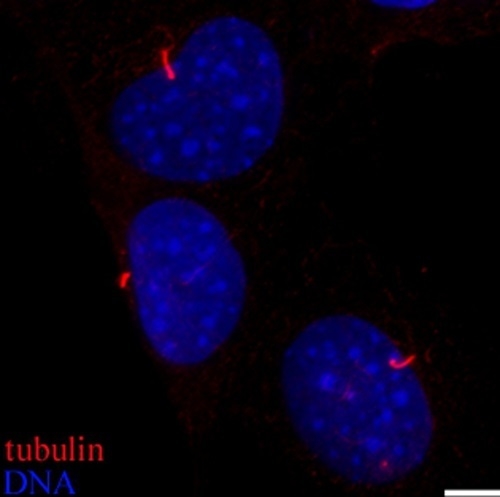
Biological and Chemical Removal of Primary Cilia Affects Mechanical Activation of Chondrogenesis Markers in Chondroprogenitors and Hypertrophic Chondrocytes
Abstract
:1. Introduction
2. Results
2.1. Disrupting Primary Cilia Structure Inhibits Cyclic Loading-Induced Mechanosensitive Genes in ATDC5 Chondroprogenitor Cells
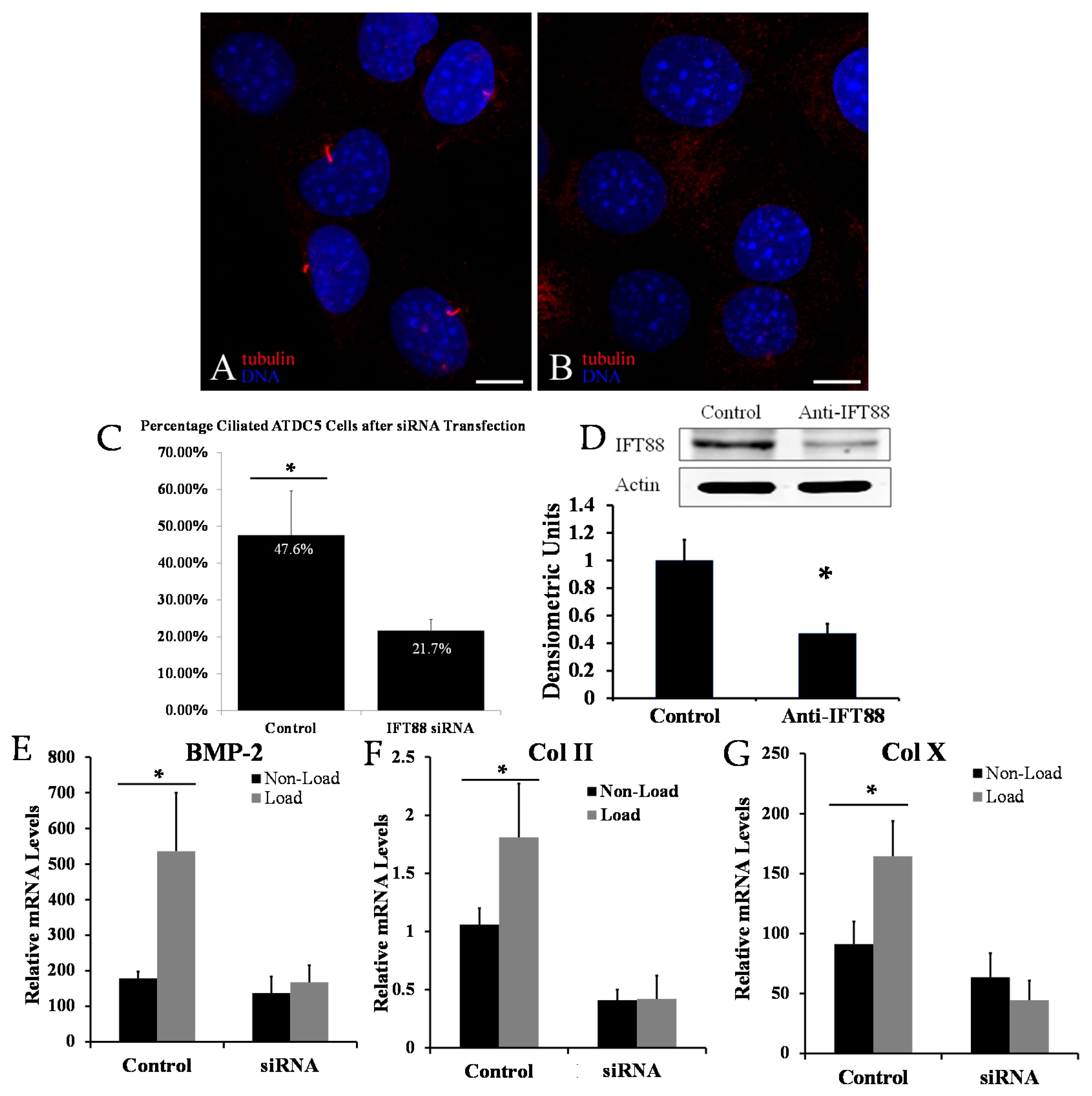
2.2. Biological Reduction of the Percentage of Ciliated Chondrocytes Decreased but Did Not Abolish Cyclic Loading Stimulation of Chondrocyte Hypertrophy
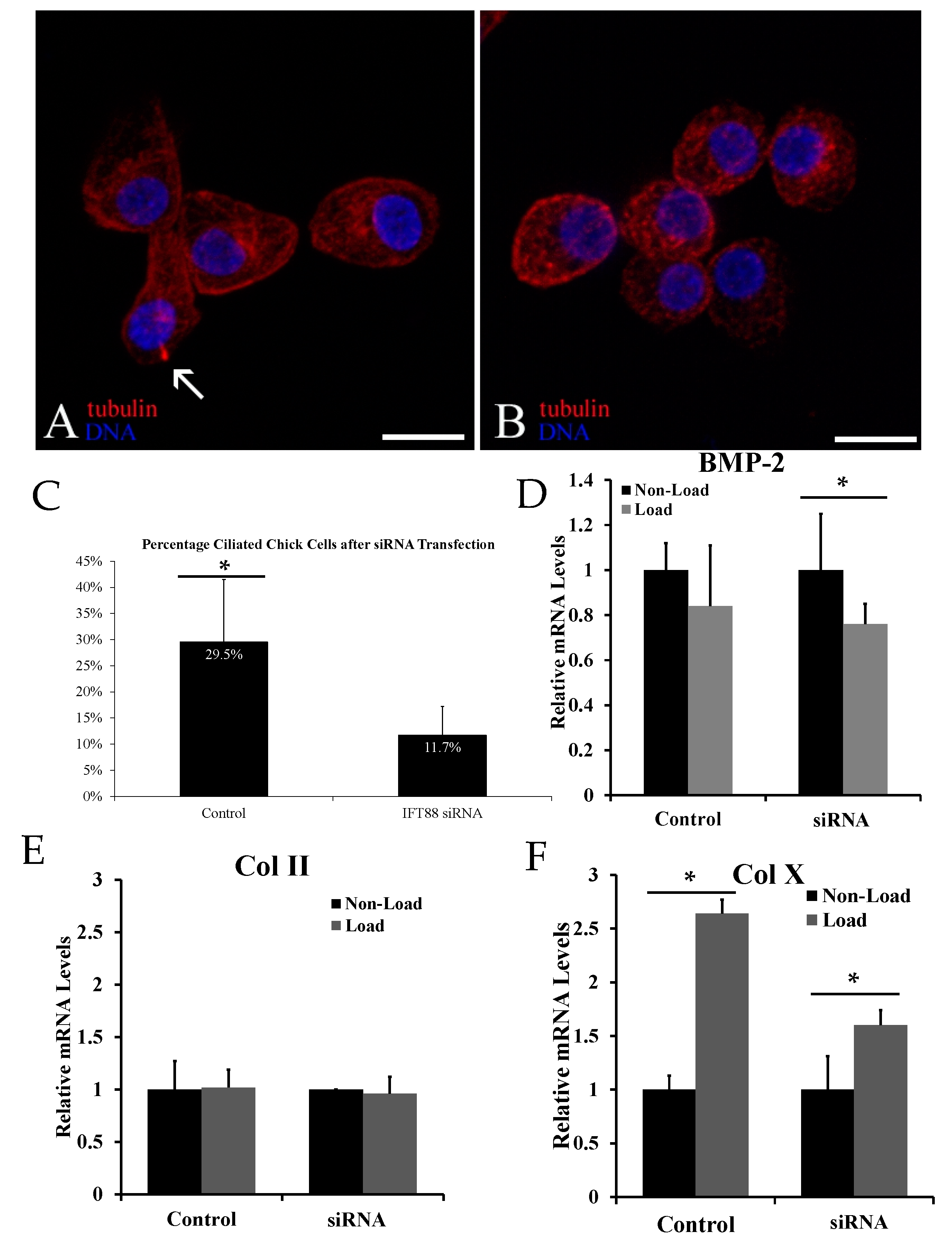
2.3. Chemical Removal of Primary Cilia Inhibits Cyclic Loading-Induced Type X Collagen (Col X) mRNA in Hypertrophic Chondrocytes
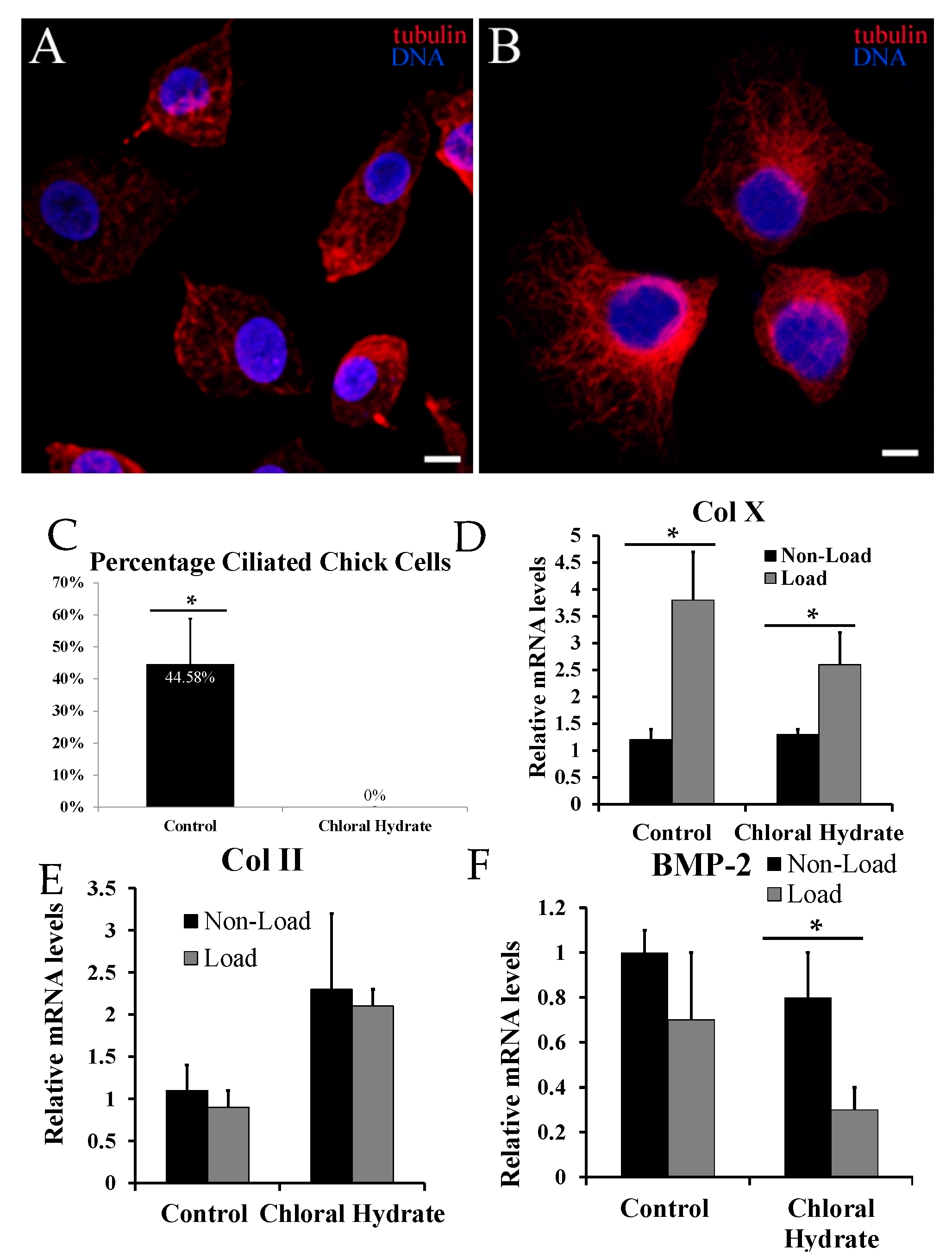
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Mechanical Stimulation
4.2. Chemical Abrogation of Primary Cilia
4.3. Transient Transfection
4.4. Immunohistochemistry
4.5. Western Blotting
4.6. Real Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carter, D.R.; Beaupré, G.S.; Wong, M.; Smith, R.L.; Andriacchi, T.P.; Schurman, D.J. The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. 2004, 427, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K.R. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 2003, 12, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.M.D.; Anderson, C.T.; Tummala, P.; Kwon, R.Y.; Johnston, T.R.; Stearns, T.; Jacobs, C.R. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 13325–13330. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Smith, U.M.; Logan, C.V.; Johnson, C.A. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J. Med. Genet. 2008, 45, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Witman, G.B. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003, 15, 105–110. [Google Scholar] [CrossRef]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.R.; Yoder, B.K. An incredible decade for the primary cilium: A look at a once-forgotten organelle. Am. J. Physiol. Ren. Physiol. 2005, 289, F1159–F1169. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K.R. A physiological view of the primary cilium. Annu. Rev. Physiol. 2005, 67, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Badano, J.L.; Mitsuma, N.; Beales, P.L.; Katsanis, N. The ciliopathies: An emerging class of human genetic disorders. Annu. Rev. Genom. Hum. Genet. 2006, 7, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Hierck, B.P.; van der Heiden, K.; Alkemade, F.E.; van de Pas, S.; van Thienen, J.V.; Groenendijk, B.C.; Bax, W.H.; van der Laarse, A.; Deruiter, M.C.; Horrevoets, A.J.; et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 2008, 237, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Veland, I.R.; Awan, A.; Pedersen, L.B.; Yoder, B.K.; Christensen, S.T. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron. Physiol. 2009, 111, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Berbari, N.F.; O’Connor, A.K.; Haycraft, C.J.; Yoder, B.K. The primary cilium as a complex signaling center. Curr. Biol. 2009, 19, R526–R535. [Google Scholar] [CrossRef] [PubMed]
- Haycraft, C.J.; Serra, R. Cilia involvement in patterning and maintenance of the skeleton. Curr. Top. Dev. Biol. 2008, 85, 303–332. [Google Scholar] [PubMed]
- Haycraft, C.J.; Zhang, Q.; Song, B.; Jackson, W.S.; Detloff, P.J.; Serra, R.; Yoder, B.K. Intraflagellar transport is essential for endochondral bone formation. Development 2007, 134, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Brailov, I.; Bancila, M.; Brisorgueil, M.J.; Miquel, M.C.; Hamon, M.; Vergé, D. Localization of 5-HT6 receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000, 872, 271–275. [Google Scholar] [CrossRef]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, H.W.; Buelens, L.A.; Vortkamp, A. Hedgehog signaling in skeletal development. Birth Defects Res. C Embryo Today 2006, 78, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Espinha, L.C.; Hoey, D.A.; Fernandes, P.R.; Rodrigues, H.C.; Jacobs, C.R. Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskeleton 2014, 71, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Haycraft, C.J.; Seo, H.S.; Yoder, B.K.; Serra, R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007, 305, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Serra, R. Role of intraflagellar transport and primary cilia in skeletal development. Anat. Rec. 2008, 291, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.K.; Tousson, A.; Millican, L.; Wu, J.H.; Bugg, C.E., Jr.; Schafer, J.A.; Balkovetz, D.F. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Ren. Physiol. 2002, 282, F541–F552. [Google Scholar] [CrossRef] [PubMed]
- Temiyasathit, S.; Tang, W.J.; Leucht, P.; Anderson, C.T.; Monica, S.D.; Castillo, A.B.; Helms, J.A.; Stearns, T.; Jacobs, C.R. Mechanosensing by the primary cilium: Deletion of Kif3A reduces bone formation due to loading. PLoS ONE 2012, 7, e33368. [Google Scholar]
- Koyama, E.; Young, B.; Nagayama, M.; Shibukawa, Y.; Enomoto-Iwamoto, M.; Iwamoto, M.; Maeda, Y.; Lanske, B.; Song, B.; Serra, R.; et al. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Dev. Camb. Engl. 2007, 134, 2159–2169. [Google Scholar] [CrossRef]
- Yang, X.; Vezeridis, P.S.; Nicholas, B.; Crisco, J.J.; Moore, D.C.; Chen, Q. Differential expression of type X collagen in a mechanically active 3D chondrocyte culture system: A quantitative study. J. Orthop. 2006, 1, 15. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Schatten, H.; Mitchell, K.D.; Crosser, M.; Taylor, M. Chloral hydrate alters the organization of the ciliary basal apparatus and cell organelles in sea urchin embryos. Cell Tissue Res. 1998, 293, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wilsman, N.J.; Farnum, C.E.; Reed-Aksamit, D.K. Incidence and morphology of equine and murine chondrocytic cilia. Anat. Rec. 1980, 197, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, D.N.; Wang, A.M.; Strugnell, G.E. Expression of primary cilia in mammalian cells. Cell Biol. Int. 1996, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.A.; Zhang, Z.J.; Ross, J.M. The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J. Anat. 2001, 199, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, O.V.; Pugacheva, E.N.; Golemis, E.A. Aurora A kinase activity influences calcium signaling in kidney cells. J. Cell Biol. 2011, 193, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- De Andrea, C.E.; Wiweger, M.; Prins, F.; Bovée, J.V.; Romeo, S.; Hogendoorn, P.C. Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab. Investig. J. Tech. Methods Pathol. 2010, 90, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, M.-G.; Lenox, M.; Farnum, C. Analysis of the orientation of primary cilia in growth plate cartilage: A mathematical method based on multiphoton microscopical images. J. Struct. Biol. 2007, 158, 293–306. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, S.R.; Cluett, E.C.; Jensen, C.G.; Poole, C.A. Primary cilia in osteoarthritic chondrocytes: From chondrons to clusters. Dev. Dyn. 2008, 237, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Hoey, D.A.; Tormey, S.; Ramcharan, S.; Ramcharan, S.; O’Brien, F.J.; Jacobs, C.R. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells Dayt. Ohio 2012, 30, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Jacobs, C.R. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-J.; Yang, X.; Wei, L.; Chen, Q. miR-365: A mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011, 25, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deren, M.E.; Yang, X.; Guan, Y.; Chen, Q. Biological and Chemical Removal of Primary Cilia Affects Mechanical Activation of Chondrogenesis Markers in Chondroprogenitors and Hypertrophic Chondrocytes. Int. J. Mol. Sci. 2016, 17, 188. https://doi.org/10.3390/ijms17020188
Deren ME, Yang X, Guan Y, Chen Q. Biological and Chemical Removal of Primary Cilia Affects Mechanical Activation of Chondrogenesis Markers in Chondroprogenitors and Hypertrophic Chondrocytes. International Journal of Molecular Sciences. 2016; 17(2):188. https://doi.org/10.3390/ijms17020188
Chicago/Turabian StyleDeren, Matthew E., Xu Yang, Yingjie Guan, and Qian Chen. 2016. "Biological and Chemical Removal of Primary Cilia Affects Mechanical Activation of Chondrogenesis Markers in Chondroprogenitors and Hypertrophic Chondrocytes" International Journal of Molecular Sciences 17, no. 2: 188. https://doi.org/10.3390/ijms17020188