The Role of Docosahexaenoic Acid (DHA) in the Control of Obesity and Metabolic Derangements in Breast Cancer
Abstract
:1. Introduction
Relation between Obesity, Insulin-Resistance, Inflammation, and Breast Cancer Development
2. Relation between Overweight and Obesity during and after Treatment for BC and the Relative Risk of Recurrence
3. Effect of ω-3 PUFAs and DHA on Inflammation in BC
4. Effect of ω-3 PUFAs and DHA on Insulin-Resistance and Obesity in BC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Ghose, A.; Kundu, R.; Toumeh, A.; Hornbeck, C.; Mohamed, I. A review of obesity, insulin resistance and the role of exercise in breast cancer patients. Nutr. Cancer 2015, 67, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Chandran, U.; Bandera, E.V.; Parekh, N. Dietary fat in breast cancer survival. Annu. Rev. Nutr. 2013, 33, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, C.; Salvagno, F.; Canuto, E.M.; Gennarelli, G. Obesity and female malignancies. Best Pract. Res. Clin. Obst. Gyn. 2015, 29, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Turk, H.F.; Liddle, D.M.; de Boer, A.A.; Power, K.A.; Ma, D.W.; Robinson, L.E. n-3 polyunsaturated fatty acids and mechanisms to mitigate inflammatory paracrine signaling in obesity-associated breast cancer. Nutrients 2014, 6, 4760–4793. [Google Scholar] [PubMed]
- Ferguson, R.D.; Gallagher, E.J.; Scheinman, E.J.; Damouni, R.; LeRoith, D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam. Horm. 2013, 93, 51–98. [Google Scholar] [PubMed]
- Rose, D.P.; Vona-Davis, L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. BioFactors 2014, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, V.M.; Nicholls, J.E.; Gledhill, J.; Rowlands, M.G.; Dowsett, M.; Ashworth, A. Biological effects of stable overexpression of aromatase in human hormone-dependent breast cancer cells. Br. J. Cancer 1994, 69, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Nichols, J.E.; Bulun, S.E. Aromatase gene expression in adipose tissue: Relationship to breast cancer. Int. J. Fertil. Menopausal Stud. 1994, 39, S75–S83. [Google Scholar]
- Daling, J.R.; Malone, K.E.; Doody, D.R.; Johnson, L.G.; Gralow, J.R.; Porter, P.L. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 2001, 92, 720–729. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Chabbert-Buffet, N.; Fabre, A.; Guillas, G.; Boutron-Ruault, M.C.; Mesrine, S.; Clavel-Chapelon, F. Hip circumference is associated with the risk of premenopausal ER-/PR-breast cancer. Int. J. Obes. 2012, 36, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Rianda, S.; Molfino, A.; Rossi Fanelli, F. Omega-3 fatty acid in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Maheo, K.; Couet, C.; Chevalier, S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog. Lipid Res. 2010, 49, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le, F.O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Flatt, S.W.; Newman, V.A.; Natarajan, L.; Rock, C.L.; Thomson, C.A.; Caan, B.J.; Parker, B.A.; Pierce, J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr. 2011, 141, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, Z.; Kristal, A.R.; Gulati, R.; Luick, B.; Bersamin, A.; Boyer, B.; Mohatt, G.V. Associations of very high intakes of eicosapentaenoic and docosahexaenoic acids with biomarkers of chronic disease risk among Yup’ik Eskimos. Am. J. Clin. Nutr. 2011, 91, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.; Freedman, L.; Clifford, C.; Midthune, D. Effect of different types and amounts of fat on the development of mammary tumors in rodents: A review. Cancer Res. 1997, 57, 3979–3988. [Google Scholar] [PubMed]
- Carmichael, A.R. Obesity and prognosis of breast cancer. Obes. Rev. 2006, 7, 333–340. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast cancer statistics, 2011. CA Cancer J. Clin. 2011, 61, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aiello, E.; McTiernan, A. Weight loss in breast cancer patient management. J. Clin. Oncol. 2002, 20, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast 2013, 22, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.; Wald, A. Interventions for the management of weight and body composition changes in women with breast cancer. Clin. J. Oncol. Nurs. 2007, 11, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Norat, T. Obesity and breast cancer: not only a risk factor of the disease. Curr. Treat. Opt. Oncol. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Heideman, W.H.; Russell, N.S.; Gundy, C.; Rookus, M.A.; Voskuil, D.W. The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. Eur. J. Cancer 2009, 45, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Cadmus, L.A.; Emond, J.A.; Pierce, J.P. Physical activity, diet, adiposity and female breast cancer prognosis: A review of the epidemiologic literature. Maturitas 2010, 66, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.T.; Ibrahim, J.G.; Stevens, J.; Cleveland, R.; Abrahamson, P.E.; Satia, J.A.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 2012, 23, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, S.C.; Frasor, J. Inflammation: An instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol. Endocrinol. 2012, 26, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70, i104–i108. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Hotamisligil, G.S. Linking the inflammasome to obesity-related disease. Nat. Med. 2011, 17, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, P.; Berrino, F.; de Petris, M.; Venturelli, E.; Mastroianni, A.; Panico, S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int. J. Cancer 2006, 119, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.; Stratton, I.M.; Horn, R.; Holman, R.R.; Turner, R.; Brabant, G. UKPDS 20: Plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 1997, 82, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Jarde, T.; Caldefie-Chezet, F.; Damez, M.; Mishellany, F.; Penault-Llorca, F.; Guillot, J.; Vasson, M.P. Leptin and leptin receptor involvement in cancer development: A study on human primary breast carcinoma. Oncol. Rep. 2008, 19, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Seelaender, M.; Sanchez-Lara, K.; Gioulbasanis, I.; Molfino, A.; Rossi Fanelli, F. Beyond anorexia-cachexia. Nutrition and modulation of cancer patients’ metabolism: Supplementary, complementary or alternative anti-neoplastic therapy? Eur. J. Pharmacol. 2011, 668, s87–s90. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Muller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.J.; Hasty, A.H.; Saraswathi, V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J. Nutr. Biochem. 2011, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Gaugler, T.L.; Fleming, J.A.; Kris-Etherton, P.M. Effects of supplemental long-chain omega-3 fatty acids and erythrocyte membrane fatty acid content on circulating inflammatory markers in a randomized controlled trial of healthy adults. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Sangster, K.; Arends, M.J. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J. Pathol. 1998, 185, 61–70. [Google Scholar] [CrossRef]
- Grammatikos, S.I.; Subbaiah, P.V.; Victor, T.A.; Miller, W.M. n-3 and n-6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br. J. Cancer 1994, 70, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Schemmel, R.A.; Dugan, L., Jr.; Gray, J.I.; Welsch, C.W. Dietary fish oil inhibits human breast carcinoma growth: A function of increased lipid peroxidation. Lipids 1993, 28, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; le Guennec, J.Y.; Galano, J.M.; Thireau, J.; Bultel-Poncé, V.; Demion, M.; Oger, C.; Lee, J.C.; Durand, T. Non-enzymatic cyclic oxygenated metabolites of omega-3 polyunsaturated fatty acid: Bioactive drugs? Biochimie 2016, 120, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Oliveira, L.T.; Oger, C.; Galano, J.M.; Bultel-Poncé, V.; Richard, S.; Guimaraes, A.G.; Vilela, J.M.; Andrade, M.S.; Durand, T.; et al. Polymeric nanocapsules prevent oxidation of core-loaded molecules: Evidence based on the effects of docosahexaenoic acid and neuroprostane on breast cancer cells proliferation. J. Exp. Clin. Cancer Res. 2015, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago-Dominguez, M.; Yuan, J.M.; Sun, C.L.; Lee, H.P.; Yu, M.C. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: The Singapore Chinese Health Study. Br. J. Cancer 2003, 89, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Takezaki, T.; Hamajima, N.; Miura, S.; Tajima, K. Dietary factors protective against breast cancer in Japanese premenopausal and postmenopausal women. Int. J. Cancer 2003, 107, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Tamakoshi, K.; Date, C.; Fukui, M.; Suzuki, S.; Lin, Y.; Niwa, Y.; Nishio, K.; Yatsuya, H.; Kondo, T.; et al. Dietary intakes of fat and fatty acids and risk of breast cancer: A prospective study in Japan. Cancer Sci. 2005, 96, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Chajes, V.; Torres-Mejía, G.; Biessy, C.; Ortega-Olvera, C.; Angeles-Llerenas, A.; Ferrari, P.; Lazcano-Ponce, E.; Romieu, I. ω-3 and ω-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: Impact of obesity status. Cancer Epidemiol. Biomark. Prev. 2011, 21, 319–326. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, Z.; Poorolajal, J.; Hashem, T.; Esmailnasab, N.; Doosti Irani, A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS ONE 2012, 7, e51446. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, A.C.; Chajes, V.; Gerber, M.; Boutron-Ruault, M.C.; Joulin, V.; Lenoir, G.; Berrino, F.; Riboli, E.; Benichou, J.; Clavel-Chapelon, F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer 2009, 124, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, M.B.; Clarke, S.E.; Perez, K.; Wood, G.A.; Muller, W.J.; Kang, J.X.; Ma, D.W. Mammary tumor development is directly inhibited by lifelong n-3 polyunsatured fatty acids. J. Nutr. Biochem. 2013, 24, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Molfino, A.; Rossi Fanelli, F. Cancer treatment toxicity: Can nutrition help? Nat. Rev. Clin. Oncol. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Giovannucci, E.L. Diet and cancer prevention. Oncogene 2004, 23, 6349–6364. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Rogers, C.J.; Prabhu, K.S.; Kris-Etherton, P.M. Immunometabolic role of long-chain omega-3 fatty acids in obesity-induced inflammation. Diabetes Metab. Res. Rev. 2013, 29, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17. [Google Scholar] [CrossRef] [PubMed]


| Research on modifiable risk factors in breast cancer is receiving clinical relevance because they affect the prognosis of the disease [1,2]. |
| Data indicate that obesity increases breast cancer risk in part due to hormonal interactions and in part to inflammatory and insulin-resistance mechanisms [3,4,5,6]. |
| Omega-3 fatty acids are metabolically active lipids with anti-inflammatory properties [13,14,15,16]. |
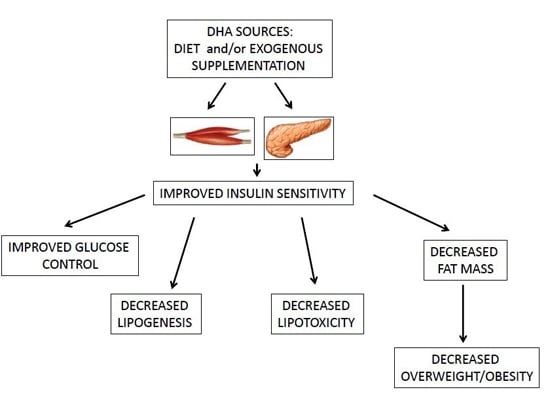
| Omega-3 fatty acids and, in particular, DHA ameliorate obesity-induced inflammation and insulin-resistance [4,36,37]. |
| Considering the role of inflammation in breast cancer, there is an increasing rationale for the use of DHA in combination with anticancer therapies [14,15,16,64]. |
| Additional evidence is needed to assess the protective role of DHA breast cancer. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Amabile, M.I.; Monti, M.; Arcieri, S.; Rossi Fanelli, F.; Muscaritoli, M. The Role of Docosahexaenoic Acid (DHA) in the Control of Obesity and Metabolic Derangements in Breast Cancer. Int. J. Mol. Sci. 2016, 17, 505. https://doi.org/10.3390/ijms17040505
Molfino A, Amabile MI, Monti M, Arcieri S, Rossi Fanelli F, Muscaritoli M. The Role of Docosahexaenoic Acid (DHA) in the Control of Obesity and Metabolic Derangements in Breast Cancer. International Journal of Molecular Sciences. 2016; 17(4):505. https://doi.org/10.3390/ijms17040505
Chicago/Turabian StyleMolfino, Alessio, Maria Ida Amabile, Massimo Monti, Stefano Arcieri, Filippo Rossi Fanelli, and Maurizio Muscaritoli. 2016. "The Role of Docosahexaenoic Acid (DHA) in the Control of Obesity and Metabolic Derangements in Breast Cancer" International Journal of Molecular Sciences 17, no. 4: 505. https://doi.org/10.3390/ijms17040505