The Vitamin D Analog, MART-10, Attenuates Triple Negative Breast Cancer Cells Metastatic Potential
Abstract
:1. Introduction
2. Result
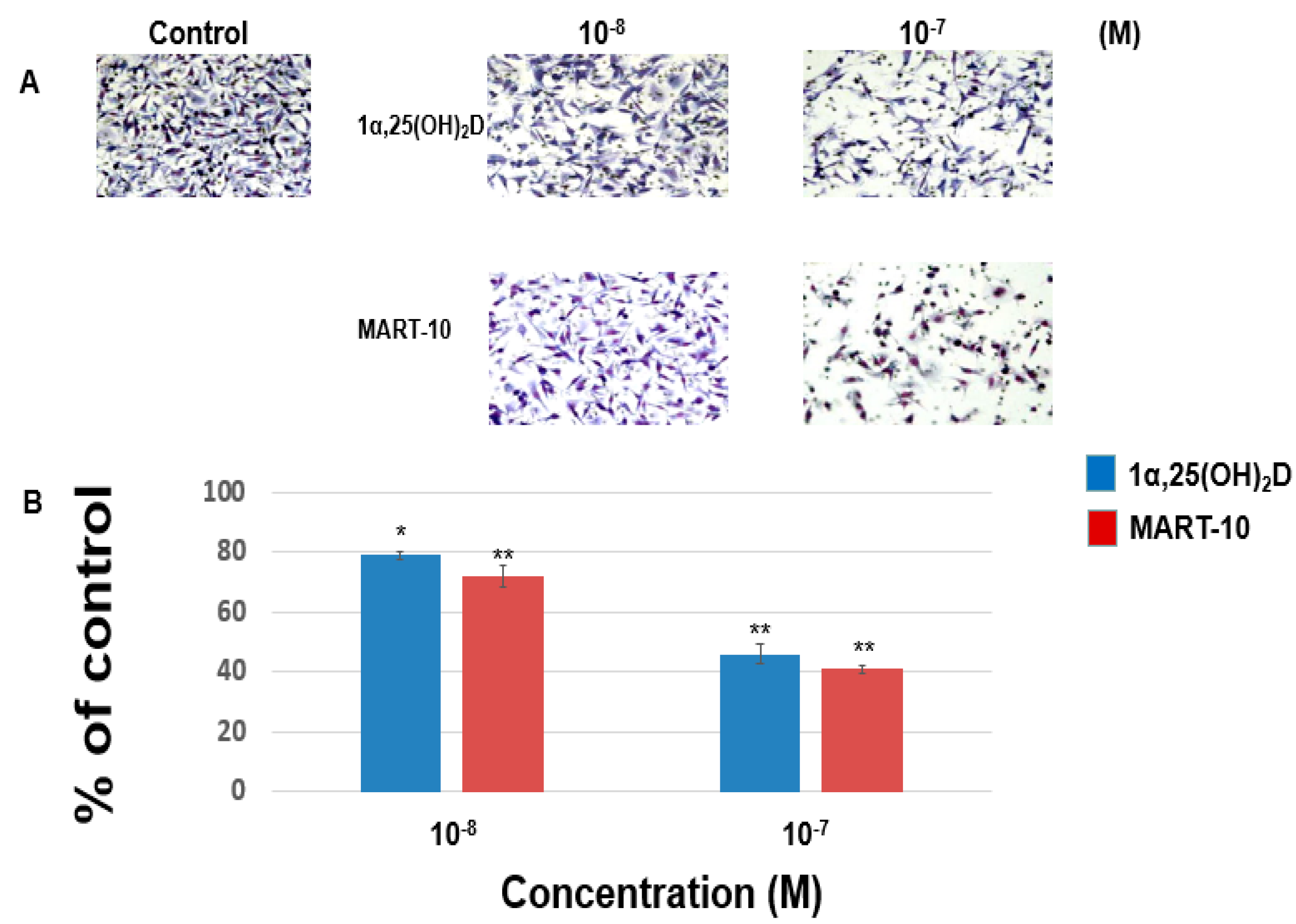
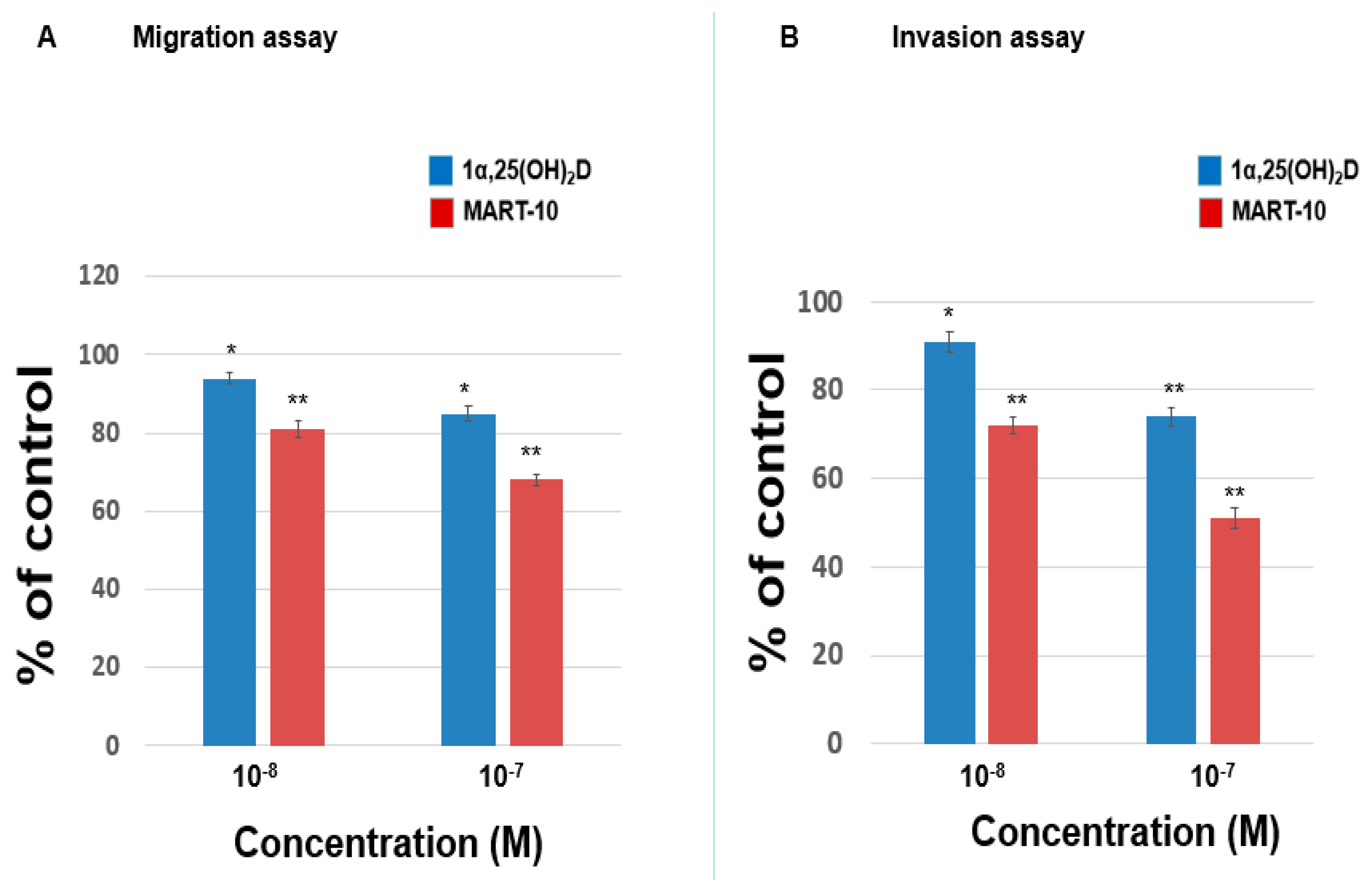
2.1. Inhibition of MDA-MB-231 Cell Invasion and Migration by 1α,25(OH)2D3 and MART-10
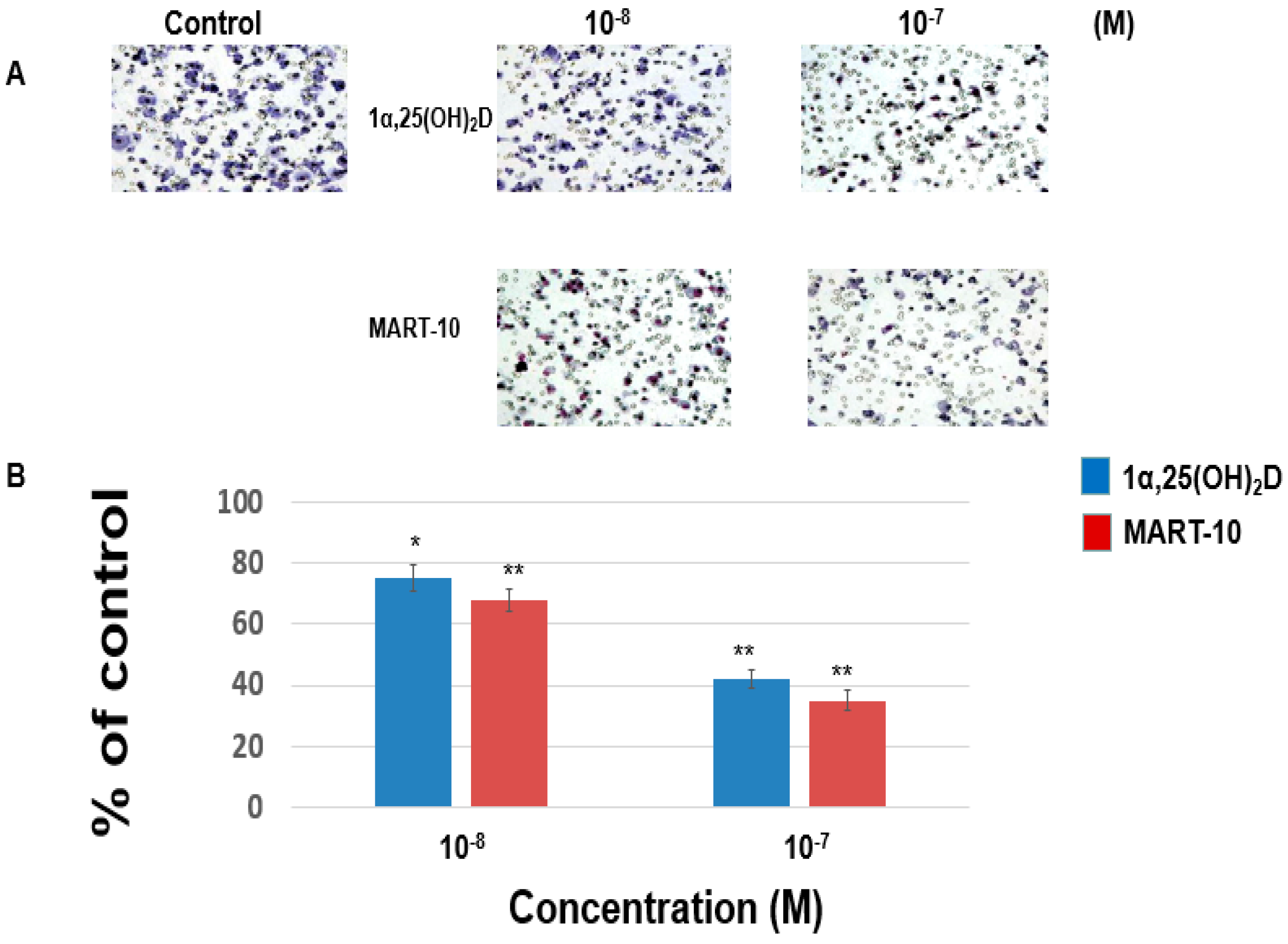
2.2. Inhibition of MDA-MB-453 Cell Invasion and Migration by 1α,25(OH)2D3 and MART-10
2.3. Evaluation of 1α,25(OH)2D3 and MART-10 Effects on E-, N-, and P-Cadherin of MDA-MB-231 Cells
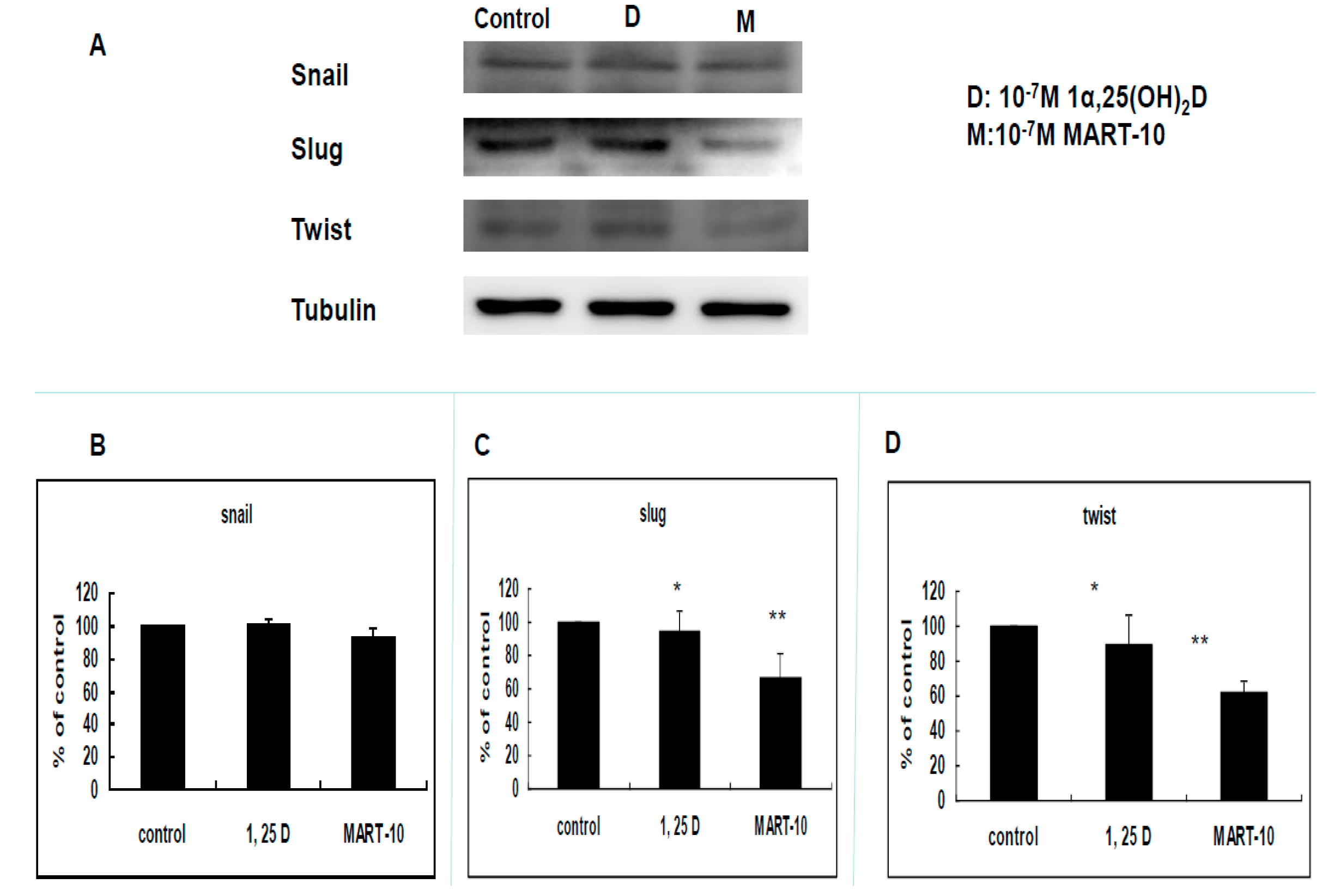
2.4. Evaluation of 1α,25(OH)2D3 and MART-10 Effects on the Expression of Epithelial-Mesenchymal Transition (EMT)-Related Transcription Factors, Zeb1 and 2, Snail, Slug, and Twist of MDA-MB-231 Cells
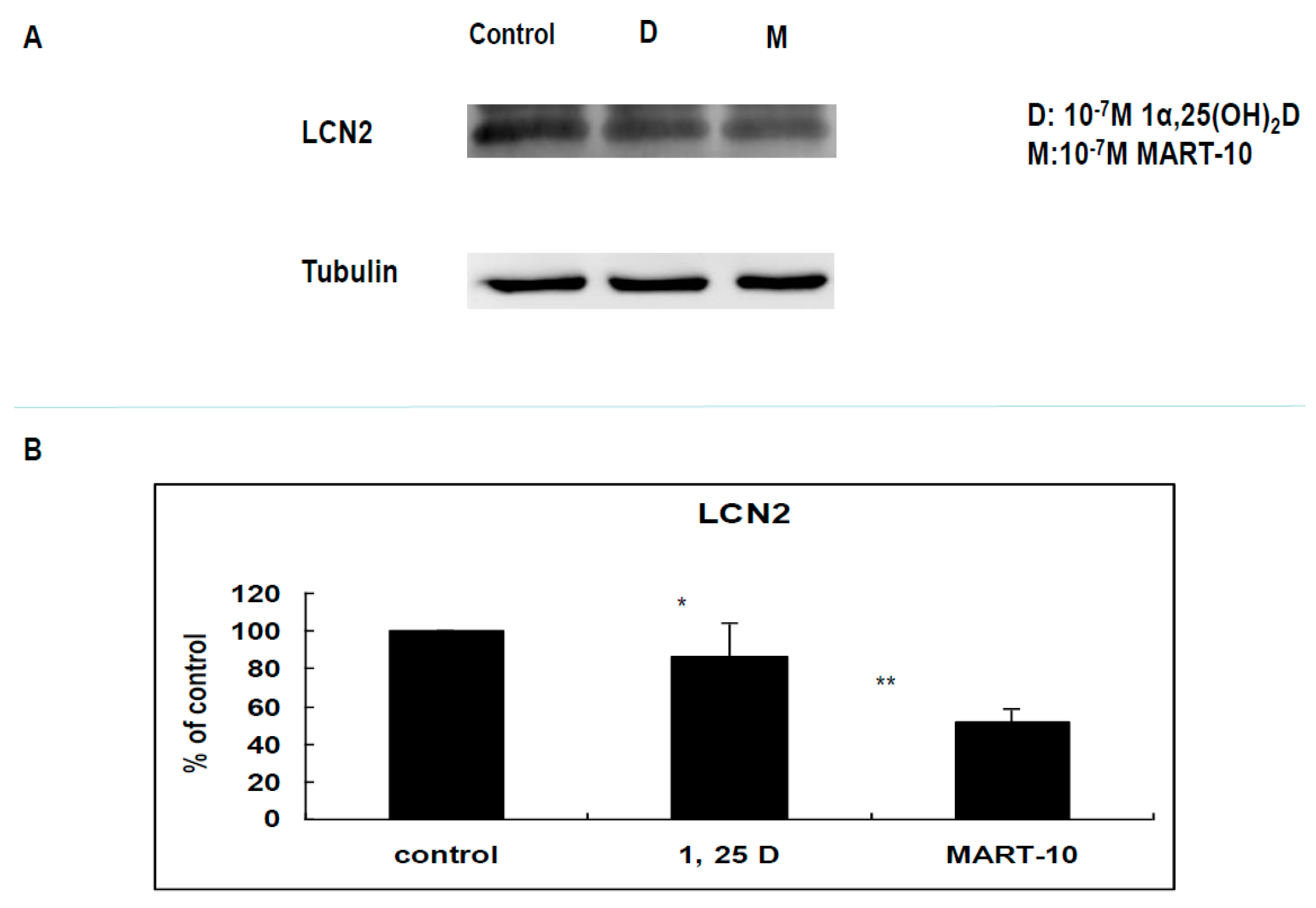
2.5. Evaluation LCN2 Expression of MDA-MB-231 Cells after 1α,25(OH)2D3 and MART-10 Treatment
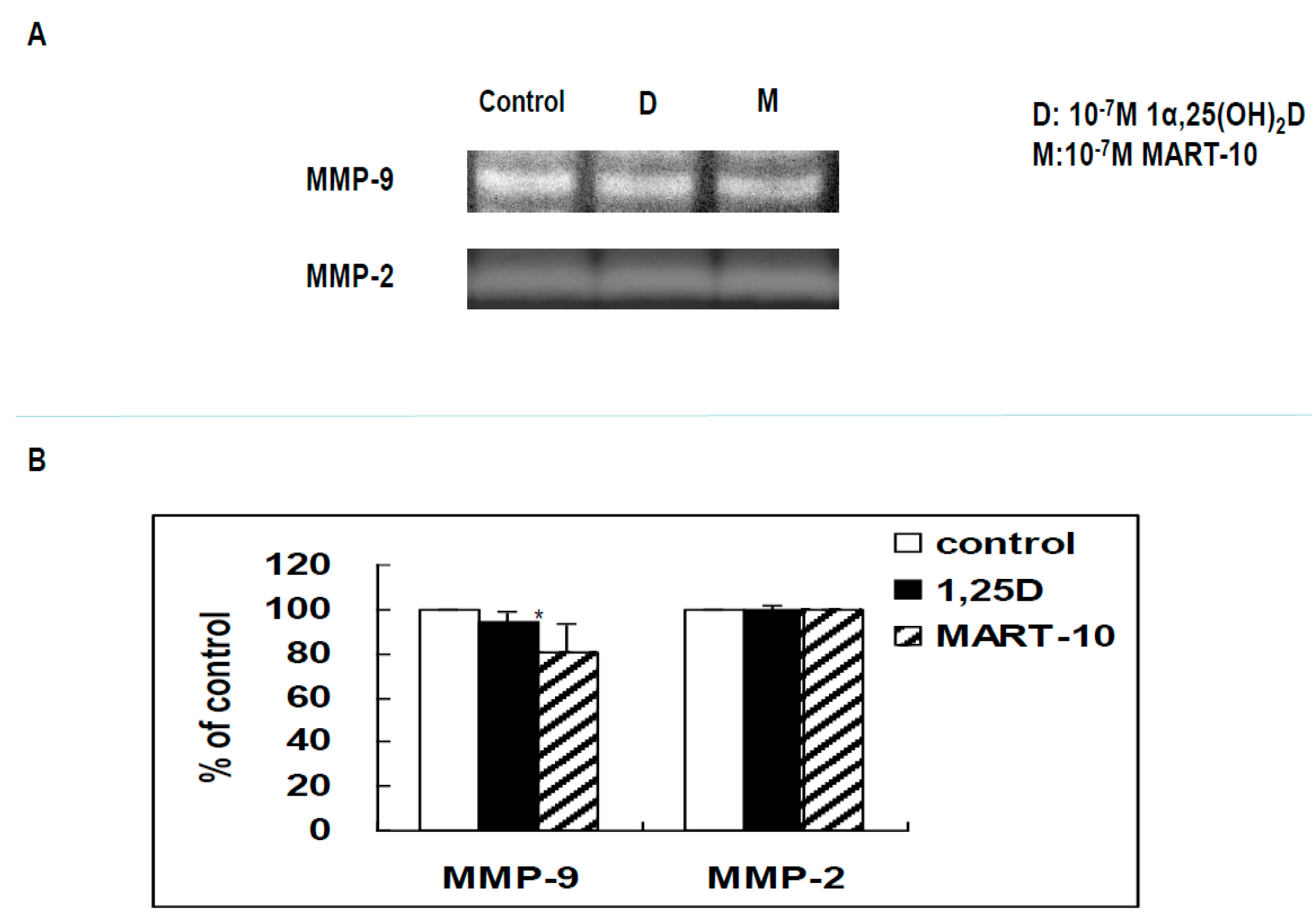
2.6. Functional Assay of MMPs by Zymography
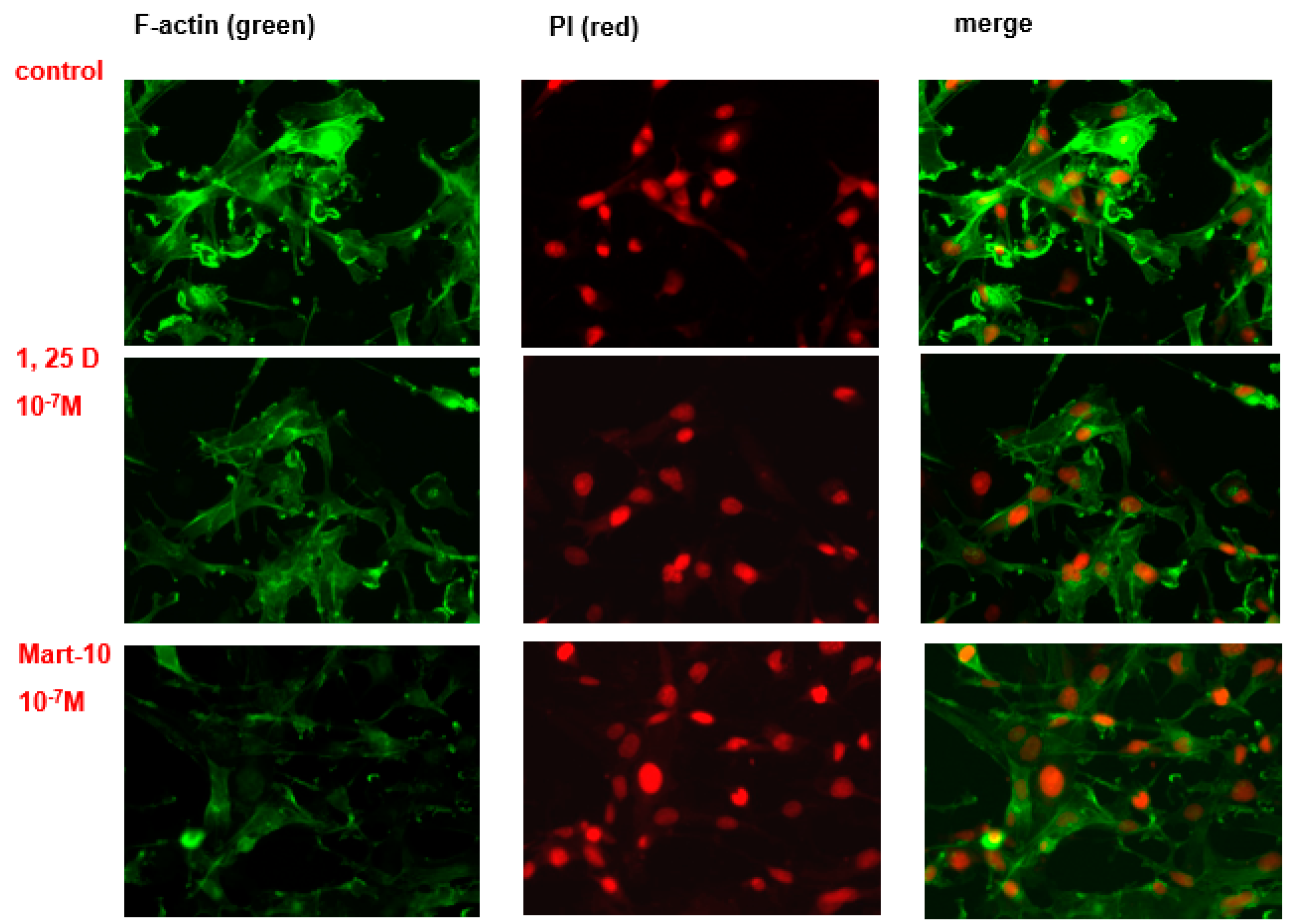
2.7. Evaluation of 1α,25(OH)2D3 and MART-10 Effect on F-Actin Synthesis in MDA-MB-231 Cells
3. Discussion
4. Material and Method
4.1. Vitamin D Compounds
4.2. Cell Culture
4.3. Matrigel Invasion Assay
4.4. Trans-Well Filter Migration Assay
4.5. Gelatin Zymography
4.6. Western Blot
4.7. F-Actin Staining
4.8. Statistics Method
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.A.; Mazar, A.P. Evaluating distant metastases in breast cancer: From biology to outcomes. Cancer Metastasis Rev. 2007, 26, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.D.; Carey, L.A.; Ahmed, A.A.; Caldas, C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol. 2005, 23, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [PubMed]
- Chen, T.C.; Holick, M.F. Vitamin d and prostate cancer prevention and treatment. Trends Endocrinol. Metab. 2003, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Zheng, S.; Chiang, K.C.; Kittaka, A.; Sakaki, T.; Nakabayashi, S.; Zhao, X.; Spanjaard, R.A.; Persons, K.S.; Mathieu, J.S.; et al. Evaluation of 19-nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Res. 2009, 29, 3547–3553. [Google Scholar] [PubMed]
- Chiang, K.C.; Persons, K.S.; Istfan, N.W.; Holick, M.F.; Chen, T.C. Fish oil enhances the antiproliferative effect of 1α,25-dihydroxyvitamin D3 on liver cancer cells. Anticancer Res. 2009, 29, 3591–3596. [Google Scholar] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Chen, H.Y.; Lee, J.M.; Juang, H.H.; Chen, M.F.; Takano, M.; Kittaka, A.; Chen, T.C. 19-nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 (MART-10) is a potent cell growth regulator with enhanced chemotherapeutic potency in liver cancer cells. Steroids 2011, 76, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1α,25-dihydroxyvitamin D3 on the G1–S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [PubMed]
- Sundaram, S.; Chaudhry, M.; Reardon, D.; Gupta, M.; Gewirtz, D.A. The vitamin D3 analog EB 1089 enhances the antiproliferative and apoptotic effects of adriamycin in MCF-7 breast tumor cells. Breast Cancer Res. Treat. 2000, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.; Sundaram, S.; Gennings, C.; Carter, H.; Gewirtz, D.A. The vitamin D3 analog, ILX-23–7553, enhances the response to adriamycin and irradiation in MCF-7 breast tumor cells. Cancer Chemother. Pharmacol. 2001, 47, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Abe-Hashimoto, J.; Kikuchi, T.; Matsumoto, T.; Nishii, Y.; Ogata, E.; Ikeda, K. Antitumor effect of 22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic mice implanted with human breast carcinoma and its synergism with tamoxifen. Cancer Res. 1993, 53, 2534–2537. [Google Scholar] [PubMed]
- Koshizuka, K.; Koike, M.; Asou, H.; Cho, S.K.; Stephen, T.; Rude, R.K.; Binderup, L.; Uskokovic, M.; Koeffler, H.P. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res. Treat. 1999, 53, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.; Colston, K.W.; Stein, R.C.; Hedley, A.; Gazet, J.C.; Ford, H.T.; Combes, R.C. Topical calcipotriol treatment in advanced breast cancer. Lancet 1991, 337, 701–702. [Google Scholar] [CrossRef]
- Gulliford, T.; English, J.; Colston, K.W.; Menday, P.; Moller, S.; Coombes, R.C. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br. J. Cancer 1998, 78, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L.; Sommerfeld, J.L.; Pramanik, B.C.; Gardner, R.; Sherry, A.D.; Partridge, J.J.; Uskokovic, M.R.; Horst, R.L. 19-nor-10-ketovitamin D derivatives: Unique metabolites of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3. Biochemistry 1983, 22, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Perlman, K.L.; Sicinski, R.R.; Schnoes, H.K.; DeLuca, H.F. 1α,25-dihydroxy-19-norvitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Lett. 1990, 31, 1823–1824. [Google Scholar] [CrossRef]
- Kittaka, A.; Saito, N.; Honzawa, S.; Takenouchi, K.; Ishizuka, S.; Chen, T.C.; Peleg, S.; Kato, S.; Arai, M.A. Creative synthesis of novel vitamin D analogs for health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshida, A.; Saito, N.; Fujishima, T.; Honzawa, S.; Suhara, Y.; Kishimoto, S.; Sugiura, T.; Waku, K.; Takayama, H.; et al. Efficient synthesis of 2-modified 1α,25-dihydroxy-19-norvitamin D3 with julia olefination: High potency in induction of differentiation on HL-60 cells. J. Org. Chem. 2003, 68, 7407–7415. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Persons, K.S.; Zheng, S.; Mathieu, J.; Holick, M.F.; Lee, Y.F.; Bao, B.; Arai, M.A.; Kittaka, A. Evaluation of C-2-substituted 19-nor-1α,25-dihydroxyvitamin D3 analogs as therapeutic agents for prostate cancer. J. Steroid Biochem. Mol. Biol. 2007, 103, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Gato, D.; Zheng, S.; Flanagan, J.N.; Jiang, L.; Kittaka, A.; Sakaki, T.; Yamamoto, K.; Itoh, T.; Lebrasseur, N.K.; Norstedt, G.; et al. Substitution at carbon 2 of 19-nor-1α,25-dihydroxyvitamin D3 with 3-hydroxypropyl group generates an analogue with enhanced chemotherapeutic potency in PC-3 prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2011, 127, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chen, T.C. The anti-cancer actions of vitamin D. Anticancer Agents Med. Chem. 2013, 13, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Hsu, J.T.; Yeh, T.S.; Jan, Y.Y.; Wu, C.T.; Chen, H.Y.; Jwo, S.C.; Takano, M.; Kittaka, A.; et al. Evaluation of the potential therapeutic role of a new generation of vitamin D analog, MART-10, in human pancreatic cancer cells in vitro and in vivo. Cell Cycle 2013, 12, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Hsu, J.T.; Jan, Y.Y.; Chen, L.W.; Kuo, S.F.; Takano, M.; Kittaka, A.; Chen, T.C.; Chen, W.T.; et al. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial-mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014, 354, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Chen, S.C.; Shen, S.C.; Hsu, J.T.; Yeh, T.S.; Pang, J.H.; Su, L.J.; Takano, M.; Kittaka, A.; et al. MART-10, a new generation of vitamin d analog, is more potent than 1α,25-dihydroxyvitamin D3 in inhibiting cell proliferation and inducing apoptosis in ER+ MCF-7 breast cancer cells. Evid.-Based Complement. Altern. Med.: eCAM 2012, 2012, 310872. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chen, S.C.; Yeh, C.N.; Pang, J.H.; Shen, S.C.; Hsu, J.T.; Liu, Y.Y.; Chen, L.W.; Kuo, S.F.; Takano, M.; et al. Mart-10, a less calcemic vitamin D analog, is more potent than 1α,25-dihydroxyvitamin D3 in inhibiting the metastatic potential of MCF-7 breast cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2014, 139, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Ding, T.; Lin, H.; Wang, Y.; Hu, L.; Hu, J.; Feig, B.; Zhang, W.; Pusztai, L.; Symmans, W.F.; et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009, 69, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Honzawa, S.; Kittaka, A. Recent results on a-ring modification of 1α,25-dihydroxyvitamin D3: Design and synthesis of VDR-agonists and antagonists with high biological activity. Curr. Top. Med. Chem. 2006, 6, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Frixen, U.H.; Behrens, J.; Sachs, M.; Eberle, G.; Voss, B.; Warda, A.; Lochner, D.; Birchmeier, W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 1991, 113, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tsanou, E.; Peschos, D.; Batistatou, A.; Charalabopoulos, A.; Charalabopoulos, K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 2008, 28, 3815–3826. [Google Scholar] [PubMed]
- Derycke, L.D.; Bracke, M.E. N-cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004, 48, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Satyamoorthy, K.; Herlyn, M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001, 61, 3819–3825. [Google Scholar] [PubMed]
- Tran, N.L.; Adams, D.G.; Vaillancourt, R.R.; Heimark, R.L. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J. Biol. Chem. 2002, 277, 32905–32914. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Doi, R.; Toyoda, E.; Tsuji, S.; Wada, M.; Koizumi, M.; Tulachan, S.S.; Ito, D.; Kami, K.; Mori, T.; et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004, 10, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Correia, A.L.; Ribeiro, A.S.; Albergaria, A.; Milanezi, F.; Schmitt, F.C. P-cadherin expression in breast cancer: A review. Breast Cancer Res. 2007, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.; Eaton, E.N.; Li, S.H.; Chaffer, C.L.; Reinhardt, F.; Kah, K.J.; Bell, G.; Guo, W.; Rubin, J.; Richardson, A.L.; et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 2011, 145, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.J.; Rajasekaran, A.K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006, 66, 8319–8326. [Google Scholar] [CrossRef] [PubMed]
- Koli, K.; Keski-Oja, J. 1α,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000, 11, 221–229. [Google Scholar] [PubMed]
- Polette, M.; Clavel, C.; Cockett, M.; Girod de Bentzmann, S.; Murphy, G.; Birembaut, P. Detection and localization of mRNAs encoding matrix metalloproteinases and their tissue inhibitor in human breast pathology. Invasion Metastasis 1993, 13, 31–37. [Google Scholar] [PubMed]
- Ueno, H.; Nakamura, H.; Inoue, M.; Imai, K.; Noguchi, M.; Sato, H.; Seiki, M.; Okada, Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997, 57, 2055–2060. [Google Scholar] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chan, Y.R. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine 2011, 56, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Lin, K.J.; Su, L.J.; Yen, T.C.; Pang, J.H.; Kittaka, A.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; et al. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a chemical-induced animal model. Oncotarget 2014, 5, 3849–3861. [Google Scholar] [CrossRef] [PubMed]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-actin cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Chung, L.C.; Feng, T.H.; Chang, P.L.; Juang, H.H. Upregulation of prostate-derived ETS factor by luteolin causes inhibition of cell prolifertation and cell invasion in prostate carcinoma cells. Int. J. Cancer 2012, 130, 2812–2823. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, K.-C.; Yeh, T.-S.; Chen, S.-C.; Pang, J.-H.S.; Yeh, C.-N.; Hsu, J.-T.; Chen, L.-W.; Kuo, S.-F.; Takano, M.; Kittaka, A.; et al. The Vitamin D Analog, MART-10, Attenuates Triple Negative Breast Cancer Cells Metastatic Potential. Int. J. Mol. Sci. 2016, 17, 606. https://doi.org/10.3390/ijms17040606
Chiang K-C, Yeh T-S, Chen S-C, Pang J-HS, Yeh C-N, Hsu J-T, Chen L-W, Kuo S-F, Takano M, Kittaka A, et al. The Vitamin D Analog, MART-10, Attenuates Triple Negative Breast Cancer Cells Metastatic Potential. International Journal of Molecular Sciences. 2016; 17(4):606. https://doi.org/10.3390/ijms17040606
Chicago/Turabian StyleChiang, Kun-Chun, Ta-Sen Yeh, Shin-Cheh Chen, Jong-Hwei S. Pang, Chun-Nan Yeh, Jun-Te Hsu, Li-Wei Chen, Sheng-Fong Kuo, Masashi Takano, Atsushi Kittaka, and et al. 2016. "The Vitamin D Analog, MART-10, Attenuates Triple Negative Breast Cancer Cells Metastatic Potential" International Journal of Molecular Sciences 17, no. 4: 606. https://doi.org/10.3390/ijms17040606





