Do Variants in GSTs Modify the Association between Traffic Air Pollution and Asthma in Adolescence?
Abstract
:1. Introduction
2. Results
2.1. Main Environmental and Genetic Effects
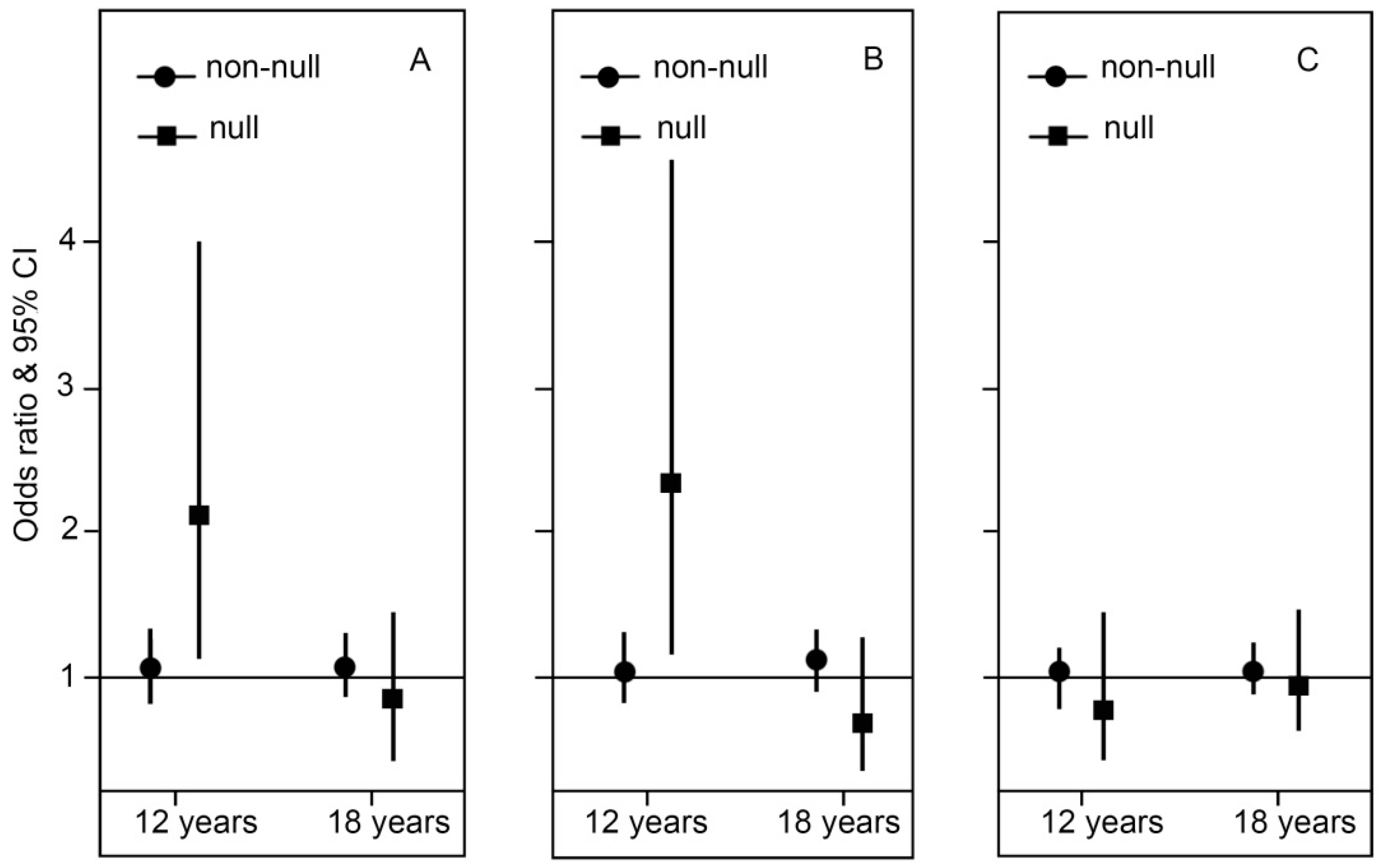
2.2. Genotype Stratification and Interaction Effect of GSTT1
2.3. Genotype Stratification and Interaction Effect of GSTP1
2.4. Genotype Stratification and Interaction Effect of GSTM1
3. Discussion
4. Materials and Methods
4.1. Health Data Collection
4.2. Definitions of Health Outcomes
4.3. Genetic Data
4.4. Address Data and Exposure Assessment
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GSTs | Glutahione S transferaese genes |
| GSTT1 | Glutahione S transferaese theta1 |
| GSTM1 | Glutahione S transferaese mu1 |
| GSTP1 | Glutahione S transferaese pi1 |
| MACS | Melbourne Atopy Cohort Study |
| NO2 | Nitrogen dioxide |
| ROS | Reactive Oxygen Species |
| TRAP | Traffic Related Air Pollution |
References
- Braman, S.S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef] [PubMed]
- Gowers, A.M.; Cullinan, P.; Ayres, J.G.; Anderson, H.R.; Strachan, D.P.; Holgate, S.T.; Mills, I.C.; Maynard, R.L. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence: A review. Respirology 2012, 17, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Zhang, Q.; Qiu, Z.; Chung, K.F. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J. Thorac. Dis. 2015, 7, 23–33. [Google Scholar] [PubMed]
- Guarnieri, M.; Balmes, J. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Vawda, S.; Mansour, R.; Takeda, A.; Funnell, P.; Kerry, S.; Mudway, I.; Jamaludin, J.; Shaheen, S.; Griffiths, C.; Walton, R. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: A huge systematic review. Am. J. Epidemiol. 2014, 179, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Tsai, C.-H.; Chen, C.-H.; Tung, K.-Y.; Lee, Y.L. Glutathione s-transferase, incense burning and asthma in children. Eur. Respir. J. 2011, 37, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Li, Y.-F.; Saxon, A.; Diaz-Sanchez, D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: Randomised, placebo-controlled crossover study. Lancet 2004, 363, 119–125. [Google Scholar] [CrossRef]
- Islam, T.; Berhane, K.; McConnell, R.; Gauderman, W.J.; Avol, E.; Peters, J.M.; Gilliland, F.D. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax 2009, 64, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Minelli, C.; Wei, I.; Sagoo, G.; Jarvis, D.; Shaheen, S.; Burney, P. Interactive effects of antioxidant genes and air pollution on respiratory function and airway disease: A huge review. Am. J. Epidemiol. 2011, 173, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Lin, Y.C.; Lee, Y.C.; Wang, J.Y.; Hsiue, T.R.; Guo, Y.L. Glutathione S-transferase P1 gene polymorphism and air pollution as interactive risk factors for childhood asthma. Clin. Exp. Allergy 2004, 34, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Lin, P.C.; Avol, E.L.; Gauderman, W.J.; Gilliland, F.D. Microsomal epoxide hydrolase, glutathione S-transferase P1, traffic and childhood asthma. Thorax 2007, 62, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Arjomandi, M.; Tager, I.B.; Holland, N.; Balmes, J.R. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur. Respir. J. 2007, 30, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; McConnell, R.; Gauderman, W.J.; Avol, E.; Peters, J.M.; Gilliland, F.D. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am. J. Respir. Crit. Care Med. 2008, 177, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Melen, E.; Nyberg, F.; Lindgren, C.M.; Berglind, N.; Zucchelli, M.; Nordling, E.; Hallberg, J.; Svartengren, M.; Morgenstern, R.; Kere, J.; et al. Interactions between glutathione S-transferase P1, tumor necrosis factor, and traffic-related air pollution for development of childhood allergic disease. Environ. Health Perspect. 2008, 116, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Schroer, K.T.; Biagini Myers, J.M.; Ryan, P.H.; LeMasters, G.K.; Bernstein, D.I.; Villareal, M.; Lockey, J.E.; Reponen, T.; Grinshpun, S.; Khurana Hershey, G.K. Associations between multiple environmental exposures and glutathione S-transferase P1 on persistent wheezing in a birth cohort. J. Pediatr. 2009, 154, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Naidoo, R.N.; Robins, T.G.; Mentz, G.; Li, H.; London, S.J.; Batterman, S. GSTM1 and GSTP1 gene variants and the effect of air pollutants on lung function measures in south african children. Am. J. Ind. Med. 2012, 55, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- London, S.J.; Romieu, I. Gene by environment interaction in asthma. Annu. Rev. Public Health 2009, 30, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Su, M.W.; Tsai, C.H.; Tung, K.Y.; Hwang, B.F.; Liang, P.H.; Chiang, B.L.; Yang, Y.H.; Lee, Y.L. GSTP1 is a hub gene for gene-air pollution interactions on childhood asthma. Allergy 2013, 68, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, E.; DePalma, G.; Mozzoni, P.; Vanni, S.; Vettori, M.; Broeckaert, F.; Bernard, A.; Mutti, A. Polymorphism of quinone-metabolizing enzymes and susceptibility to ozone-induced acute effects. Am. J. Respir. Crit. Care Med. 2001, 163, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wei, X.; Gong, C.; Wei, J.; Chen, Z.; Chen, X.; Wang, Z.; Deng, J. Significant association between asthma risk and the GSTM1 and GSTT1 deletion polymorphisms: An updated meta-analysis of case-control studies. Respirology 2013, 18, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Liu, Z.; Ye, Y.; Mao, M. Significant association between GSTT1 null genotype and risk of asthma during childhood in caucasians. Mol. Biol. Rep. 2013, 40, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Castro-Giner, F.; Künzli, N.; Jacquemin, B.; Forsberg, B.; de Cid, R.; Sunyer, J.; Jarvis, D.; Briggs, D.; Vienneau, D.; et al. Traffic-related air pollution, oxidative stress genes, and asthma (ECHRS). Environ. Health Perspect. 2009, 117, 1919–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.C.; Hwang, S.S.; Kim, J.H.; Lee, K.H.; Lee, H.J.; Lee, K.H.; Yu, S.D.; Kim, D.S. Metals in particulate pollutants affect peak expiratory flow of schoolchildren. Environ. Health Perspect. 2007, 115, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Froom, J.; Culpepper, L.; Green, L.A.; de Melker, R.A.; Grob, P.; Heeren, T.; van Balen, F. A cross-national study of acute otitis media: Risk factors, severity, and treatment at initial visit. Report from the international primary care network (IPCN) and the ambulatory sentinel practice network (ASPN). J. Am. Board Fam. Pract. 2001, 14, 406–417. [Google Scholar] [PubMed]
- Wright, R.J.; Brunst, K.J. Programming of respiratory health in childhood: Influence of outdoor air pollution. Curr. Opin. Pediatr. 2013, 25, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, C.; Worm, M.; Leynaert, B. Impact of gender on asthma in childhood and adolescence: A GA2LEN review. Allergy 2008, 63, 47–57. [Google Scholar] [CrossRef] [PubMed]
- David, G.L.; Romieu, I.; Sienra-Monge, J.J.; Collins, W.J.; Ramirez-Aguilar, M.; del Rio-Navarro, B.E.; Reyes-Ruiz, N.I.; Morris, R.W.; Marzec, J.M.; London, S.J. Nicotinamide adenine dinucleotide (phosphate) reduced:Quinone oxidoreductase and glutathione S-transferase M1 polymorphisms and childhood asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Ramirez-Aguilar, M.; Sienra-Monge, J.J.; Moreno-Macias, H.; del Rio-Navarro, B.E.; David, G.; Marzec, J.; Hernandez-Avila, M.; London, S. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur. Respir. J. 2006, 28, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Sienra-Monge, J.; Ramirez-Aguilar, M.; Moreno-Macias, H.; Reyes-Ruiz, N.; Estela del Río-Navarro, B.; Hernandez-Avila, M.; London, S. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in mexico city. Thorax 2004, 59, 8–10. [Google Scholar] [PubMed]
- Rose, N.; Cowie, C.; Gillett, R.; Marks, G.B. Weighted road density: A simple way of assigning traffic-related air pollution exposure. Atmos. Environ. 2009, 43, 5009–5014. [Google Scholar] [CrossRef]
- Piacentini, S.; Polimanti, R.; Porreca, F.; Martinez-Labarga, C.; de Stefano, G.F.; Fuciarelli, M. GSTT1 and GSTM1 gene polymorphisms in European and African populations. Mol. Biol. Rep. 2011, 38, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Kellen, E.; Hemelt, M.; Broberg, K.; Golka, K.; Kristensen, V.N.; Hung, R.J.; Matullo, G.; Mittal, R.D.; Porru, S.; Povey, A.; et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile 105Val polymorphism and bladder cancer: A HuGE-GSEC review. Am. J. Epidemiol. 2007, 165, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Environmetal Protection Agency Victoria. Future Air Quality in Victoria—Final Report; EPA Victoria: Melbourne, Australia, 2013.
- Filipiak, B.; Zutavern, A.; Koletzko, S.; von Berg, A.; Brockow, I.; Grubl, A.; Berdel, D.; Reinhardt, D.; Bauer, C.P.; Wichmann, H.E.; et al. Solid food introduction in relation to eczema: Results from a four-year prospective birth cohort study. J. Pediatr. 2007, 151, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mihrshahi, S.; Ampon, R.; Webb, K.; Almqvist, C.; Kemp, A.S.; Hector, D.; Marks, G.B. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin. Exp. Allergy 2007, 37, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Lowe, A.J.; Allen, K.J.; Zaloumis, S.; Gurrin, L.C.; Matheson, M.C.; Axelrad, C.; Welsh, L.; Bennett, C.M.; Hopper, J.; et al. Childhood wheeze phenotypes show less than expected growth in FEV1 across adolescence. Am. J. Respir. Crit. Care Med. 2014, 189, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Hosking, C.S.; Bennett, C.M.; Allen, K.J.; Axelrad, C.; Carlin, J.B.; Abramson, M.J.; Dharmage, S.C.; Hill, D.J. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2011, 128, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Carlin, J.B.; Bennett, C.M.; Abramson, M.J.; Hosking, C.S.; Hill, D.J.; Dharmage, S.C. Atopic disease and breast-feeding—Cause or consequence? J. Allergy Clin. Immunol. 2006, 117, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, J.; Tang, Q.; Zhang, S.; Yuan, B.; Li, J.; Wu, D.; Sun, H.; Lu, C.; Xia, Y.; et al. GSTM1 and GSTT1 null polymorphisms and male infertility risk: An updated meta-analysis encompassing 6934 subjects. Sci. Rep. 2013, 3, 3302. [Google Scholar] [CrossRef]
- Environmetal Protection Agency Victoria. Data Product Description, Vicmap Transport; Victorian Government Department of Environment and Primary Industries Melbourne: Melbourne, Australia, 2013.
- Karner, A.A.; Eisinger, D.S.; Niemeier, D.A. Near-roadway air quality: Synthesizing the findings from real-world data. Environ. Sci. Technol. 2010, 44, 5334–5344. [Google Scholar] [CrossRef] [PubMed]
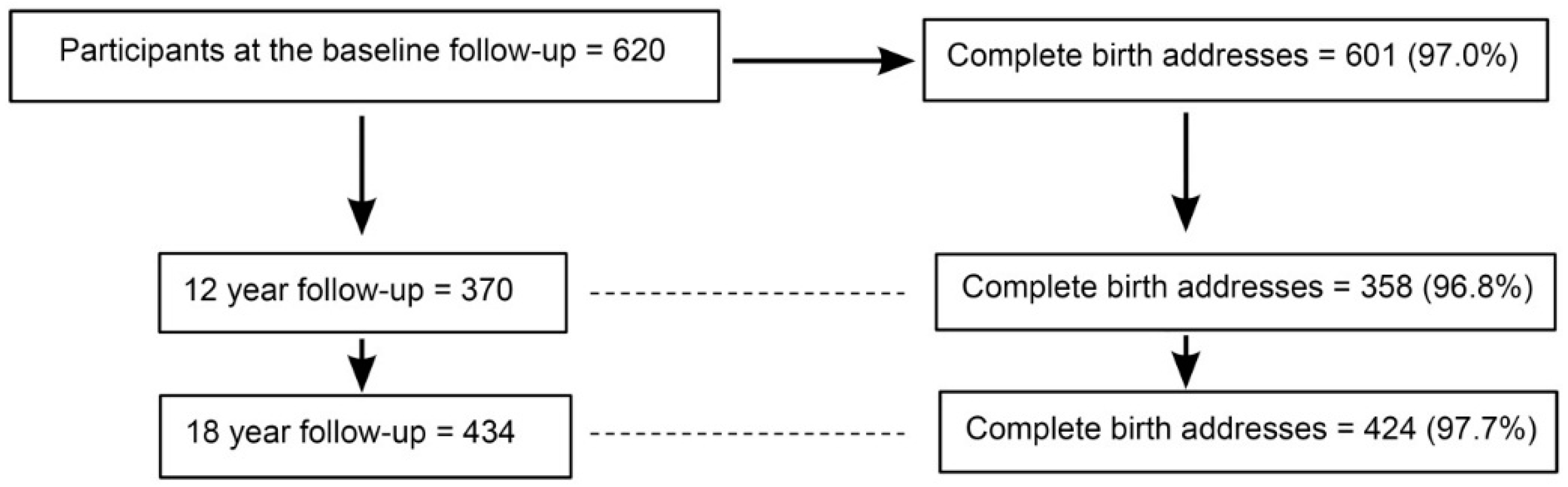


| Characteristics | During the First Year of Life | At 12 Years | At 18 Years | ||||
|---|---|---|---|---|---|---|---|
| Baseline Participants (n = 620) | Participants (n = 370) | Non-Participants (n = 250) | p | Participants (n = 434) | Non-Participants (n = 186) | p | |
| Female sex | 303 (48.9%) | 175 (47.3%) | 128 (51.2%) | 0.34 * | 215 (49.5%) | 88 (47.3%) | 0.61 * |
| Mother’s education | |||||||
| Primary | 50 (8.0%) | 24 (6.5%) | 26 (10.4%) | <0.05 # | 30 (7.0%) | 20 (10.5%) | <0.05 # |
| Secondary | 206 (33.2%) | 113 (30.5%) | 93 (37.2%) | 129 (29.7%) | 77 (41.4%) | ||
| University | 364 (58.7%) | 233 (63.0%) | 131 (52.4%) | 275 (63.3%) | 89 (47.8%) | ||
| Father’s education | |||||||
| Primary | 68 (11.0%) | 42 (11.4%) | 31 (12.5%) | <0.05 # | 24 (5.5%) | 30 (16.1%) | <0.05 # |
| Secondary | 172 (27.9%) | 125 (33.8%) | 65 (26.2%) | 113 (26.0%) | 61 (32.3%) | ||
| University | 377 (61.1%) | 268 (72.4%) | 152 (61.3%) | 233 (53.7%) | 92 (49.5%) | ||
| Parental smoking | 107 (17.5%) | 75 (20.3%) | 47 (19.1%) | 0.71 * | 63 (14.5%) | 44 (24.0%) | <0.05 * |
| Parent asthma | 379 (61.3%) | 233 (63.0%) | 146 (58.4%) | 0.25 * | 261 (60.0%) | 118 (63.4%) | 0.43 * |
| Living ≤150 m from a freeway or highway | 97 (16.0%) | 51 (13.8%) | 46 (18.4%) | 0.12 * | 64 (14.7%) | 33 (17.7%) | 0.35 * |
| Major road length within 150 m buffer | |||||||
| Median/m | 261 | 261 | 262 | – | 262 | 254 | – |
| Inter quartile range/m | 102 | 105 | 92 | – | 110 | 122 | – |
| Gene Polymorphism | Outcome | 12 Years | 18 Years | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p | OR | 95% CI | p | ||
| GSTP1 rs1695 | Current asthma | 1.16 | 0.66, 2.05 | 0.61 | 0.82 | 0.52, 1.29 | 0.39 |
| Current wheeze | 0.82 | 0.49, 1.38 | 0.46 | 0.95 | 0.61, 1.49 | 0.83 | |
| Current hay fever | 1.14 | 0.70, 1.87 | 0.60 | 1.25 | 0.85, 1.86 | 0.26 | |
| GSTT1 | Current asthma | 0.79 | 0.36, 1.73 | 0.55 | 0.80 | 0.43, 1.50 | 0.49 |
| Current wheeze | 1.31 | 0.67, 2.55 | 0.44 | 0.99 | 0.55, 1.78 | 0.97 | |
| Current hay fever | 0.74 | 0.38, 1.46 | 0.39 | 0.85 | 0.51, 1.43 | 0.55 | |
| GSTM1 | Current asthma | 1.00 | 0.57, 1.77 | 0.99 | 1.22 | 0.76, 1.94 | 0.41 |
| Current wheeze | 1.28 | 0.76, 2.17 | 0.35 | 1.46 | 0.92, 2.32 | 0.11 | |
| Current hay fever | 0.92 | 0.56, 1.51 | 0.74 | 1.16 | 0.78, 1.72 | 0.47 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowatte, G.; Lodge, C.J.; Lowe, A.J.; Erbas, B.; Dennekamp, M.; Marks, G.B.; Perret, J.; Hui, J.; Wjst, M.; Gurrin, L.C.; et al. Do Variants in GSTs Modify the Association between Traffic Air Pollution and Asthma in Adolescence? Int. J. Mol. Sci. 2016, 17, 485. https://doi.org/10.3390/ijms17040485
Bowatte G, Lodge CJ, Lowe AJ, Erbas B, Dennekamp M, Marks GB, Perret J, Hui J, Wjst M, Gurrin LC, et al. Do Variants in GSTs Modify the Association between Traffic Air Pollution and Asthma in Adolescence? International Journal of Molecular Sciences. 2016; 17(4):485. https://doi.org/10.3390/ijms17040485
Chicago/Turabian StyleBowatte, Gayan, Caroline J. Lodge, Adrian J. Lowe, Bircan Erbas, Martine Dennekamp, Guy B. Marks, Jennifer Perret, Jennie Hui, Matthias Wjst, Lyle C. Gurrin, and et al. 2016. "Do Variants in GSTs Modify the Association between Traffic Air Pollution and Asthma in Adolescence?" International Journal of Molecular Sciences 17, no. 4: 485. https://doi.org/10.3390/ijms17040485








