Google Scholar] [CrossRef]
- Baeza, E.; Catalan, K.; Villena, J.; Carrasco, H.; Cuellar, M.; Espinoza, L. Synthesis and cytotoxic activity of geranylmethoxyhydroquinone derivatives. J. Chil. Chem. Soc. 2012, 57, 1219–1223. [Google Scholar] [CrossRef]
- Taborga, L.; Vergara, A.; Osorio, M.; Carvajal, M.; Madrid, A.; Marilaf, F.; Carrasco, H.; Espinoza, L. Synthesis and NMR structure determination of new linear geranylphenols by direct geranylation of activated phenols. J. Chil. Chem. Soc. 2013, 58, 1790–1796. [Google Scholar] [CrossRef]
- Espinoza, L.; Taborga, L.; Diaz, K.; Olea, A.F.; Peña-Cortes, H. Synthesis of linear geranylphenols and their effect on mycelial growth of plant pathogen Botrytis cinerea. Molecules 2014, 19, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.I.; Soto, M.; Taborga, L.; Diaz, K.; Olea, A.F.; Bay, C.; Pena-Cortes, H.; Espinoza, L. Synthesis and in vitro antifungal activity against Botrytis cinerea of geranylated phenols and their phenyl acetate derivatives. Int. J. Mol. Sci. 2015, 16, 19130–19152. [Google Scholar] [CrossRef] [PubMed]
- Taborga, L.; Diaz, K.; Olea, A.F.; Reyes-Bravo, P.; Flores, M.E.; Pena-Cortes, H.; Espinoza, L. Effect of polymer micelles on antifungal activity of geranylorcinol compounds against Botrytis cinerea. J. Agric. Food Chem. 2015, 63, 6890–6896. [Google Scholar] [CrossRef] [PubMed]
- Taborga, L.; Espinoza, L.; Moller, A.; Carrasco, H.; Cuellar, M.; Villena, J. Antiproliferative effect and apoptotic activity of linear geranylphenol derivatives from phloroglucinol and orcinol. Chem. Biol. Interact. 2016, 247, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Evenses, K. Physiological aspects of resistance to Botrytis cinerea. Phytopathology 1995, 85, 637–643. [Google Scholar] [CrossRef]
- Latorre, B.A.; Flores, V.; Sara, A.M.; Roco, A. Dicarboximide-resistant isolates of Botrytis-cinerea from table grape in Chile—survey and characterization. Plant Dis. 1994, 78, 990–994. [Google Scholar] [CrossRef]
- Latorre, B.A.; Spadaro, I.; Rioja, M.E. Occurrence of resistant strains of Botrytis cinerea to anilinopyrimidine fungicides in table grapes in Chile. Crop Prot. 2002, 21, 957–961. [Google Scholar] [CrossRef]
- Cotoras, M.; Folch, C.; Mendoza, L. Characterization of the antifungal activity on Botrytis cinerea of the natural diterpenoids kaurenoic acid and 3β-hydroxy-kaurenoic acid. J. Agric. Food Chem. 2004, 52, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Espinoza, P.; Urzua, A.; Vivanco, M.; Cotoras, M. In vitro antifungal activity of the diterpenoid 7a-hydroxy-8(17)-labden-15-oic acid and its derivatives against Botrytis cinerea. Molecules 2009, 14, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Araya-Maturana, R.; Cardona, W.; Delgado-Castro, T.; Garcia, C.; Lagos, C.; Cotoras, M. In vitro sensitivity of Botrytis cinerea to anthraquinone and anthrahydroquinone derivatives. J. Agric. Food Chem. 2005, 53, 10080–10084. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.L.; Jurd, L.; Manners, G. Transformations of geraniol in aqueous acid solutions. Tetrahedron 1972, 28, 1939–1944. [Google Scholar] [CrossRef]
- Manners, G.; Jurd, L.; Stevens, K. Biogenetic-type syntheses of isoprenoid and diisoprenoid derivatives of orcinol. Tetrahedron 1972, 28, 2949–2959. [Google Scholar] [CrossRef]
- Chukicheva, I.Y.; Fedorova, I.V.; Koroleva, A.A.; Kuchin, A.V. Synthesis of natural geranyhidroqunone analogs. Chem. Nat. Compd. 2015, 51, 1056–1058. [Google Scholar] [CrossRef]
- Hou, Z.; Yang, R.; Zhang, C.; Zhu, L.; Miao, F.; Yang, X.; Zhou, L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as novel antifungal lead compounds: Biological evaluation and structure-activity relationships. Molecules 2013, 18, 10413–10424. [Google Scholar] [CrossRef] [PubMed]

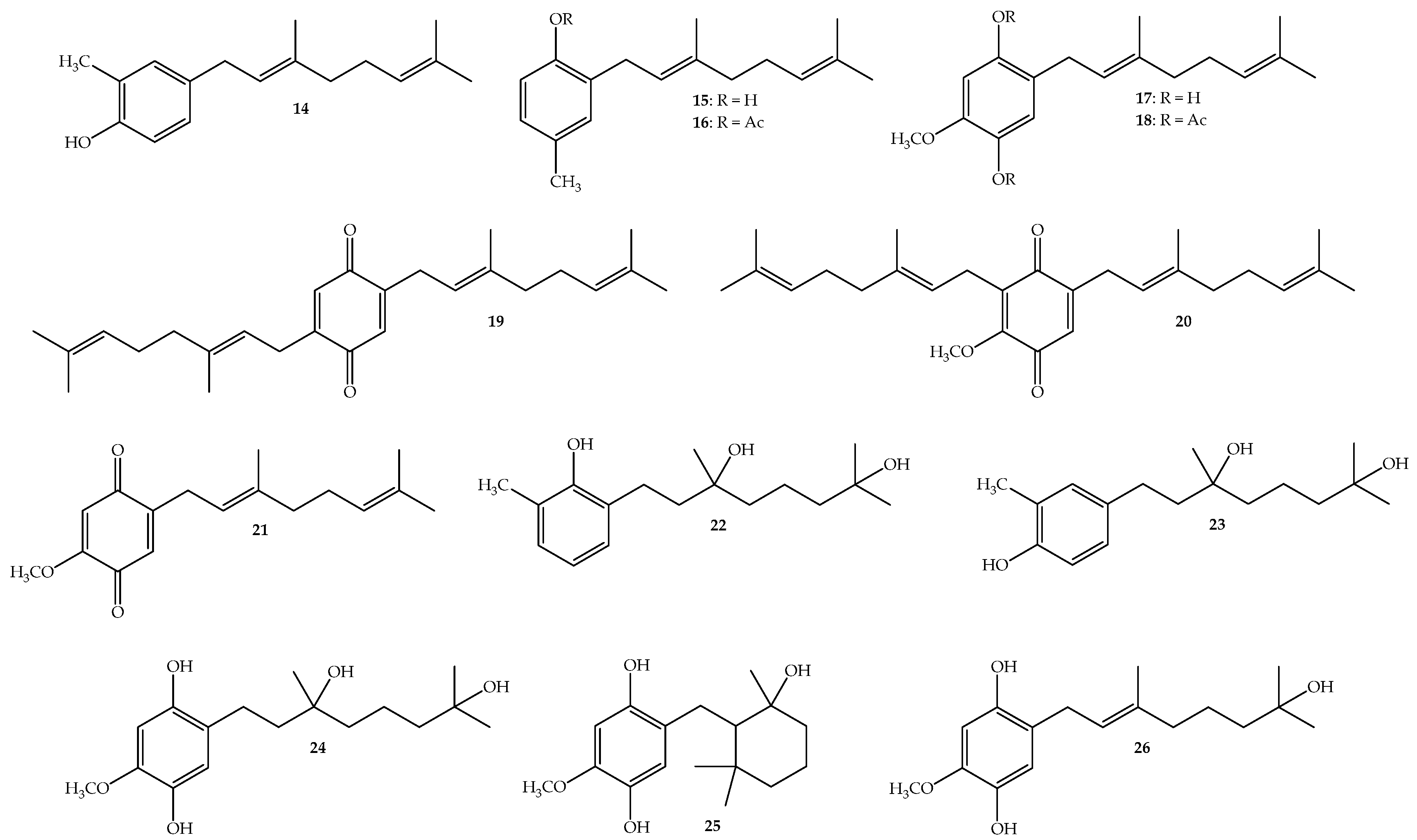







| Compounds | Percentage of Inhibition on Mycelial Growth of B. cinerea in Vitro (%) | ||
|---|---|---|---|
| 50 mg/L | 150 mg/L | 250 mg/L | |
| 14 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 15 | 9 ± 4 | 6 ± 3 | 8 ± 5 |
| 16 | 0 ± 0 | 0 ± 0 | 9 ± 0 |
| 17 | 49 ± 2 | 56 ± 2 | 56 ± 0 |
| 18 | 36 ± 3 | 48 ± 3 | 52 ± 2 |
| 19 | 30 ± 2 | 51 ± 1 | 69 ± 1 |
| 20 | 43 ± 8 | 58 ± 8 | 73 ± 8 |
| 21 | 36 ± 0 | 64 ± 0 | 75 ± 0 |
| 22 | 0 ± 0 | 30 ± 2 | 53 ± 3 |
| 23 | 0 ± 0 | 0 ± 0 | 28 ± 7 |
| 24 | 36 ± 0 | 66 ± 4 | 67 ± 5 |
| 25 | 50 ± 6 | 81 ± 5 | 90 ± 1 |
| 26 | 81 ± 0 | 91 ± 0 | 94 ± 0 |
| C− 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| C+ 2 | 94 ± 5 | 94 ± 0 | 99 ± 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, M.; Espinoza, L.; Chávez, M.I.; Díaz, K.; Olea, A.F.; Taborga, L. Synthesis of New Hydrated Geranylphenols and in Vitro Antifungal Activity against Botrytis cinerea. Int. J. Mol. Sci. 2016, 17, 840. https://doi.org/10.3390/ijms17060840
Soto M, Espinoza L, Chávez MI, Díaz K, Olea AF, Taborga L. Synthesis of New Hydrated Geranylphenols and in Vitro Antifungal Activity against Botrytis cinerea. International Journal of Molecular Sciences. 2016; 17(6):840. https://doi.org/10.3390/ijms17060840
Chicago/Turabian StyleSoto, Mauricio, Luis Espinoza, María I. Chávez, Katy Díaz, Andrés F. Olea, and Lautaro Taborga. 2016. "Synthesis of New Hydrated Geranylphenols and in Vitro Antifungal Activity against Botrytis cinerea" International Journal of Molecular Sciences 17, no. 6: 840. https://doi.org/10.3390/ijms17060840