The Utilization of the Immune System in Lung Cancer Treatment: Beyond Chemotherapy
Abstract
:1. Introduction
2. Cancer Treatment—Why Should We Go Beyond Chemotherapy?
2.1. Mechanistic Aspects of Tumor Removal by Chemotherapeutic Drugs
2.2. Drawbacks of Chemotherapy
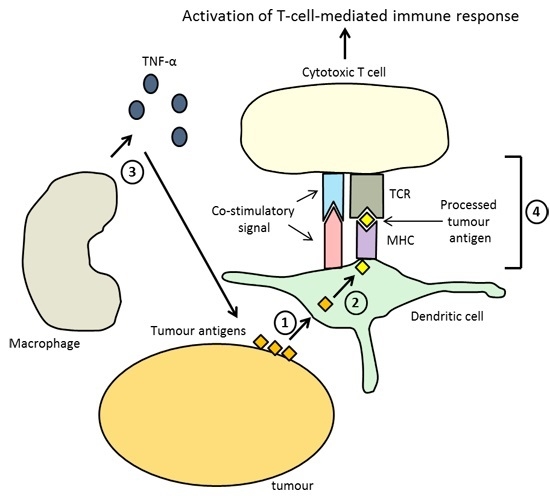
3. Immunotherapy—Harnessing the Immune System to Remove Tumors
4. Cancer Vaccines in Immunotherapy
5. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Ridge, C.A.; McErlean, A.M.; Ginsberg, M.S. Epidemiology of lung cancer. Semin. Interv. Radiol. 2013, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed on 5 January 2016).
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. Inflammatory cytokines in acute renal failure. Kidney Int. Suppl. 2004, 66, S56–S61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Houshyar, R.; Bhosale, P.; Choi, J.I.; Gulati, R.; Lall, C. Chemotherapy induced liver abnormalities: An imaging perspective. Clin. Mol. Hepatol. 2014, 20, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol. Biol. 2010, 596, 467–488. [Google Scholar] [PubMed]
- Bidet, M.; Tomico, A.; Martin, P.; Guizouam, H.; Mollat, P.; Mus-Veteau, I. The Hedgehog receptor patched functions in multidrug transport and chemotherapy resistance. Mol. Cancer Res. 2012, 10, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Turcotte, S.; Rosenberg, S.A. Immunotherapy for metastatic solid cancers. Adv. Surg. 2011, 45, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Eichbaum, C.; Meyer, A.S.; Wang, N.; Bischofs, E.; Steinborn, A.; Bruckner, T.; Brodt, P.; Sohn, C.; Eichbaum, M.H. Breast cancer cell-derived cytokines, macrophages and cell adhesion: Implications for metastasis. Anticancer Res. 2011, 31, 3219–3227. [Google Scholar] [PubMed]
- Jiang, T.; Zhou, C. The past, present and future of immunotherapy against tumor. Transl. Lung Cancer Res. 2015, 4, 253–264. [Google Scholar] [PubMed]
- Yang, S.C.; Owen-Schaub, L.; Mendiguren-Rodriguez, A.; Grimm, E.A.; Hong, W.K.; Roth, J.A. Combination immunotherapy for non-small cell lung cancer. Results with interleukin-2 and tumor necrosis factor-alpha. J. Thorac. Cardiovasc. Surg. 1990, 99, 8–12. [Google Scholar] [PubMed]
- Staveley-O’Carroll, K.; Sotomayor, E.; Montgomery, J.; Borrello, I.; Hwang, L.; Fein, S.; Pardoll, D.; Levitsky, H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA 1998, 95, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Neal, J.; Lu, H.; Cuillerot, J.M.; et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012, 30, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, N.; Hirsch, F.R.; Luft, A.V.; Szczesna, A.; Ciuleanu, T.E.; Dediu, M.; Ramlau, R.; Galiulin, R.K.; Bálint, B.; Losonczy, G.; et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): An open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015, 16, 763–774. [Google Scholar] [CrossRef]
- Kimura, H.; Matsui, Y.; Ishikawa, A.; Nakajima, T.; Yoshino, M.; Sakairi, Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol. Immunother. 2015, 64, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Buti, S.; Camisa, R.; Brighenti, M.; Lazzarelli, S.; Mazza, G.; Passalacqua, R. Gefitinib plus interleukin-2 in advanced non-small cell lung cancer patients previously treated with chemotherapy. Cancers 2014, 6, 2035–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, B.; Li, H.; Yu, J.; Cao, S.; Hao, X.; Ren, X. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol. Immunother. 2013, 62, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Liu, L.; Du, C.; Cao, S.; Yu, J.; Wang, S.E.; Hao, X.; Ren, X.; Li, H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: A phase II clinical study. Cancer. Immunol. Immunother. 2012, 61, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Ramlau, R.; Westeel, V.; Papai, Z.; Madroszyk, A.; Riviere, A.; Koralewski, P.; Breton, J.L.; Stoelben, E.; Braun, D.; et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2B trial. Lancet Oncol. 2011, 12, 1125–1133. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Villadolid, J.; Amin, A. Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl. Lung Cancer Res. 2015, 4, 560–575. [Google Scholar] [PubMed]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Brunsvig, P.F.; Kyte, J.A.; Kersten, C.; Sundstrom, S.; Moller, M.; Nyakas, M.; Hansen, G.L.; Gaudernack, G.; Aamdal, S. Telomerase peptide vaccination in NSCLC: A phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin. Cancer Res. 2011, 17, 6847–6857. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.; Maksymiuk, A.; Goss, G.; Soulieres, D.; Marshall, E.; Cormier, Y.; Ellis, P.M.; Price, A.; Sawhney, R.; Beier, F.; et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J. Cancer Res. Clin. Oncol. 2011, 137, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
| Drug Combination and Their Biological Activities | n | Main Findings | References | |||
|---|---|---|---|---|---|---|
| Clinical Outcome | ChemImm | Chem | ||||
| Immunotherapeutic agent used: Necitumumab; Biological activity: Block downstream signaling of EGFR that facilitates cell proliferation. Chemotherapeutic drug used: (1) Gemcitabine; Biological activity: Prevention of pyrimidine synthesis and hence DNA synthesis. (2) Cisplatin; Biological activity: Induction of DNA alkylation and hence DNA damage. | 1093 | Median OS | 11.5 months (95% CI: 10.4–12.6) | 9.9 months (95% CI: 8.9–11.1) | Thatcher et al., 2015 [16] | |
| Median PFS | 5·7 months (95% CI: 5.6–6.0) | 5·5 months (95% CI: 4.8–5.6) | ||||
| 1-year OS rate | 48% (95% CI: 43–52) | 43% (95% CI: 39–47) | ||||
| 3-month PFS rate | 79% (95% CI: 76–83) | 73% (95% CI: 68–76) | ||||
| Notes on toxicity profile of combined therapy | Frequency of serious adverse events appear higher, yet comparable, in ChemImm group than Chem group (48% vs. 38%). | |||||
| Immunotherapeutic agent used: Activated T cells and dendritic cells; Biological activity: Removal of metastatic tumors through an immune response. Chemotherapeutic drug used: Chemotherapeutic drugs used in the study were not reported. | 101 | 2-year OS rate | 93.4% (95% CI: 80.8–97.8) | 66.0% (95% CI: 50.4–77.7) | Kimura et al., 2015 [17] | |
| 2-year RFS rate | 68.5% (95% CI: 53.2–79.7) | 41.4% (95% CI 27.5–54.7) | ||||
| Notes on toxicity profile of combined therapy | 44% of patients (22 out of 50) experienced at least one adverse event in the form of shivering, chills and fever. | |||||
| Immunotherapeutic agent used: Interleukin-2; Biological activity: Stimulation of the growth and differentiation of T cells, for the generation of an immune response against tumors. Chemotherapeutic drug used: Gefitinib; Biological activity: Inhibition of EGFR signaling and prevent cell proliferation. | 70 | Median OS | 20.1 months (95% CI: 5.1–35.1) | 6.9 months (95% CI: 4.9–8.9) | Bersanelli et al., 2014 [18] | |
| RR | 16.1% (95% CI not reported) | 5.1% (95% CI not reported) | ||||
| DCR | 41.9% (95% CI not reported) | 41.0% (95% CI not reported) | ||||
| Notes on toxicity profile of combined therapy | Grade 2-3 toxicity experienced by patients in the ChemImm group, with fever (46%), fatigue (23%) and arthralgias (13%) being the most common adverse events. | |||||
| Immunotherapeutic agent used: Cytokine-induced killer cells (CIK); Biological activity: exhibition of cytotoxicity against tumor cells. Chemotherapeutic drug used: (1) Navelbine; Biological activity: Induction of microtubule depolymerization, causing the inability of cells to form mitotic spindles and divide. (2) Cisplatin; Biological activity: Induction of DNA alkylation and hence DNA damage. | 122 | 1-year OS rate | 57.2% (95% CI not reported) | 37.3% (95% CI not reported) | Yang et al., 2013 [19] | |
| Objective RR | 18% (95% CI not reported) | 17% (95% CI not reported) | ||||
| DCR | 69% (95% CI not reported) | 49% (95% CI not reported) | ||||
| Notes on toxicity profile of combined therapy | No severe adverse events reported, despite the occurrence of usual side effects as a result of chemotherapy. | |||||
| Immunotherapeutic agent used: Cytokine-induced killer cells (CIK); Biological activity: exhibition of cytotoxicity against tumor cells. Chemotherapeutic drug used: (1) Paclitaxel; Biological activity: Induction of microtubule depolymerization, causing the inability of cells to form mitotic spindles and divide. (2) Navelbine; Biological activity: Induction of microtubule depolymerization, causing the inability of cells to form mitotic spindles and divide. (3) Gemcitabine; Biological activity: Prevention of pyrimidine synthesis and hence DNA synthesis. (4) Cisplatin; Biological activity: Induction of DNA alkylation and hence DNA damage. | 174 | Median OS | 48 months (95% CI: 29–67) | 18 months (95% CI: 11–25) | Li et al., 2012 [20] | |
| Median PFS | 24 months (95% CI: 14–34) | 12 months (95% CI: 8–16) | ||||
| 3-year OS rate | 61% (95% CI: 55–67) | 39% (95% CI: 36–42) | ||||
| 3-year PFS rate | 39% (95% CI: 36–42) | 32% (95% CI: 30–34) | ||||
| Notes on toxicity profile of combined therapy | Toxicity profile of the combined therapy was not reported in the study. | |||||
| Immunotherapeutic agent used: TG-4010; Biological activity: A cancer vaccine against the MUC1 protein, a protein overexpressed in tumors. Chemotherapeutic drug used: (1) Gemcitabine; Biological activity: Prevention of pyrimidine synthesis and hence DNA synthesis. (2) Cisplatin; Biological activity: Induction of DNA alkylation and hence DNA damage. | 148 | Median OS | 10.7 months (95% CI: 8.8–18.0) | 10.3 months (95% CI: 8.3–12.5) | Quoix et al., 2011 [21] | |
| 6-month PFS rate | 43.2% (95% CI: 33.4–53.5) | 35.1% (95% CI: 25.9–45.3) | ||||
| RR | 41.9% (95% CI: 30.5–53.9) | 28.4% (95% CI: 18.5–40.1) | ||||
| Notes on toxicity profile of combined therapy | Occurrence of more than 1 serious adverse events are comparable between ChemImm and Chem groups (52.1% vs. 47.2%). | |||||
| Clinical Outcome | Borghaei et al., 2015 [15] (n = 582) | Brahmer et al., 2015 [22] (n = 272) | ||
|---|---|---|---|---|
| Nivolumab | Docetaxel | Nivolumab | Docetaxel | |
| Median OS | 12.2 months (95% CI: 9.7–15.1) | 9.4 months (95% CI: 8.1–10.7) | 9.2 months (95% CI: 7.3–13.3) | 6.0 months (95% CI: 5.1–7.3) |
| HR of death | 0.73 (96% CI: 0.59–0.89; p = 0.002) | 0.59 (95% CI: 0.44–0.79; p < 0.001) | ||
| 1-year OS rate | 51% (95% CI: 45–56) | 39% (95% CI: 33–45) | 42% (95% CI: 34–50) | 24% (95% CI: 17–31) |
| Median PFS | 2.3 months (95% CI: 2.2–3.3) | 4.2 months (95% CI: 3.5–4.9) | 3.5 months (95% CI: 2.1–4.9) | 2.8 months (95% CI: 2.1–3.5) |
| 1-year PFS rate | 19% (95% CI: 14–23) | 8% (95% CI: 5–12) | 21% (95% CI: 14–28) | 6% (95% CI: 3–12) |
| RR | 19% (95% CI: 15–24) | 12% (95% CI: 9–17) | 20% (95% CI: 14–28) | 9% (95% CI: 5–15) |
| Frequency of adverse events | Events of any grade: 69%; Events of grade 3 or 4: 10% | Events of any grade: 88%; Events of grade 3 or 4: 54% | Events of any grade: 58%; Events of grade 3 or 4: 7% | Events of any grade: 86%; Events of grade 3 or 4: 55% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.W.H.; Tsui, S.K.W.; Law, B.M.H.; So, W.K.W.; Tang, F.W.K.; Wong, C.-L. The Utilization of the Immune System in Lung Cancer Treatment: Beyond Chemotherapy. Int. J. Mol. Sci. 2016, 17, 286. https://doi.org/10.3390/ijms17030286
Chan CWH, Tsui SKW, Law BMH, So WKW, Tang FWK, Wong C-L. The Utilization of the Immune System in Lung Cancer Treatment: Beyond Chemotherapy. International Journal of Molecular Sciences. 2016; 17(3):286. https://doi.org/10.3390/ijms17030286
Chicago/Turabian StyleChan, Carmen W. H., Stephen K. W. Tsui, Bernard M. H. Law, Winnie K. W. So, Fiona W. K. Tang, and Cho-Lee Wong. 2016. "The Utilization of the Immune System in Lung Cancer Treatment: Beyond Chemotherapy" International Journal of Molecular Sciences 17, no. 3: 286. https://doi.org/10.3390/ijms17030286