Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Triterpenoids 1–3
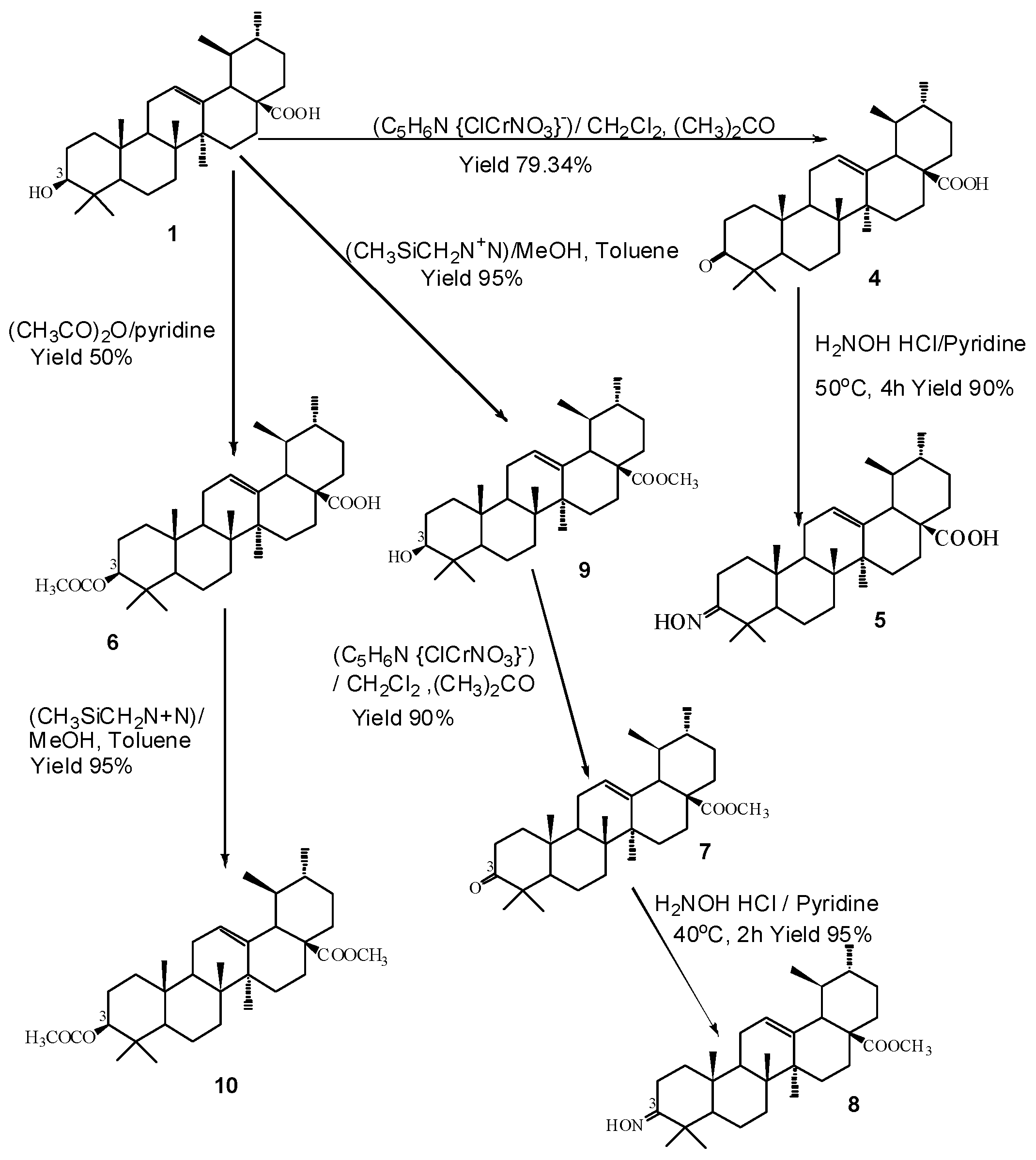
2.2. Synthesis
2.3. Hyaluronidase Inhibitory Activity
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substitutional Pattern | a IC50 | ||||||||||||
| Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | |
| 1 | OH | CH3 | COOH | CH3 | H | H | H | H | H | H | H | H | 103.18 ± 1.70 ** |
| 2 | OH | CH2OH | COOH | CH3 | H | H | H | H | OH | H | H | H | 286.95 ± 10.28 |
| 3 | OAc | CH3 | COOH | H | CH3 | H | H | H | H | H | H | H | 1466.5 ± 2.37 |
| 4 | =O | CH3 | COOH | CH3 | H | H | H | H | H | H | H | H | 162.83 ± 6.37 * |
| 5 | NOH | CH3 | COOH | CH3 | H | H | H | H | H | H | H | H | 190.94 ± 0.01 * |
| 6 | OAc | CH3 | COOH | CH3 | H | H | H | H | H | H | H | H | 136.92 ± 0.04 * |
| 7 | =O | CH3 | COOCH3 | CH3 | H | H | H | H | H | H | H | H | 1184.15 ± 6.63 |
| 8 | NOH | CH3 | COOCH3 | CH3 | H | H | H | H | H | H | H | H | 275.68 ± 1.42 |
| 9 | OH | CH3 | COOCH3 | CH3 | H | H | H | H | H | H | H | H | 182.51 ± 0.84 * |
| 10 | OAc | CH3 | COOCH3 | CH3 | H | H | H | H | H | H | H | H | 812.93 ± 10.29 |
| 11 | OH | CH3 | CH3 | H | COOCH3 | H | H | =O | H | H | H | H | 1750.91 ± 2.38 |
| 12 | OH | CH2OH | CH3 | CH3 | H | H | H | H | H | H | H | H | 227.97 ± 2.81 |
| 13 | OH | CH3 | CH2OH | H | CH3 | H | H | H | H | H | H | H | 206.21 ± 2.32 |
| 14 | OH | CH3 | CH3 | CH3 | H | H | H | H | H | H | H | H | 211.44 ± 3.16 * |
| 15 | OH | CH3 | COOH | H | CH3 | OH | H | H | H | H | H | H | 140.91 ± 6.71 |
| 16 | OH | CH3 | COOCH3 | H | CH3 | H | H | H | H | H | H | H | 84.52 ± 0.01 ** |
| 17 | S1 | CH2OH | COOH | H | CH3 | H | H | H | H | H | H | H | 842.54 ± 0.11 |
| 18 | OH | CH3 | CH3 | H | CH3 | H | H | H | H | H | H | H | 215.66 ± 4.27 * |
| 19 | OH | CH2OH | COOH | CH3 | H | H | OH | H | H | H | H | H | 115.96 ± 0.47 * |
| 20 | OH | CH3 | COOH | H | CH3 | H | H | H | H | H | H | H | 227.97 ± 5.99 |
| 21 | OH | CH3 | CH3 | H | COOH | H | H | =O | H | H | H | H | 146.18 ± 2.67 * |
| 22 | S2 | CH3 | CH3 | H | COOH | H | H | =O | H | H | H | H | 56.33 ± 0.01 ** |
| 23 | OH | COOH | CH3 | H | CH3 | H | H | H | H | H | H | H | 1482.56 ± 0.70 |
| 24 | OH | CH2OH | COOH | H | CH3 | H | H | H | H | H | H | H | 230.00 ± 2.17 |
| 25 | O-glucoside | CH3 | COOH | H | CH3 | OH | H | H | H | H | H | H | NA |
| 26 | OH | COOH | CH3 | CH3 | H | H | H | H | H | H | H | H | NA |
| 27 | OH | S3 | CH2OH | CH3 | H | H | OH | H | H | H | H | H | NA |
| 28 | OH | CH2OH | CH2OH | H | CH3 | OH | H | H | H | H | OH | OH | NA |
| 29 | OH | CH2OH | COOS4 | CH3 | H | H | OH | H | H | OH | H | H | NA |
| 30 | – | – | – | – | – | – | – | – | – | – | – | – | NA |
| Apigenin | – | – | – | – | – | – | – | – | – | – | – | – | 214.74 |
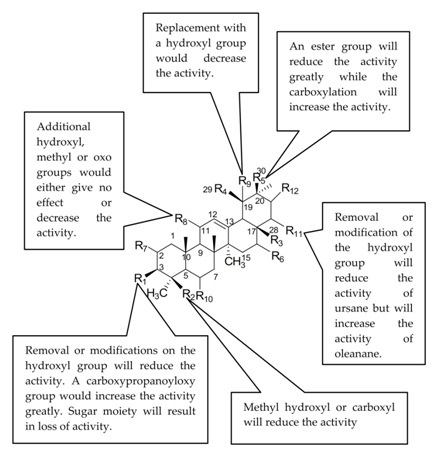
2.4. Structure Activity Relationship (SAR) of Ursolic Acid 1 and Its Analogues

2.5. QSAR Model and Its Interpretation
| Descriptor Number | Correlation Coefficient (R2) | Fisher Criteria (F) | Standard Deviation (s2) |
|---|---|---|---|
| 2 | 0.6774 | 21.13 | 0.0633 |
| 3 | 0.7992 | 19.90 | 0.0373 |
| 4 | 0.8579 | 16.80 | 0.026 |
| 5 | 0.8821 | 19.45 | 0.0167 |
| 6 | 0.9303 | 26.71 | 0.0123 |
| 7 | 0.9591 | 36.85 | 0.0065 |
| 8 | 0.9866 | 92.38 | 0.0037 |
| 9 | 0.9934 | 150.00 | 0.0021 |
| 10 | 0.9966 | 237.73 | 0.0010 |
| 11 | 0.9994 | 1020.35 | 0.0005 |
| 12 | 0.9998 | 3234.68 | 0.0002 |
| Descriptor | Symbol | t-Test | X | ΔX |
|---|---|---|---|---|
| Min partial charge for a C atom (Zefirov’s PC) | 5.8487 | 4.8595 × 102 | 8.3087 × 10 | |
| Min valency of an H atom | 7.1708 | 1.9708 × 10 | 2.7483 × 100 | |
| Max bond order of a C atom | 4.3256 | 7.0647 × 100 | 1.6332 × 100 | |
| Molecular surface area | 2.4697 | 4.0917 × 10−3 | 1.6568 × 10−3 | |
| Intercept | −4.4980 | −1.6475 × 10 | 3.6628 × 100 |
| Test Set Compound | Experimental Log IC50 | Predicted Log IC50 | Differences | Percentage Differences |
|---|---|---|---|---|
| 6 | 2.01 | 1.8 | 0.2 | 8.5 |
| 8 | 2.3 | 2.1 | 0.18 | 7.5 |
| 11 | 2.9 | 2.9 | 0.05 | 1.6 |
| 19 | 3.2 | 2.5 | 0.6 | 3.9 |
2.6. Design of a New Potential Pentacylic Triterpene

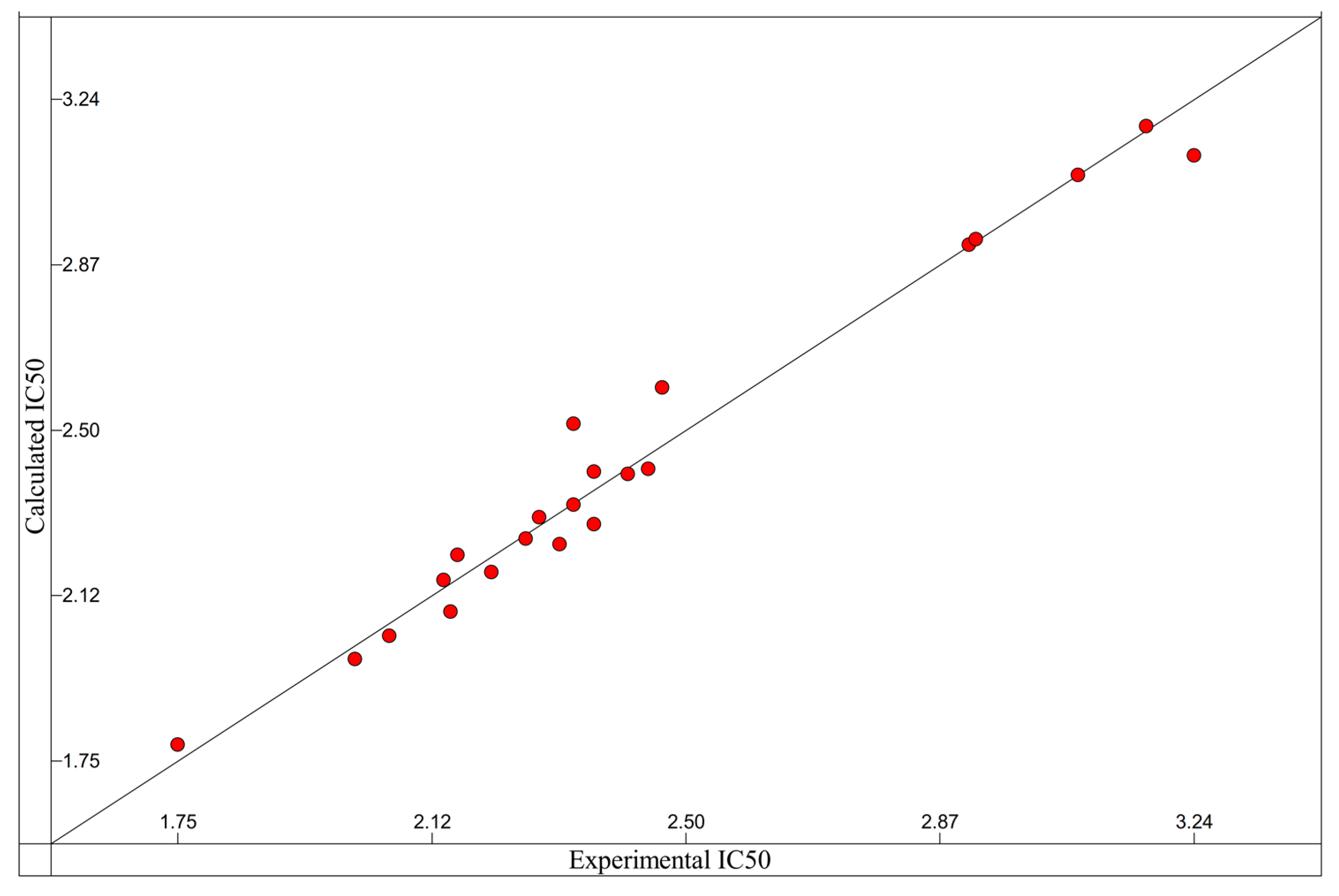
2.7. Method Validation of the Proposed Model
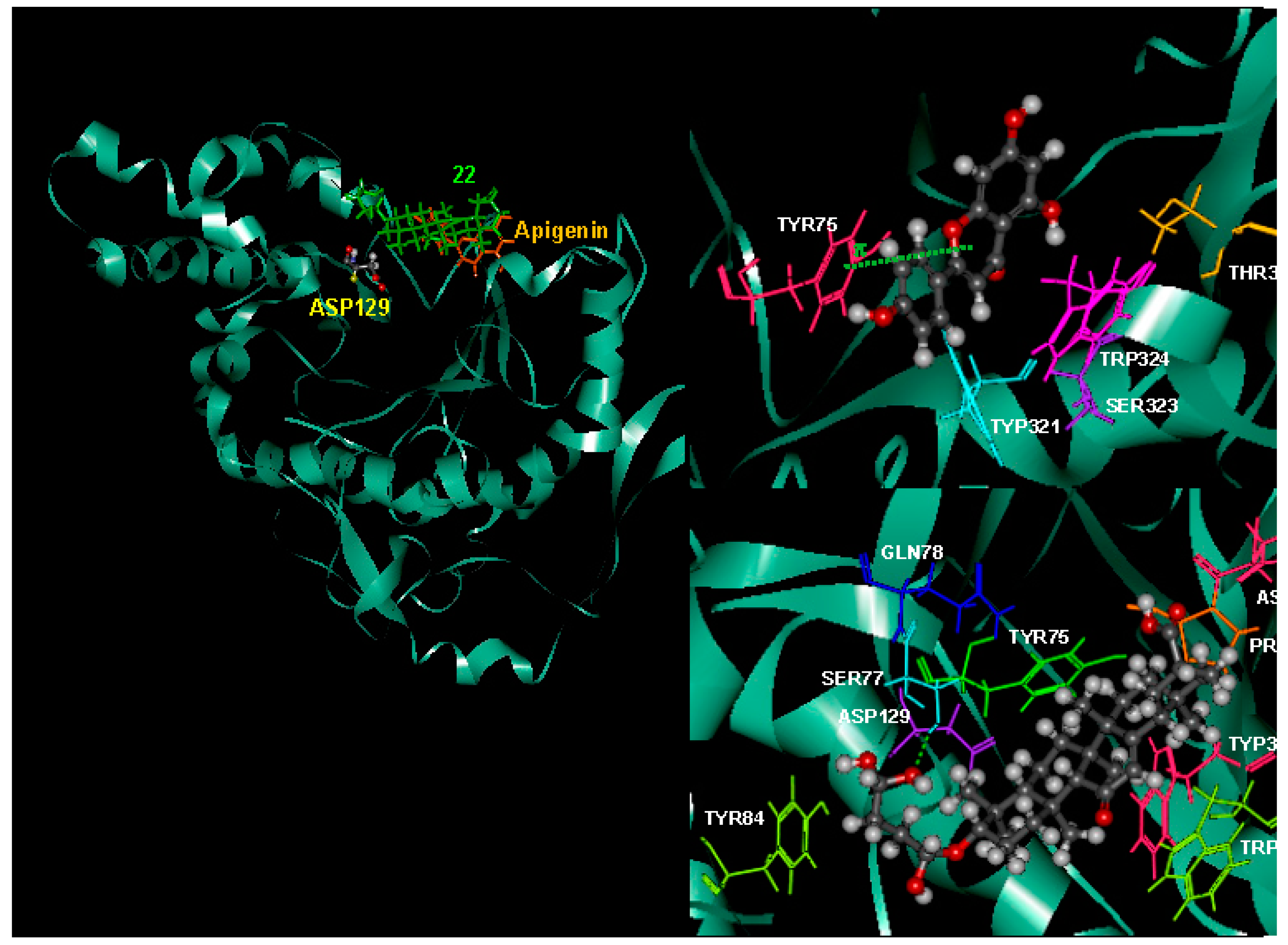
2.8. Possible Interactions from an in Silico Molecular Docking Study
| Residue | Interaction Energy (IE) | VDW | Electrostatic | Residue | Interaction Energy (IE) | VDW | Electrostatic |
|---|---|---|---|---|---|---|---|
| Apigenin | 22 | ||||||
| ALA38 | −1.03 | −0.64 | −0.39 | ASN39 | −1.81 | −0.77 | −1.04 |
| ASN39 | −1.57 | −2.05 | 0.48 | ASN61 | −5.72 | −0.56 | −5.16 |
| PRO62 | −2.93 | −2.23 | −0.71 | PRO62 | −4.00 | −2.89 | −1.11 |
| GLY63 | −1.34 | −1.94 | 0.60 | GLY63 | −2.94 | −1.94 | −1.00 |
| TYR75 | −4.22 | −4.04 | −0.18 | TYR75 | −15.15 | −6.08 | −9.07 |
| SER77 | −0.69 | −0.37 | −0.32 | SER76 | −3.83 | −2.84 | −1.00 |
| TRP321 | −4.33 | −1.51 | −2.82 | SER77 | −9.99 | −2.00 | −7.99 |
| VAL322 | 0.60 | −0.44 | 1.04 | GLN78 | −4.95 | −0.52 | −4.43 |
| SER323 | −4.57 | −0.69 | −3.88 | TYR82 | −2.31 | −0.72 | −1.59 |
| TRP324 | −7.40 | −3.80 | −3.60 | TYR84 | −5.56 | −3.08 | −2.48 |
| THR327 | −4.61 | −0.53 | −4.07 | ASP129 | −10.36 | −0.86 | −9.50 |
| GLU131 | −2.55 | −2.34 | −0.21 | ||||
| TRP321 | −8.18 | −1.51 | −6.67 | ||||
| TRP324 | −11.45 | −5.56 | −5.89 | ||||
| BE in 4 Å | −32.09 | −18.24 | −13.85 | BE in 4 Å | −88.81 | −31.66 | −57.15 |
| Total IE | −140.91 | −23.62 | −117.28 | Total IE | −191.07 | −39.23 | −151.84 |
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Synthesis of Ursolic Acid Analogues
3.4.1. 3-Oxo-urs-12-en-28-oic Acid (4)
3.4.2. 3-Hydroxyimino-urs-12-en-28-oic Acid (5)
3.4.3. 3-Acetyl-urs-12-en-28-oic Acid (6)
3.4.4. 3-Hydroxy-urs-12-en-28-oic Acid Methyl Ester (9)
3.4.5. 3-Oxo-urs-12-en-28-oic Acid Methyl Ester (7)
3.4.6. 3-Hydroxyimino-urs-12-en-28-oic Acid Methyl Ester (8)
3.4.7. 3-Acetyl-urs-12-en-28-oic Acid Methyl Ester (10)
3.5. Hyaluronidase Inhibitory Assay
3.6. Construction of a QSAR Model
3.6.1. Data Set
3.6.2. Descriptors
3.7. Molecular Docking Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Boyer, P.D. The Enzymes, 3rd ed.; Academic Press: London, UK; New York, NY, USA, 1971; Volume 5. [Google Scholar]
- El-Safory, N.S.; Fazary, A.E.; Lee, C.K. Hyaluronidases, a group of glycosidases: Current and future perspectives. Carbohydr. Polym. 2010, 81, 165–181. [Google Scholar] [CrossRef]
- Vincent, J.C.; Lenormand, H. How hyaluronan-protein complexes modulate the hyaluronidase activity: The model. Biophys. Chem. 2009, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, F.; Zhu, X.; Su, Y.; Ling, P. Expression of a novel hyaluronidase from Streptococcus zooepidemicus in Escherichia coli and its application for the preparation of HA oligosaccharides. Carbohydr. Polym. 2009, 77, 254–260. [Google Scholar] [CrossRef]
- Lemmens, R.H.M.J.; Bunyapraphatsara, N. Plant Resources of South-East Asia 12 (3), Medicinal and Poisonous Plants 3; Backhuys: Leiden, The Netherlands, 2003. [Google Scholar]
- Burkill, I.H.; Birtwistle, W.; Foxworthy, F.W.; Scrivenor, J.B.; Watson, J.G. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and cooperatives: Kuala Lumpur, Malaysia, 1966. [Google Scholar]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Topçu, G.; Ertaş, A.; Kolak, U.; Öztürk, M.; Ulubelen, A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. ARKIVOC 2007, 7, 195–208. [Google Scholar]
- Zhu, Y.M.; Shen, J.K.; Wang, H.K.; Cosentino, L.M.; Lee, K.H. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 3115–3118. [Google Scholar] [CrossRef]
- Ma, C.; Nakamura, N.; Miyashiro, H.; Hattori, M.; Shimotohno, K. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem. Pharm. Bull. 1999, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Sailer, E.R. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Šarek, J.; Klinot, J.; Džubák, P.; Klinotová, E.; Nosková, V.; Křeček, V.; Kořínková, G.; Thomson, J.O.; Janošt'áková, A.; Wang, S.; et al. New lupane derived compounds with pro-apoptotic activity in cancer cells: Synthesis and structure-activity relationships. J. Med. Chem. 2003, 46, 5402–5415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Pezzuto, J.M.; Pisha, E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg. Med. Chem. Lett. 1998, 8, 1707–1712. [Google Scholar] [CrossRef]
- Lee, K.H.; Lin, Y.M.; Wu, T.S.; Zhang, D.C.; Yamagishi, T.; Hayashi, T.; Hall, I.H.; Chang, J.J.; Wu, R.Y.; Yang, T.H. The cytotoxic principles of Prunella vulgaris, Psychotria serpens, and Hyptis capitata: Ursolic acid and related derivatives1. Planta Med. 1988, 54, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Lu, C.M.; Cheng, M.K.; Gan, K.H.; Won, S.J. The cytotoxic principles of Solanum incanum. J. Nat. Prod. 1990, 53, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Mayaux, J.F.; Bousseau, A.; Pauwels, R.; Huet, T.; Henin, Y.; Dereu, N.; Evers, M.; Soler, F.; Poujade, C.; de Clercq, E.; et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.; Poujade, C.; Soler, F.; Ribeill, Y.; James, C.; Lelièvre, Y.; Gueguen, J.C.; Reisdorf, D.; Morize, I.; Pauwels, R.; et al. Betulinic acid derivatives: A new class of human immunodeficiency virus type 1 specific inhibitors with a new mode of action. J. Med. Chem. 1996, 39, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nagao, T.; Hashimoto, A.; Ikeshiro, Y.; Okabe, H.; Cosentino, L.M.; Lee, K.H. Anti-aids agents 38. Anti-hiv activity of 3-O-acyl ursolic acid derivatives1. J. Nat. Prod. 2000, 63, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Kalani, K.; Yadav, D.; Khan, F.; Srivastava, S.; Suri, N. Pharmacophore, qsar, and adme based semisynthesis and in vitro evaluation of ursolic acid analogs for anticancer activity. J. Mol. Model. 2012, 18, 3389–3413. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, L.; Li, L.; Liu, J.; Li, H.; Zhang, L.; Chen, L.; Cheng, K.; Zheng, M.; Wen, X.; et al. Identification of pentacyclic triterpenes derivatives as potent inhibitors against glycogen phosphorylase based on 3D-QSAR studies. Eur. J. Med. Chem. 2011, 46, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Khan, F.; Bawankule, D.U.; Yadav, D.K.; Srivastava, S.K. Qsar, docking and in vivo studies for immunomodulatory activity of isolated triterpenoids from Eucalyptus tereticornis and Gentiana kurroo. Eur. J. Pharm. Sci. 2012, 47, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Xu, X.; Zhou, L. QSAR model of triterpene derivatives as potent anti-HIV agents. Mol. Simul. 2008, 34, 651–659. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Single-step preparative separation of barbinervic acid and its epimer (rotungenic acid), along with two other pentacyclic triterpene acids from the leaves of diospyros kaki using HSCCC. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 815–826. [Google Scholar] [CrossRef]
- Ling, S.K.; Tanaka, T.; Kouno, I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by & β-glucosidase in the presence of amino acids. Biol. Pharm. Bull. 2003, 26, 352–356. [Google Scholar] [PubMed]
- Katritzky, A.R.; Pacureanu, L.M.; Slavov, S.; Dobchev, D.A.; Karelson, M. QSAR study of antiplatelet agents. Bioorg. Med. Chem. 2006, 14, 7490–7500. [Google Scholar] [CrossRef] [PubMed]
- Topliss, J.G.; Costello, R.J. Chance correlations in structure-activity studies using multiple regression analysis. J. Med. Chem. 1972, 15, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Karelson, M. Molecular Descriptors in QSAR/QSPR; John Wiley and sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Hawkins, D.M.; Basak, S.C.; Mills, D. Assessing model fit by cross-validation. J. Chem. Inf. Comput. Sci. 2003, 43, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Singh, S.; Pal, M. In silico ligand based design of indolylpiperidinyl derivatives as novel histamine H(1) receptor antagonists. Drug Discov. Ther. 2012, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.T.; Roy, K. Exploring molecular shape analysis of styrylquinoline derivatives as HIV-1 integrase inhibitors. Eur. J. Med. Chem. 2008, 43, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Guha, R.; Jurs, P.C. Determining the validity of a QSAR model—A classification approach. J. Chem. Inf. Model. 2005, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.B.; Freire, R.O.; Simas, A.M.; Stewart, J.J. RM1: A reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br, and I. J. Comput. Chem. 2006, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Mopac Manual; Seiler Research Laboratory United States Air Force Academy: Colorado Springs, CO, USA, 1990. [Google Scholar]
- Awang, K.; Abdullah, N.H.; Thomas, N.F.; Ng, S.W. Methyl 3-dehydroxy-3-oxoursolate. Acta Crystallogr. Sect. E 2009. [Google Scholar] [CrossRef] [PubMed]
- Kartizky, A.R.; Lobanov, V.S.; Karelson, M. Comprehensive Descriptors for Structural and Statistical Analysis, 2nd ed.; University of Florida: Gainesville, FL, USA, 1994. [Google Scholar]
- Zefirov, N.S.; Kirpichenok, M.A.; Izmailov, F.F.; Trofimoz, M.I. Scheme for the calculation of the electronegativities of atoms in a molecule in the framework of sanderson’s principle. Dokl Akad. Nauk SSSR 1987, 296, 883–887. [Google Scholar]
- Narwal, M.; Haikarainen, T.; Fallarero, A.; Vuorela, P.M.; Lehtiö, L. Screening and structural analysis of flavones inhibiting tankyrases. J. Med. Chem. 2013, 56, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org/pdb/home/home.do (accessed on 21 December 2015).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef] [PubMed]
- Momany, F.A.; Rone, R. Validation of the general purpose QUANTA®3.2/CHARMm® force field. J. Comput. Chem. 1992, 13, 888–900. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, N.H.; Thomas, N.F.; Sivasothy, Y.; Lee, V.S.; Liew, S.Y.; Noorbatcha, I.A.; Awang, K. Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study. Int. J. Mol. Sci. 2016, 17, 143. https://doi.org/10.3390/ijms17020143
Abdullah NH, Thomas NF, Sivasothy Y, Lee VS, Liew SY, Noorbatcha IA, Awang K. Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study. International Journal of Molecular Sciences. 2016; 17(2):143. https://doi.org/10.3390/ijms17020143
Chicago/Turabian StyleAbdullah, Nor Hayati, Noel Francis Thomas, Yasodha Sivasothy, Vannajan Sanghiran Lee, Sook Yee Liew, Ibrahim Ali Noorbatcha, and Khalijah Awang. 2016. "Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study" International Journal of Molecular Sciences 17, no. 2: 143. https://doi.org/10.3390/ijms17020143
APA StyleAbdullah, N. H., Thomas, N. F., Sivasothy, Y., Lee, V. S., Liew, S. Y., Noorbatcha, I. A., & Awang, K. (2016). Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study. International Journal of Molecular Sciences, 17(2), 143. https://doi.org/10.3390/ijms17020143