Up-Regulation of PAI-1 and Down-Regulation of uPA Are Involved in Suppression of Invasiveness and Motility of Hepatocellular Carcinoma Cells by a Natural Compound Berberine
Abstract
:1. Introduction
2. Results
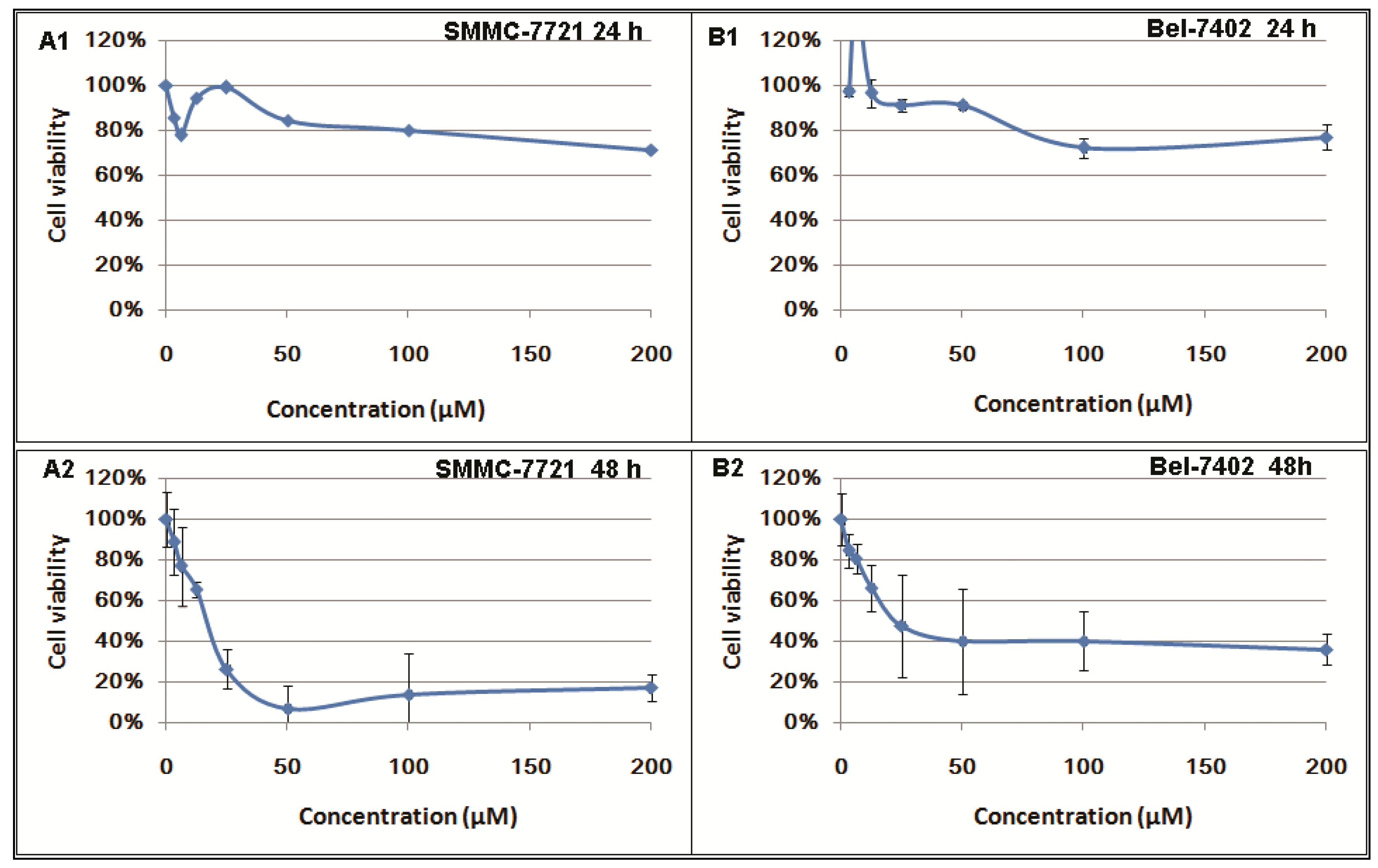
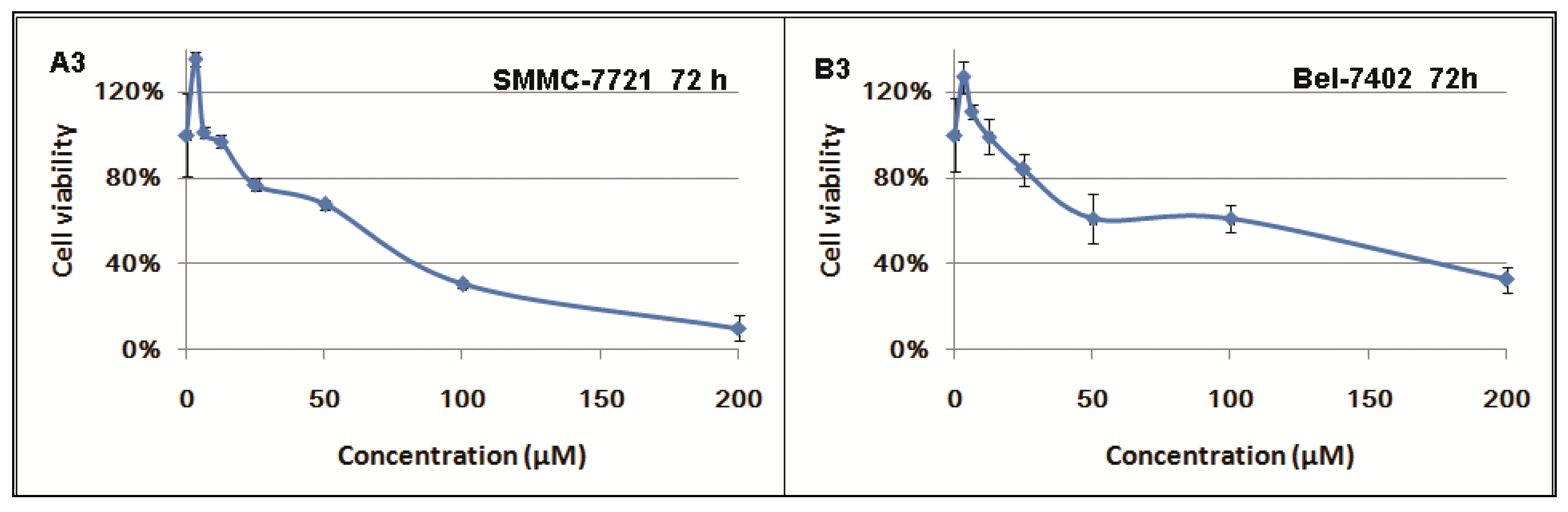
2.1. Inhibitory Effects of Berberine on Hepatocellular Carcinoma (HCC) Cells
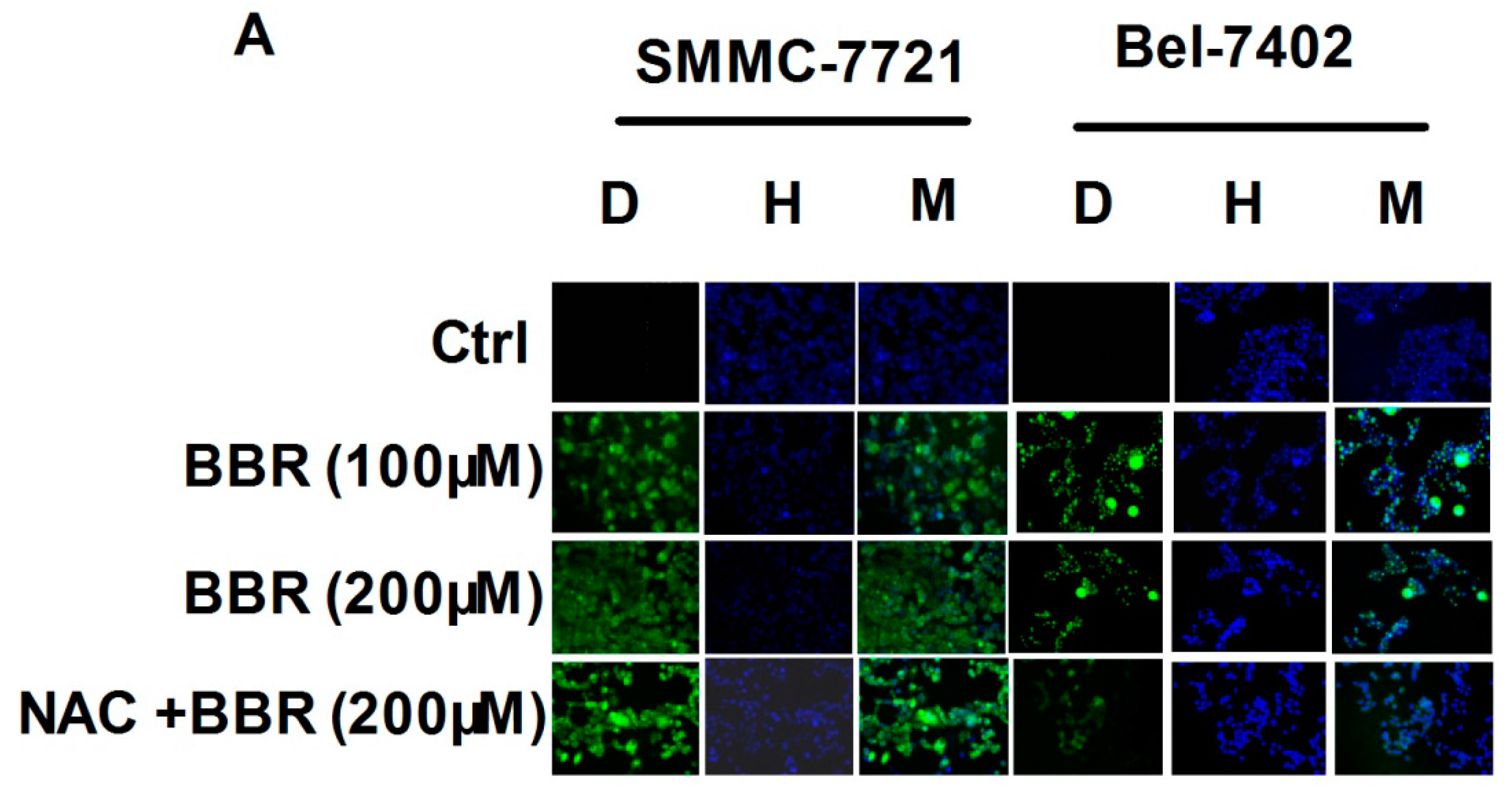
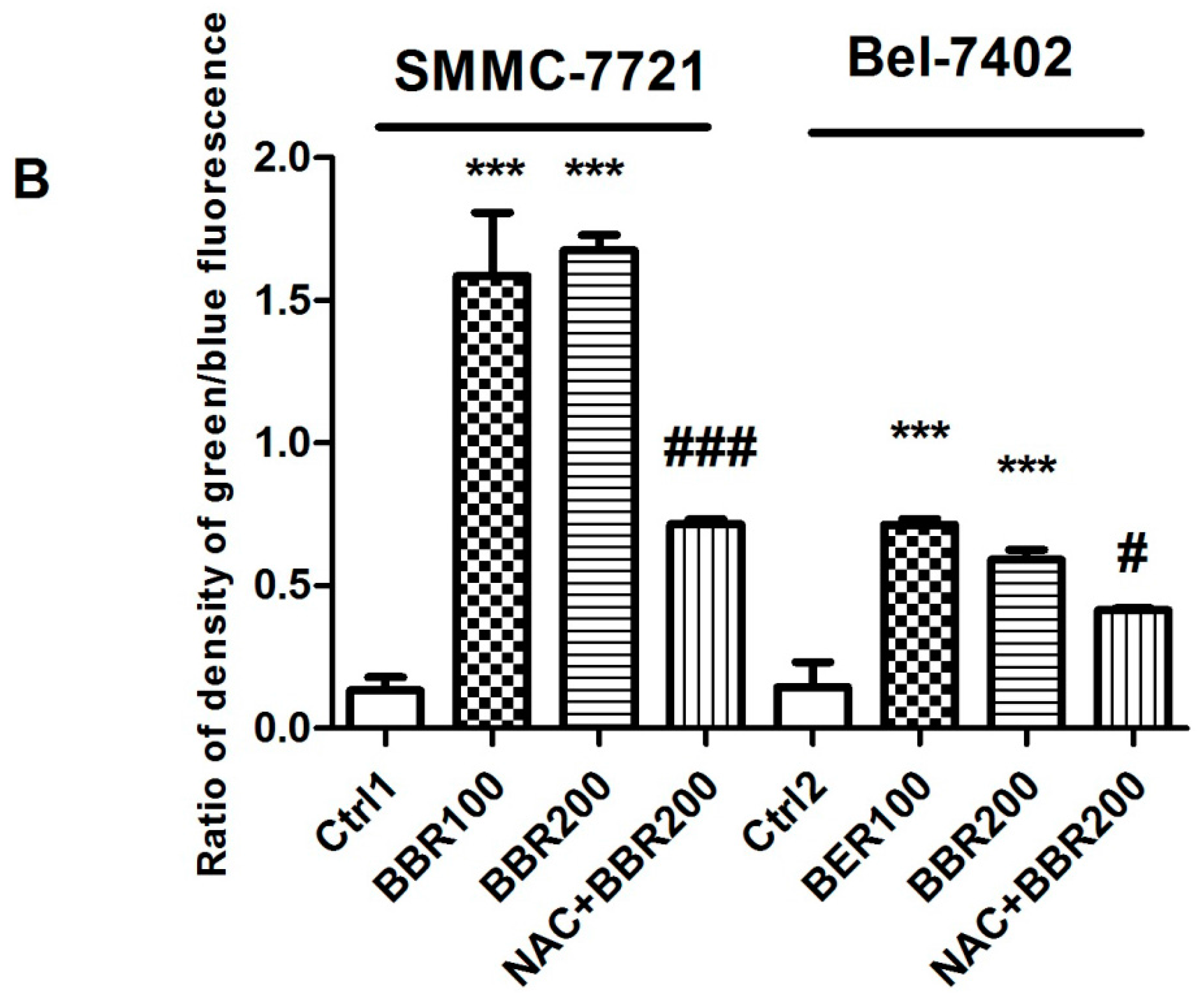
2.2. Intracellular Reactive Oxygen Species (ROS) Production by High Concentration of Berberine in HCC Cells
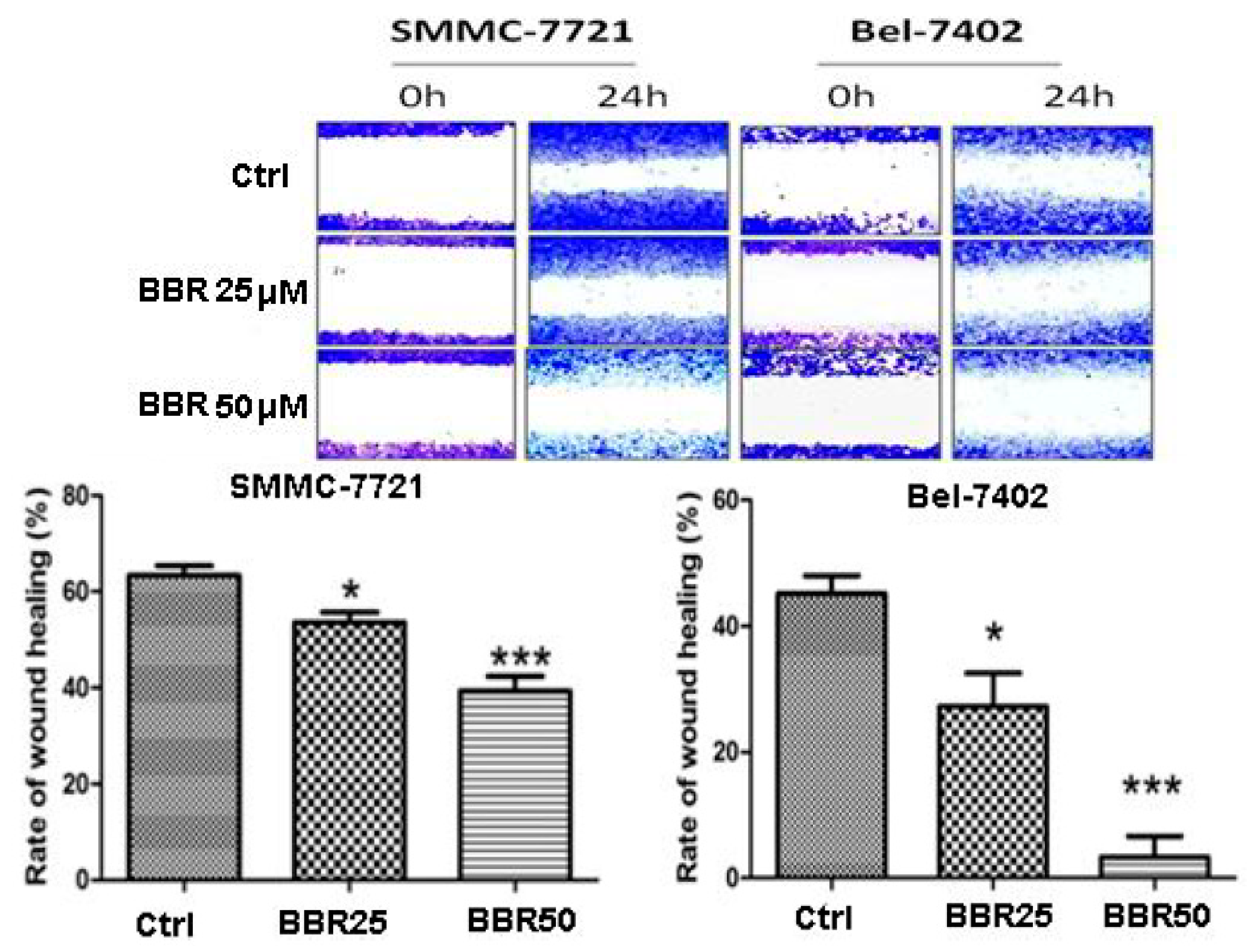
2.3. Migration Inhibition of HCC Cells by Low Dose Berberine (BBR)
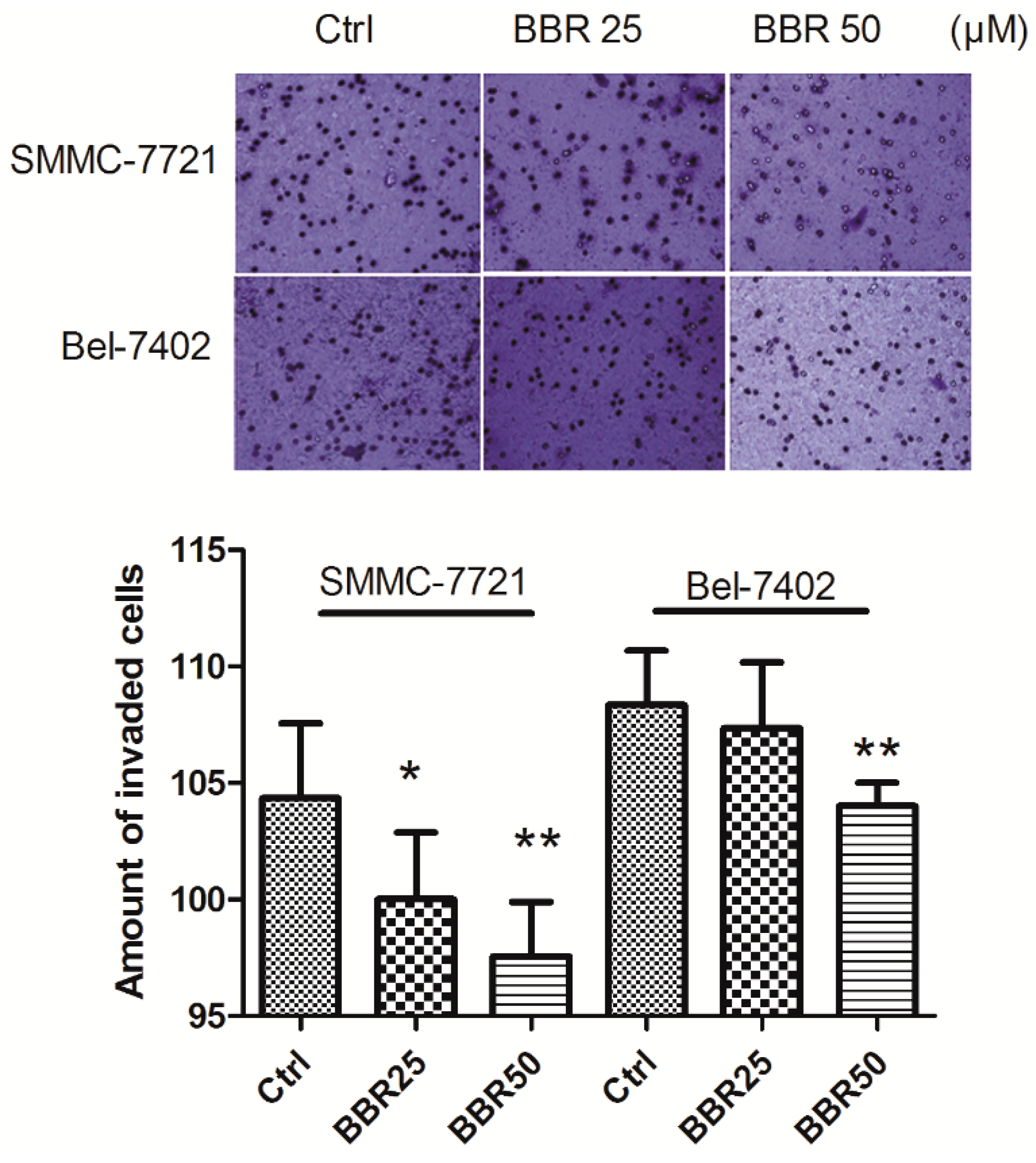
2.4. Invasion Inhibition of HCC Cells by Low Concentration of BBR
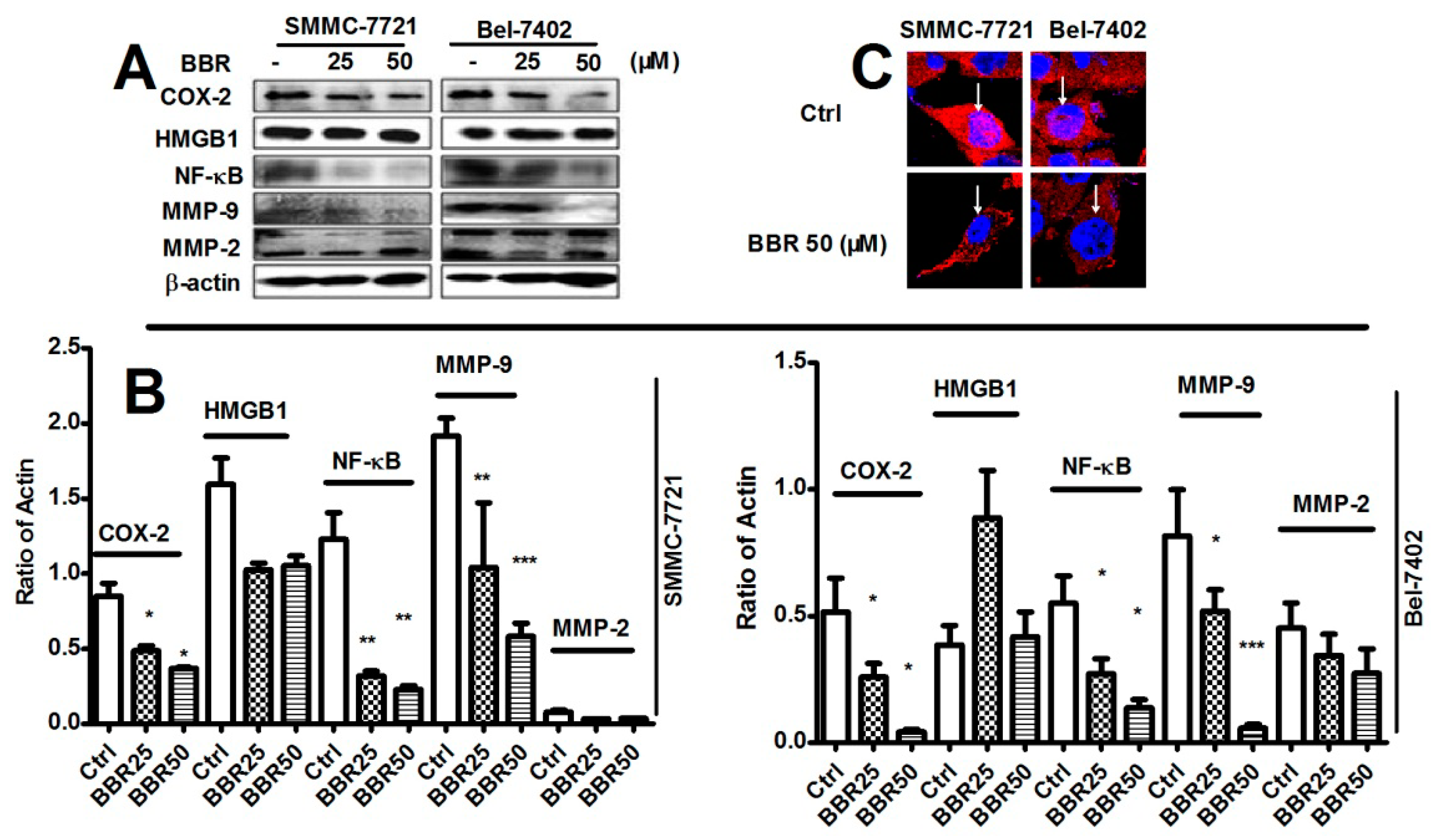
2.5. Inhibition of Cyclooxygenase-2 (COX-2), NF-κB and Matrix Metalloproteinase (MMP)-9 in HCC Cells by Low Dose BBR
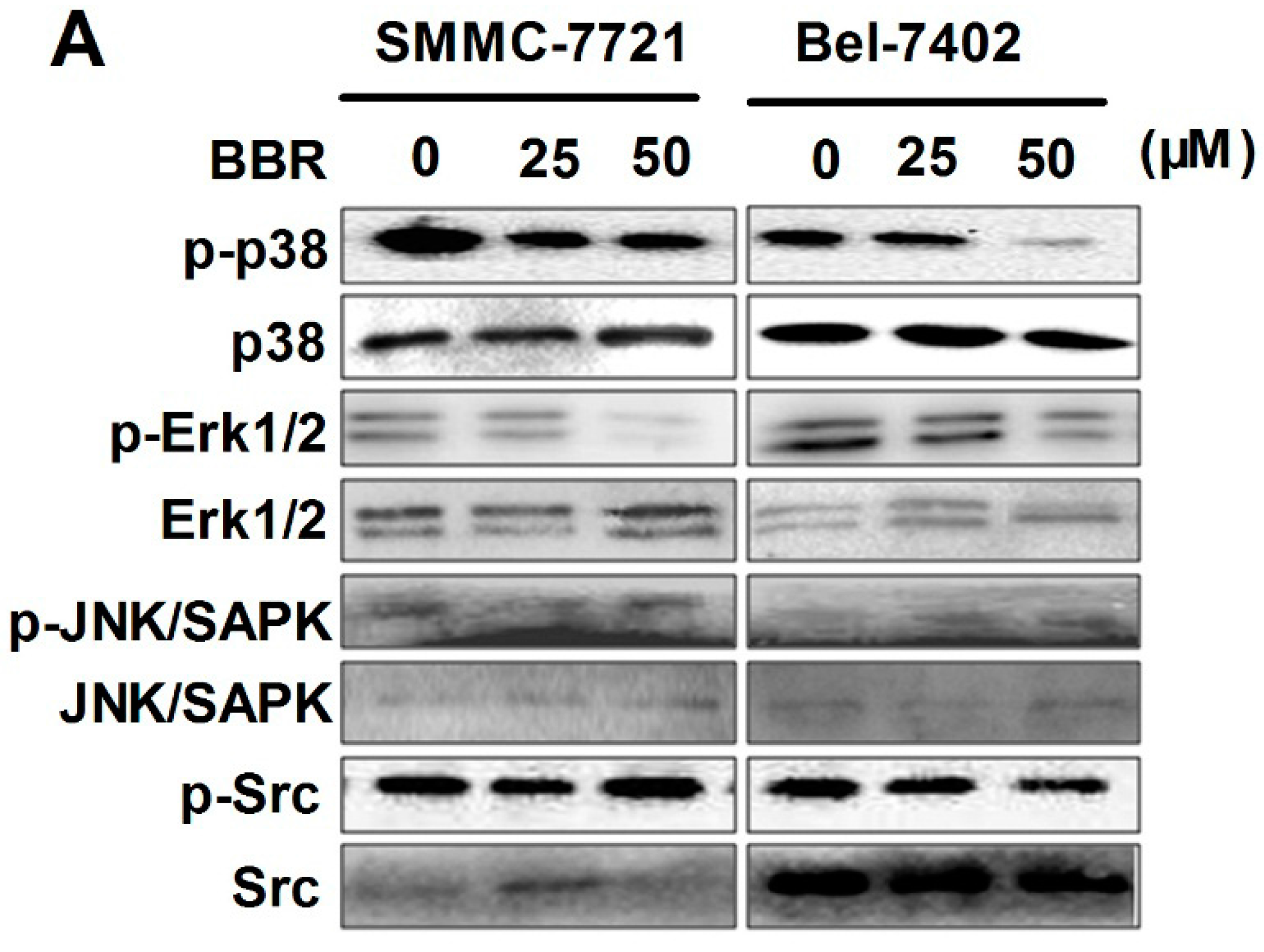
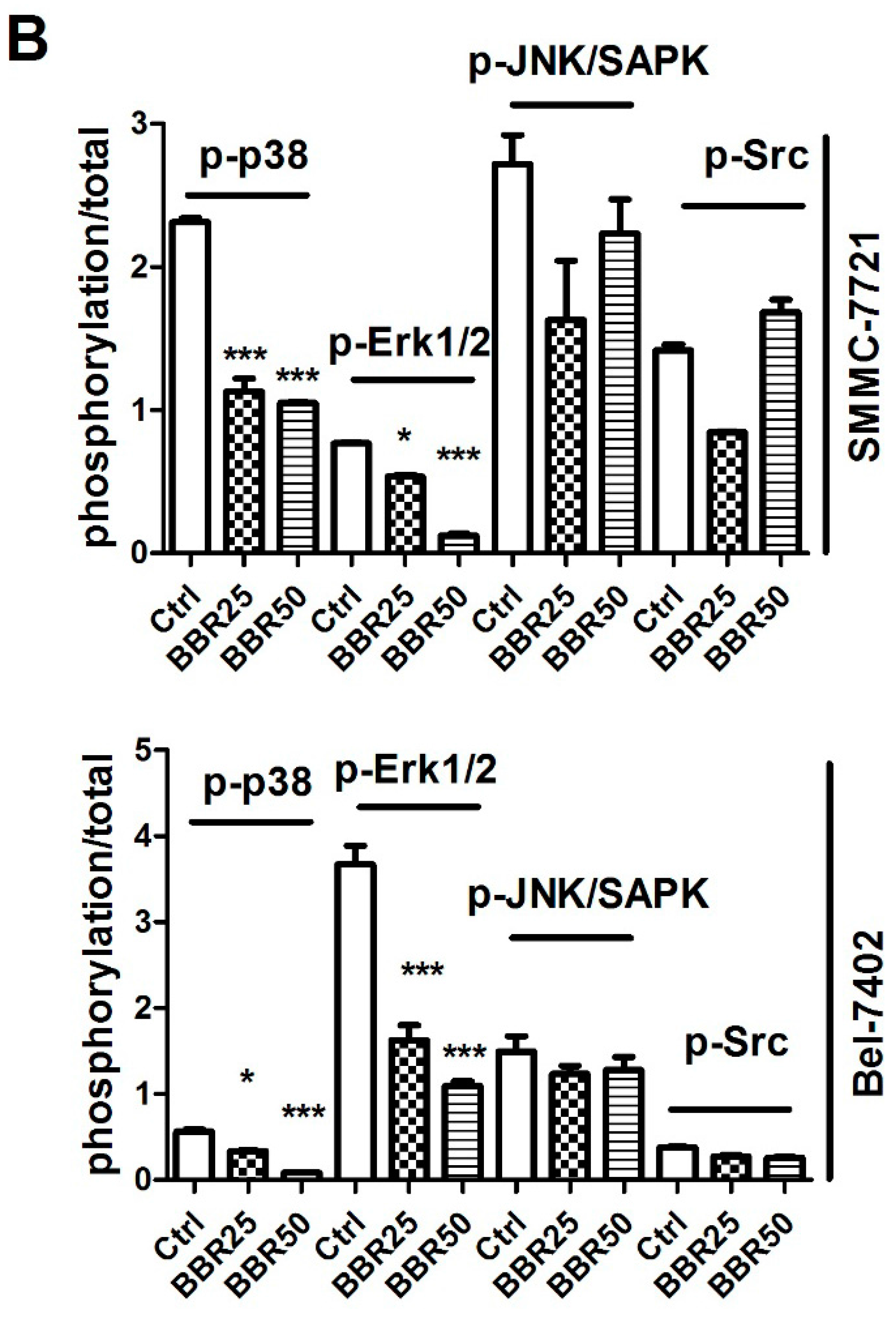
2.6. Inactivation of p38 and Erk1/2 MAPK in BBR-Treated HCC Cells
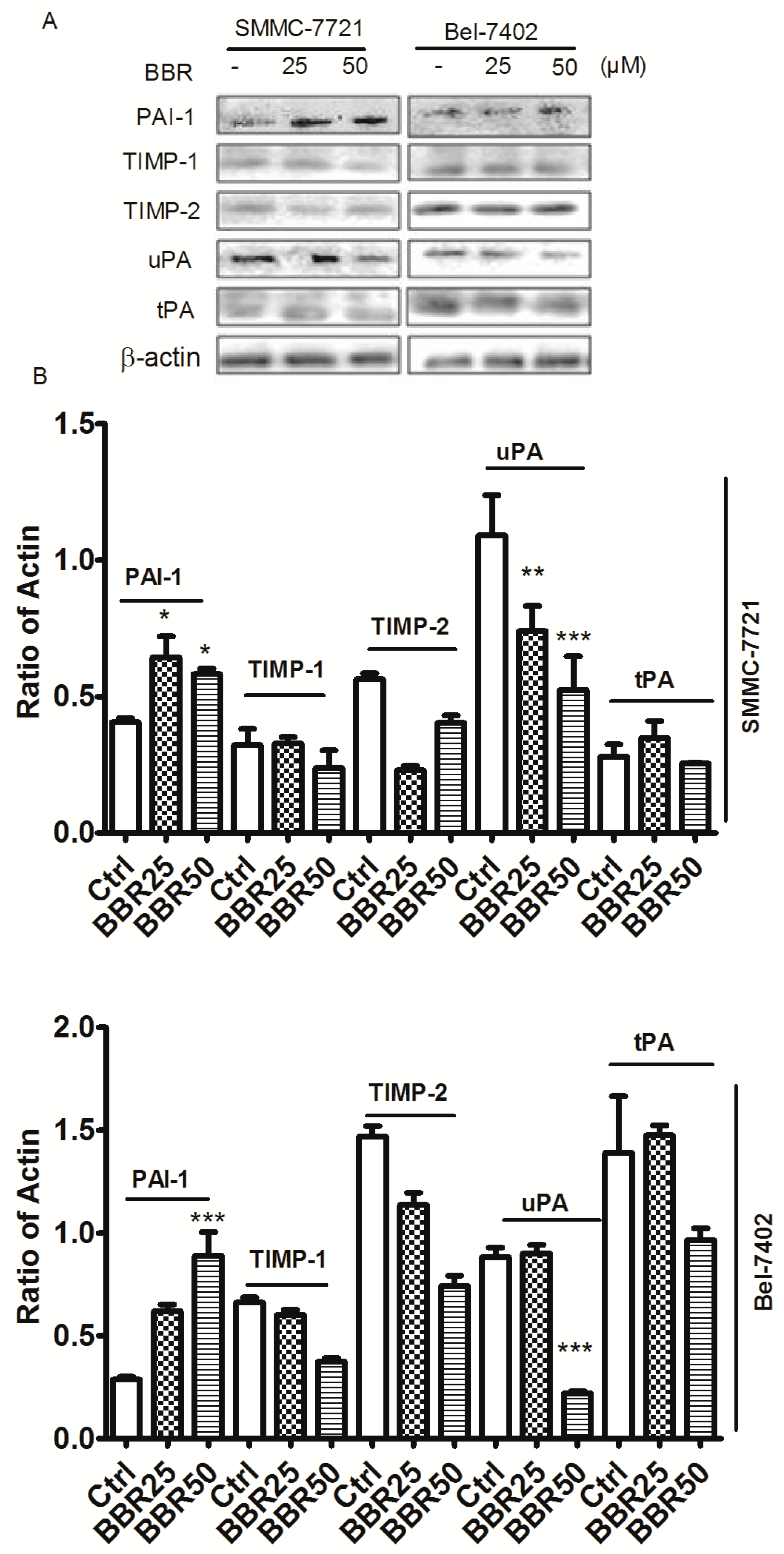
2.7. Berberine Inhibited Urokinase-Type Plasminogen Activator (uPA) Receptor via Inducing Plasminogen Activator Inhibitor-1 (PAI-1) Expression and Suppressing uPA in HCC Cells
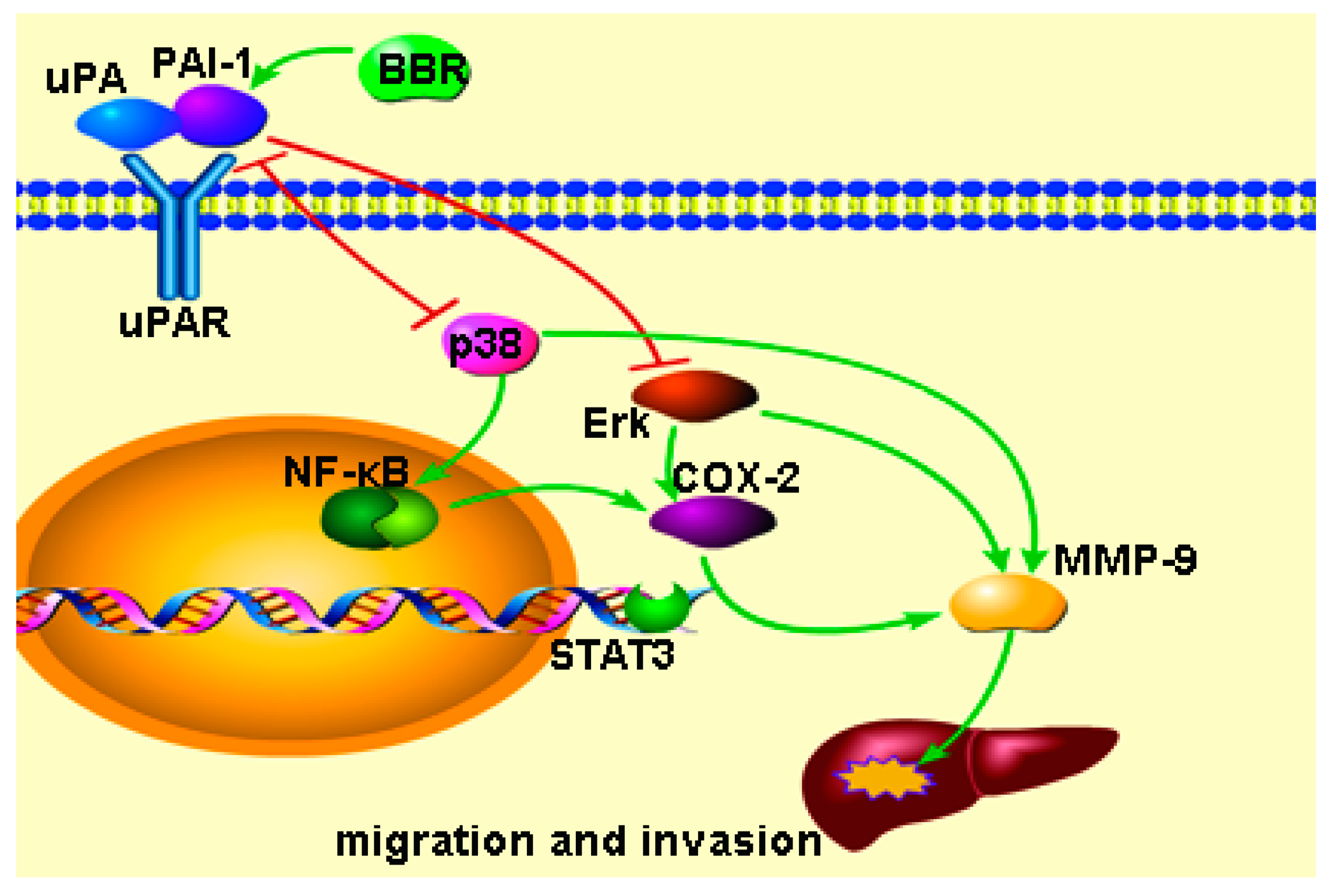
3. Discussion
4. Materials and Methods
4.1. Experimental Agents
4.2. Cell Culture and Treatments
4.3. Experimental Design
4.4. Cell Viability Assay
4.5. Intracellular Level of ROS Detection
4.6. Wound Healing Assay
4.7. Transwell Assay
4.8. Western Blot
4.9. Confocal Microscope
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA: Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Zhang, S.W. Liver cancer epidemic in China: Past, present and future. Semin. Cancer Biol. 2011, 21, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Cheung, F.; Lao, L.; Li, C.; Feng, Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: Current progress on pharmacological actions and mechanisms. J. Integr. Med. 2015, 13, 142–164. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Zhu, M.; Wang, X.; Tan, H.Y.; Tsao, S.W.; Feng, Y. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in hepatocellular carcinoma. Biochim. Biophys. Acta 2014, 1839, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Hyun, M.; Lim, S.; Lee, W.; Kim, D. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J. Cell. Biochem. 2009, 107, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Yip, N.K.; Ho, W.S. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 2013, 30, 1107–1112. [Google Scholar] [PubMed]
- Yang, X.; Huang, N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol. Med. Rep. 2013, 8, 505–510. [Google Scholar] [PubMed]
- Lopes Barreto, D.; Struijk, D.G.; Krediet, R.T. Peritoneal effluent MMP-2 and PAI-1 in encapsulating peritoneal sclerosis. Am. J. Kidney Dis. 2015, 65, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, Z.Q.; Wang, B.; Jiang, H.X.; Cheng, L.; Shen, L.M. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Goh, D.; Ong, E.S. Investigation of differentially expressed proteins due to the inhibitory effects of berberine in human liver cancer cell line HepG2. Mol. Biosyst. 2006, 2, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, D.; Han, X.; Zhang, W.; Fan, C.; Zhang, J.; Mo, C.; Yang, M.; Li, J.; Wang, Z.; et al. The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J. Cell. Biochem. 2014, 115, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Yan, A.; Gao, X.; Chen, Y.; He, X.; Hu, Z.; Mi, M.; Tang, X.; Gou, X. Berberine sensitizes rapamycin-mediated human hepatoma cell death in vitro. Mol. Med. Rep. 2014, 10, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Han, X.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Qi, Y.; Peng, J.Y. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine 2008, 15, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Jie, S.; Li, H.; Tian, Y.; Guo, D.; Zhu, J.; Gao, S.; Jiang, L. Berberine inhibits angiogenic potential of HepG2 cell line through VEGF down-regulation in vitro. J. Gastroenterol. Hepatol. 2011, 26, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Lau, E.P.W.; Tsang, C.; Ching, Y.; Man, K.; Tong, Y.; Nagamatsu, T.; Su, W.; Tsao, S. F-actin reorganization and inactivation of Rho signaling pathway involved in the inhibitory effect of Coptidis rhizoma on hepatoma cell migration. Integr. Cancer Ther. 2010, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, C.M.; Lau, E.P.W.; Di, K.; Cheung, P.Y.; Hau, P.M.; Ching, Y.P.; Wong, Y.C.; Cheung, A.L.M.; Wan, T.S.K.; Tong, Y.; et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int. J. Mol. Med. 2009, 24, 131–138. [Google Scholar] [PubMed]
- Tsang, C.M.; Cheung, K.C.; Cheung, Y.C.; Man, K.; Lui, V.W.; Tsao, S.W.; Feng, Y. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim. Biophys. Acta 2015, 1852, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.R.; Mihaly, J.; Prager, G.W. uPAR–uPA–PAI-1 interactions and signaling: A vascular biologist’s view. Thromb. Haemost. 2007, 97, 336–342. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.J.; Kwaan, H.C. Components of the plasminogen-plasmin system as biologic markers for cancer. Adv. Exp. Med. Biol. 2015, 867, 145–156. [Google Scholar] [PubMed]
- Wang, N.; Feng, Y.; Cheung, F.; Chow, O.Y.; Wang, X.; Su, W.; Tong, Y. A comparative study on the hepatoprotective action of bear bile and Coptidis rhizoma aqueous extract on experimental liver fibrosis in rats. BMC Complement. Altern. Med. 2012, 12, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Siu, K.Y.; Ye, X.; Wang, N.; Yuen, M.F.; Leung, C.H.; Tong, Y.; Kobayashi, S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin. Med. 2010, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letašiová, S.; Jantová, S.; Čipák, L.; Múčková, M. Berberine—Antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006, 239, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS ONE 2012, 7, e36418. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-T.; Lu, C.-C.; Yang, J.-S.; Chiang, J.-H.; Li, T.-C.; Ip, S.-W.; Hsia, T.-C.; Liao, C.-L.; Lin, J.-G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar] [PubMed]
- Patil, J.; Kim, J.; Jayaprakasha, G. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur. J. Pharmacol. 2010, 645, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; Hsieh, Y.; Kuo, H.; Teng, C.; Huang, H.; Wang, C.; Yang, S.; Liou, Y.; Kuo, W. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Yang, J.S.; Lee, J.H.; Hsieh, W.T.; Chung, J.G. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J. Gastroenterol. 2006, 12, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Oh, D.; Yim, M.J.; Park, J.J.; Kang, K.R.; Cho, I.A.; Moon, S.M.; Oh, J.S.; You, J.S.; Kim, C.S.; et al. Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol. Rep. 2015, 33, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Wang, N.; Cheung, F.; Tan, H.Y.; Zhong, S.; Li, C.; Kobayashi, S. Chinese medicines induce cell death: The molecular and cellular mechanisms for cancer therapy. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- To, C.; Liew, C.; Wang, X.; Wang, N.; Feng, Y. Comparison of components and anti-liver cancer activity in vitro between Huanglian and Yunlian. J. Bioequivalence Bioavailab. 2012, 4, 86–90. [Google Scholar]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Dda, C.; Durigan Rde, C.; Tibana, R.A.; Durigan, J.L.; Navalta, J.W.; Prestes, J. The response of matrix metalloproteinase-9 and -2 to exercise. Sports Med. 2015, 45, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chou, Y.E.; Chiou, H.L.; Chen, M.K.; Yang, W.E.; Hsieh, M.J.; Yang, S.F. Pterostilbene suppresses oral cancer cell invasion by inhibiting MMP-2 expression. Expert Opin. Ther. Targets 2014, 18, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Vaid, M.; Katiyar, N.; Sharma, S.; Katiyar, S.K. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin E2 and prostaglandin E2 receptors. Carcinogenesis 2011, 32, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Li, T.M.; Tan, T.W.; Fong, Y.C.; Tang, C.H. Berberine reduces the metastasis of chondrosarcoma by modulating the αvβ 3 integrin and the pkcδ, c-Src, and AP-1 signaling pathways. Evid. Based Complement. Alternat. Med. 2013, 2013, 423164. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, Q.; Ye, N.; Sui, H.; Zhou, L.; Zhu, H.; Fan, Z.; Cai, J.; Li, Q. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS ONE 2015, 10, e0123478. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, N.; Jensen, J.K.; Chi, T.F.; Samoylenko, A.; Kietzmann, T. PAI-1 modulates cell migration in a LRP1-dependent manner via β-catenin and Erk1/2. Thromb. Haemost. 2015, 113, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, K.M.; Jeon, B.H.; Choi, S. The hexane fraction of naematoloma sublateritium extract suppresses the TNF-α-induced metastatic potential of MDA-MB-231 breast cancer cells through modulation of the JNK and p38 pathways. Int. J. Oncol. 2014, 45, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, L.; Li, H.; Chen, P.; Feng, Y.; Zhang, J.; Yang, N.; Li, F.; Wang, Y.; Zhang, Q.; et al. The role of HMGB1 signaling pathway in the development and progression of hepatocellular carcinoma: A review. Int. J. Mol. Sci. 2015, 16, 22527–22540. [Google Scholar] [CrossRef] [PubMed]
- Montuori, N.; Cosimato, V.; Rinaldi, L.; Rea, V.E.; Alfano, D.; Ragno, P. uPAR regulates pericellular proteolysis through a mechanism involving integrins and fMLF-receptors. Thromb. Haemost. 2013, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.J.; Chun, J.M.; Lee, A.Y.; Kim, H.K. Anti-metastatic effects of Rheum Palmatum L. extract in human MDA-MB-231 breast cancer cells. Environ. Toxicol. Pharmacol. 2015, 40, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Coum, A.; Marinescu, V.D.; Polajeva, J.; Smits, A.; Nelander, S.; Uhrbom, L.; Westermark, B.; Forsberg-Nilsson, K.; Ponten, F.; et al. Glioma-derived plasminogen activator inhibitor-1 (PAI-1) regulates the recruitment of LRP1 positive mast cells. Oncotarget 2015, 6, 23647–23661. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peng, H.; Liu, W.; Sun, Y.; Su, N.; Tang, W.; Zhang, X.; Wang, J.; Cui, L.; Hu, P.; et al. Silencing of plasminogen activator inhibitor-1 suppresses colorectal cancer progression and liver metastasis. Surgery 2015, 158, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.L.; Hsieh, Y.S.; Wang, C.J.; Hsu, J.L.; Chou, F.P. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol. Appl. Pharmacol. 2006, 214, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Akudugu, J.; Serafin, A.; Bohm, L. Further evaluation of uPA and PAI-1 as biomarkers for prostatic diseases. J. Cancer Res. Clin. Oncol. 2015, 141, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Kraicun, D.; Bonello, S.; Gorlach, A. The ‘PAI-1 paradox’ in vascular remodeling. Thromb. Haemost. 2008, 100, 984–991. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, N.; Li, H.; Liu, M.; Cao, F.; Yu, X.; Zhang, J.; Tan, Y.; Xiang, L.; Feng, Y. Up-Regulation of PAI-1 and Down-Regulation of uPA Are Involved in Suppression of Invasiveness and Motility of Hepatocellular Carcinoma Cells by a Natural Compound Berberine. Int. J. Mol. Sci. 2016, 17, 577. https://doi.org/10.3390/ijms17040577
Wang X, Wang N, Li H, Liu M, Cao F, Yu X, Zhang J, Tan Y, Xiang L, Feng Y. Up-Regulation of PAI-1 and Down-Regulation of uPA Are Involved in Suppression of Invasiveness and Motility of Hepatocellular Carcinoma Cells by a Natural Compound Berberine. International Journal of Molecular Sciences. 2016; 17(4):577. https://doi.org/10.3390/ijms17040577
Chicago/Turabian StyleWang, Xuanbin, Ning Wang, Hongliang Li, Ming Liu, Fengjun Cao, Xianjun Yu, Jingxuan Zhang, Yan Tan, Longchao Xiang, and Yibin Feng. 2016. "Up-Regulation of PAI-1 and Down-Regulation of uPA Are Involved in Suppression of Invasiveness and Motility of Hepatocellular Carcinoma Cells by a Natural Compound Berberine" International Journal of Molecular Sciences 17, no. 4: 577. https://doi.org/10.3390/ijms17040577





