Keratinocyte Growth Factor Combined with a Sodium Hyaluronate Gel Inhibits Postoperative Intra-Abdominal Adhesions
Abstract
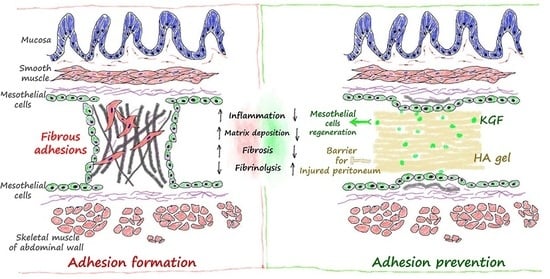
:1. Introduction
2. Results
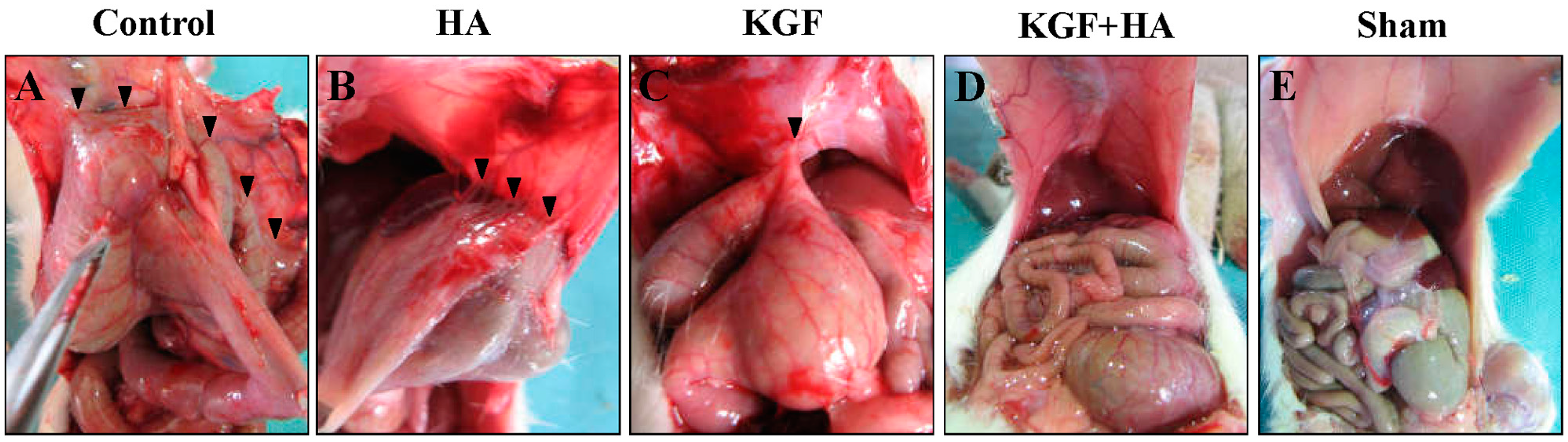
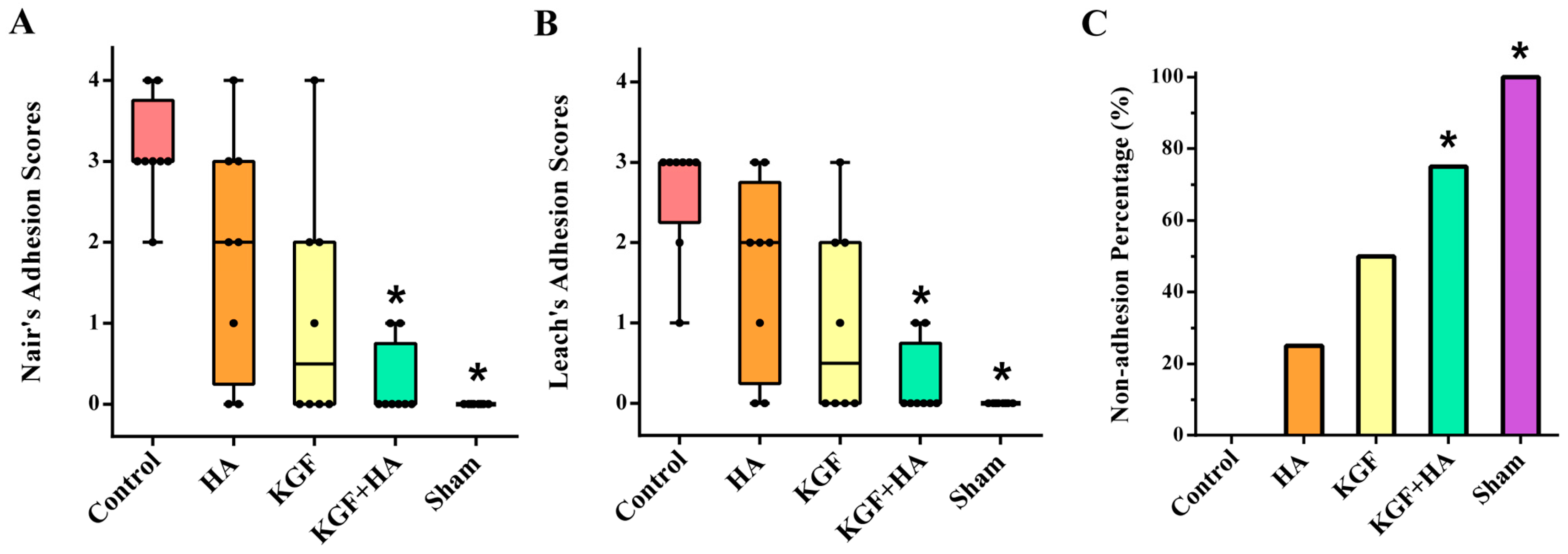
2.1. The Combined Administration of Keratinocyte Growth Factor (KGF) and Sodium Hyaluronate (HA) Significantly Reduced the Abdominal Adhesion Score in the Rat Model Using the Visual Scoring System
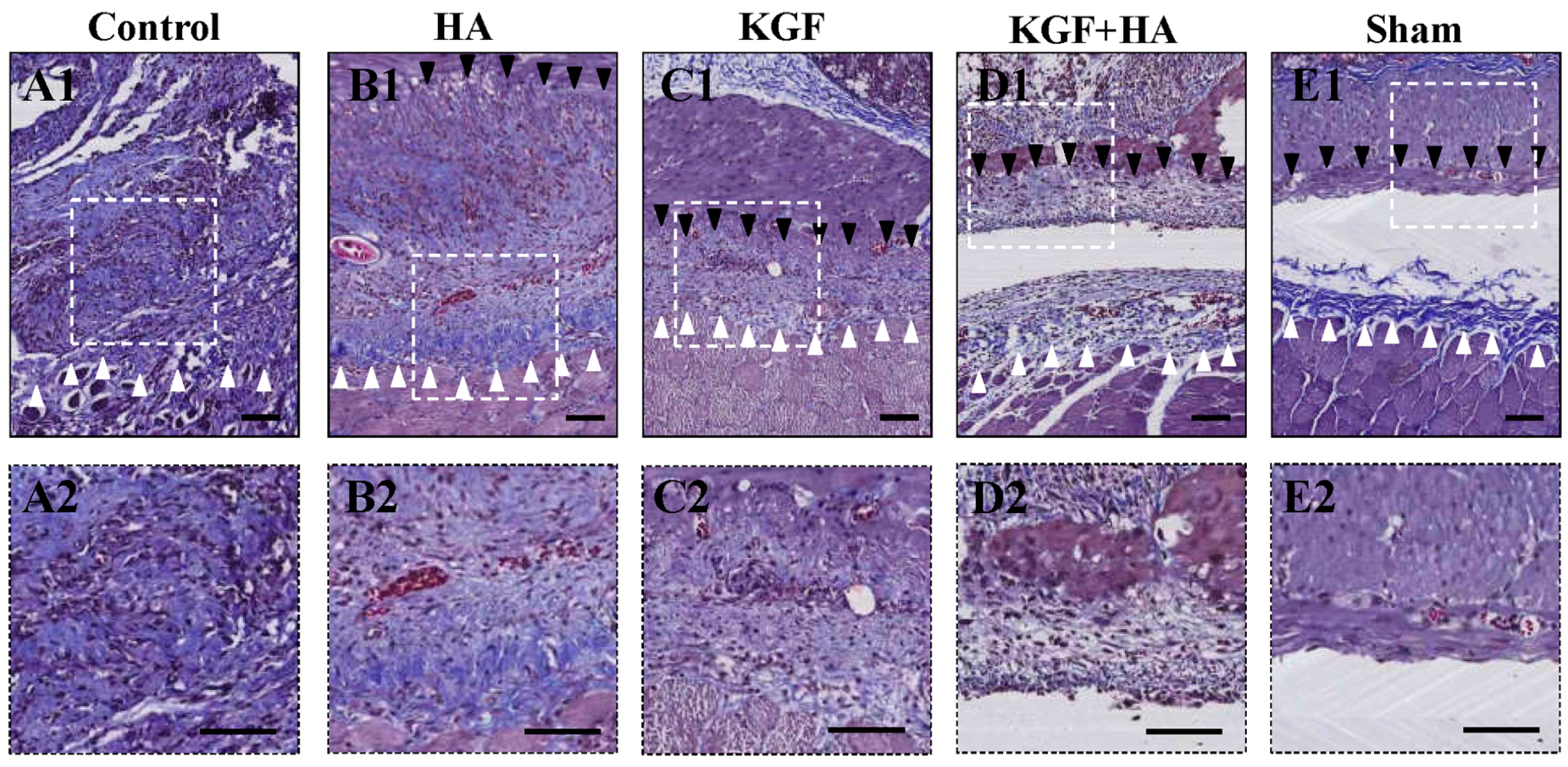
2.2. The Combined Administration of KGF and HA Decreased Collagen Deposition in the Injured Peritoneum of the Rat Model
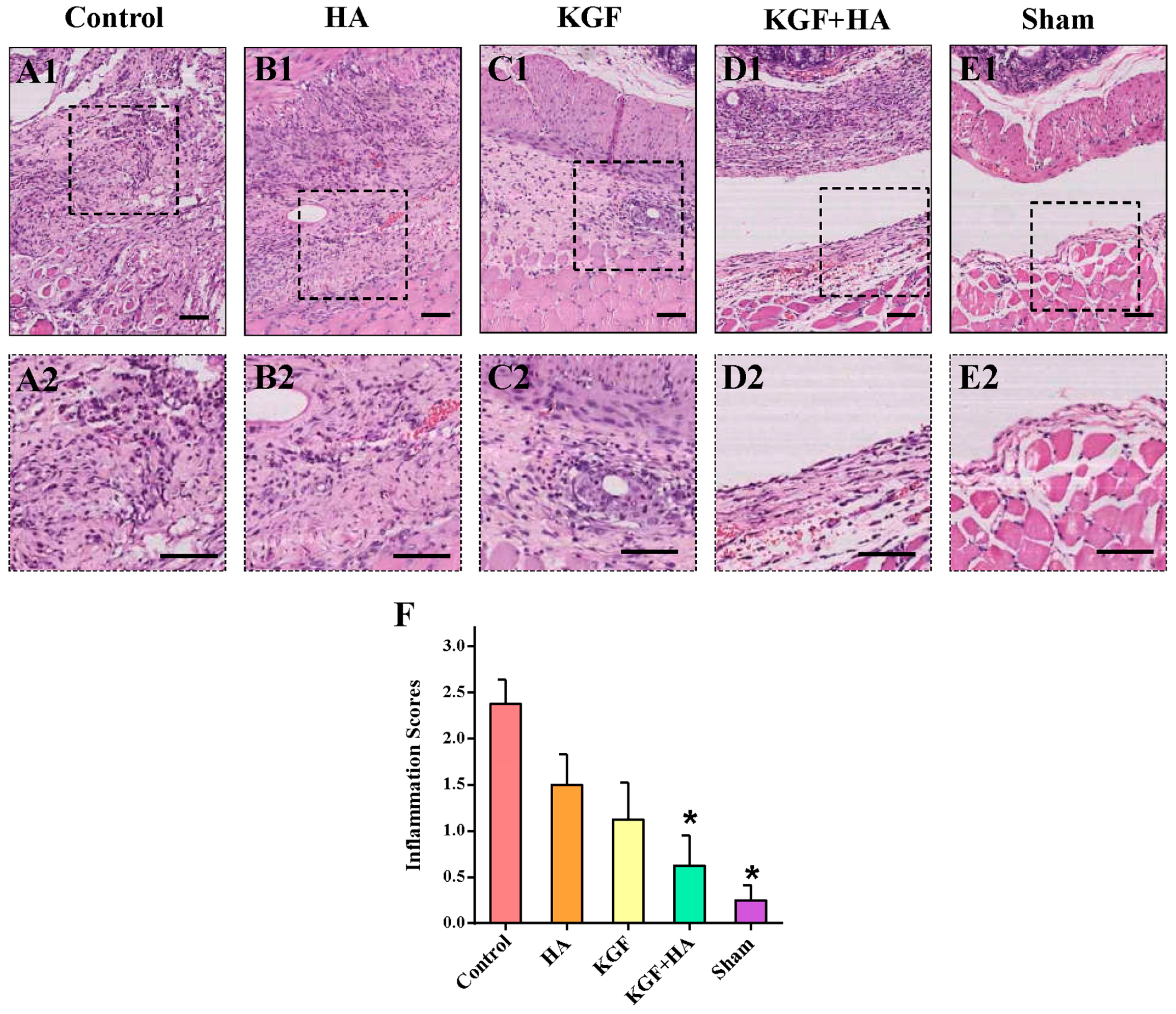
2.3. The Combined Administration of KGF and HA Can Reduce Inflammatory Infiltration in the Injured Rat Peritoneum
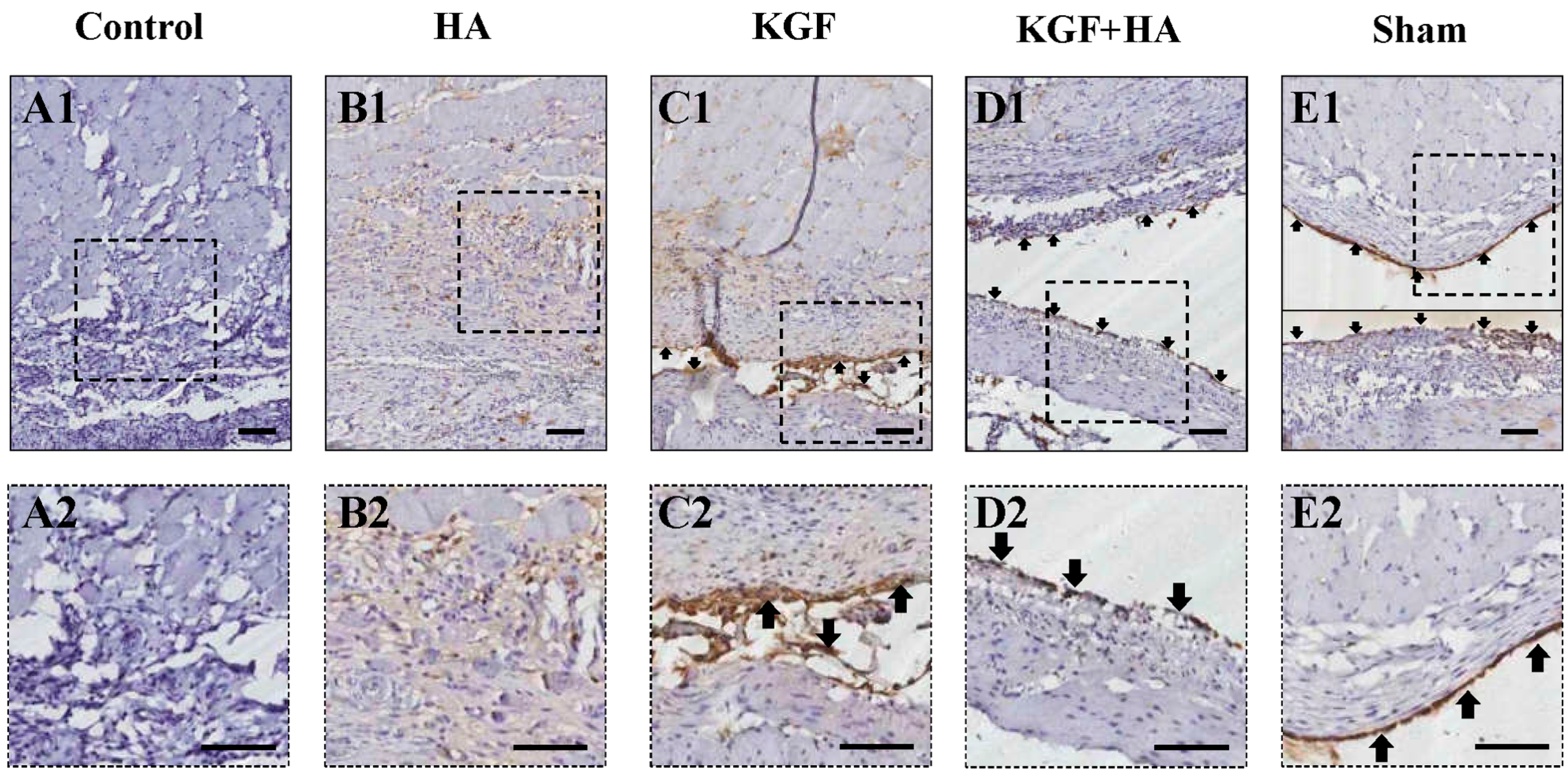
2.4. The Combined Administration of KGF and HA Promotes Mesothelial Cell Repair on the Injured Peritoneal Surface
2.5. The Combined Administration of KGF and HA Inhibited the Severity of the Fibrous Changes in the Injured Peritoneum and/or Adhesion Tissue in the Rat Model
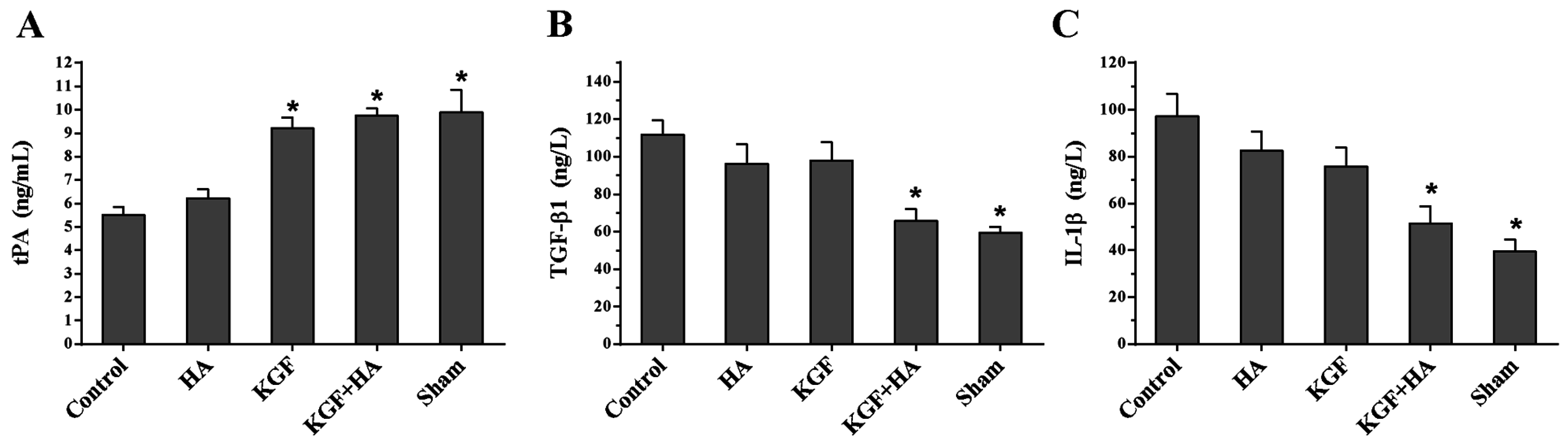
2.6. The Combined Administration of KGF and HA Suppressed the Abdominal Fluid Levels of Tissue Plasminogen Activator (tPA) and the Pro-Inflammatory Cytokines Transforming Growth Factor β1 (TGF-β1) and Interleukin 1β (IL-1β) in the Rat Model
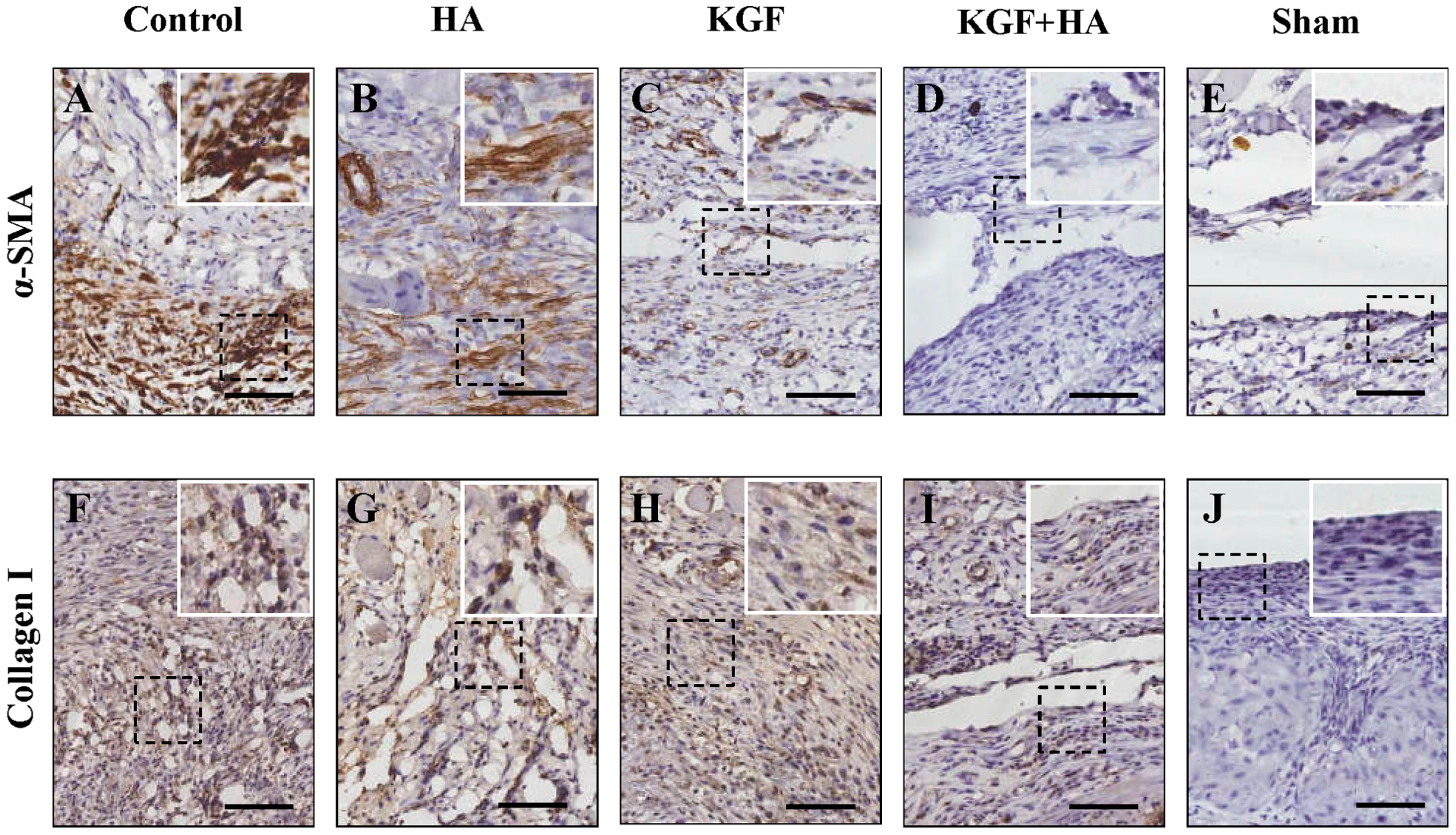
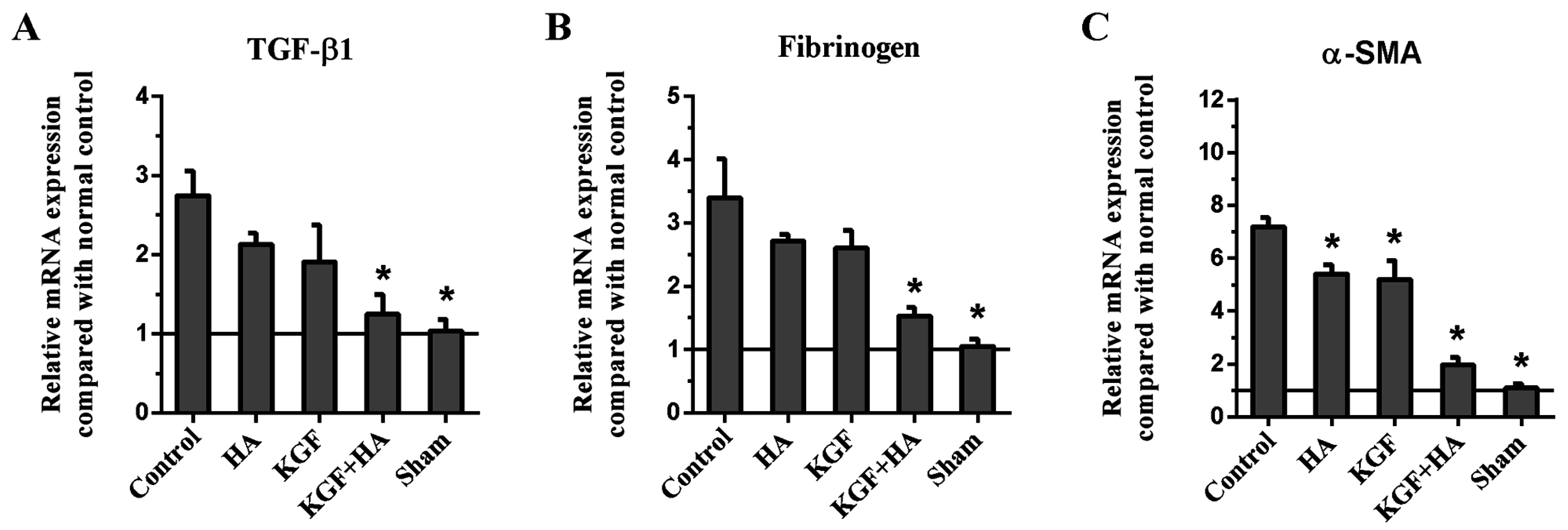
2.7. The Combined KGF and HA Treatment Inhibited Src Phosphorylation and Expression of TGF-β1, Fibrinogen, and α-Smooth Muscle Actin (α-SMA) in the Injured Peritoneum and/or Adhesion Tissue in the Rat Model
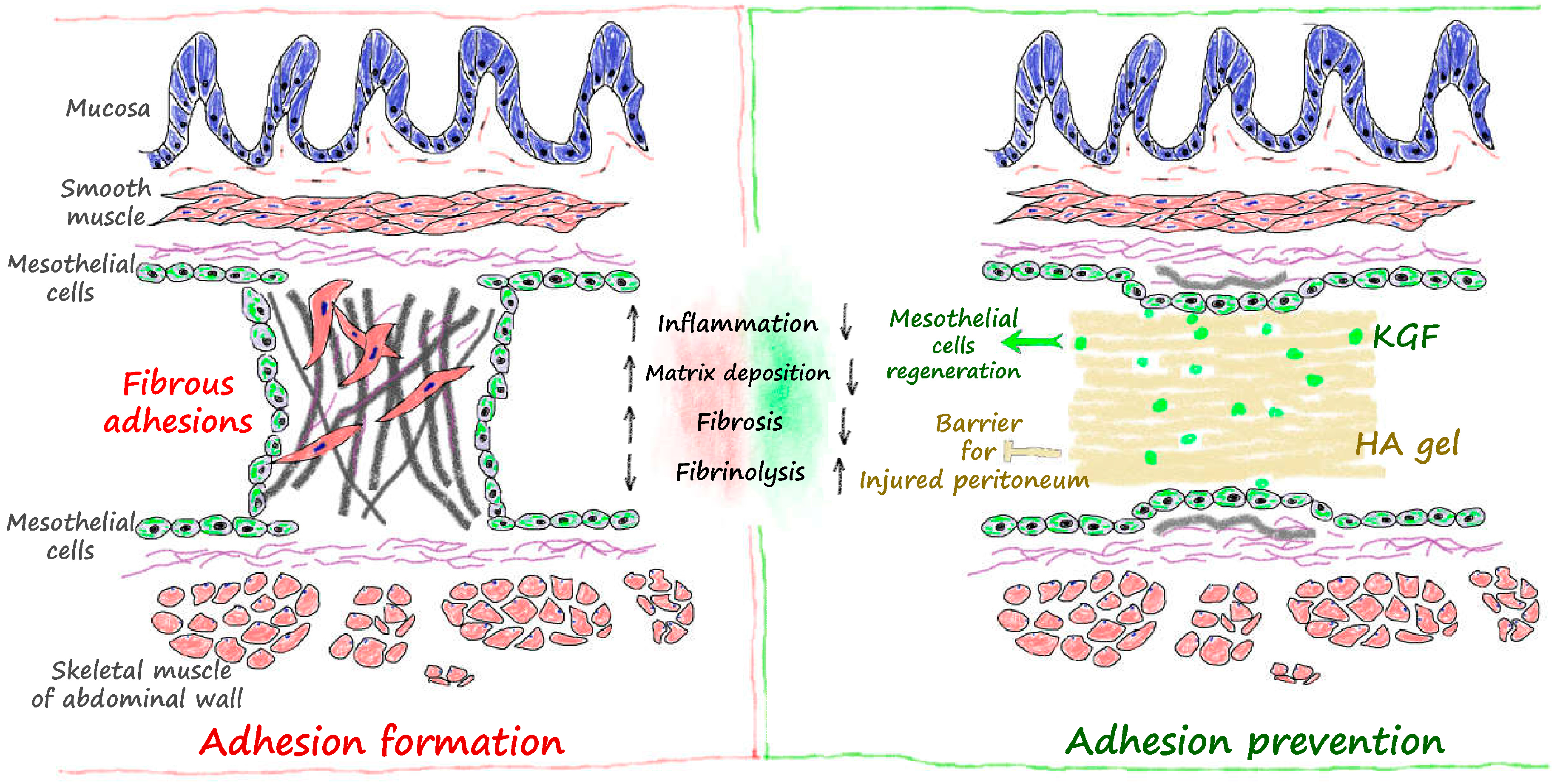
3. Discussion
4. Materials and Methods
4.1. Agents
4.2. Surgical Procedures
4.3. Adhesion Grade and Assessment
4.4. Hematoxylin and Eosin (HE) Staining and Microscopic Histological Grading of Inflammation
4.5. Picrosirius Red Staining for Collagen
4.6. Immunohistochemistry
4.7. Western Blot
4.8. Real-Time RT-PCR
4.9. ELISA Quantification of Abdominal Fluid Levels of IL-6, Tumor Necrosis Factor α (TNF-α), TGF-β1
4.10. Hydroxyproline Determination
4.11. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, J.; Fan, D.; Lin, X.; Wu, X.; He, X.; He, X.; Wu, X.; Lan, P. Safety and efficacy of sodium hyaluronate gel and chitosan in preventing postoperative peristomal adhesions after defunctioning enterostomy: A prospective randomized controlled trials. Medicine 2015, 94, e2354. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gong, C.; Zhao, X.; Zhou, S.; Li, Z.; Qi, X.; Zhong, Q.; Luo, F.; Qian, Z. Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int. J. Nanomed. 2012, 7, 547–557. [Google Scholar]
- Yuan, F.; Lin, L.X.; Zhang, H.H.; Huang, D.; Sun, Y.L. Effect of carbodiimide-derivatized hyaluronic acid gelatin on preventing postsurgical intra-abdominal adhesion formation and promoting healing in a rat model. J. Biomed. Mater. Res. A 2016, 104, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 2013, 347, f5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.; Boman, J.; Shrier, I.; Gordon, P.H. Natural history of patients with adhesive small bowel obstruction. Br. J. Surg. 2000, 87, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Alpay, Z.; Saed, G.M.; Diamond, M.P. Postoperative adhesions: From formation to prevention. Semin. Reprod. Med. 2008, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M.; Stucchi, A.F. Intra-abdominal adhesion prevention: Are we getting any closer. Ann. Surg. 2004, 240, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Kavalukas, S.L.; Barbul, A. Intra-abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr. Probl. Surg. 2015, 52, 271–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Wu, C.T.; Duan, H.F.; Wu, B.; Lu, Z.Z.; Wang, L. Adenoviral-mediated gene expression of hepatocyte growth factor prevents postoperative peritoneal adhesion in a rat model. Surgery 2006, 140, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Uguralp, S.; Akin, M.; Karabulut, A.B.; Harma, B.; Kiziltay, A.; Kiran, T.R.; Hasirci, N. Reduction of peritoneal adhesions by sustained and local administration of epidermal growth factor. Pediatr. Surg. Int. 2008, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.T.; Thao, D.T.; Thuoc, T.L. An overview on keratinocyte growth factor: From the molecular properties to clinical applications. Protein Pept. Lett. 2014, 21, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, M.; Zhu, W.; Bao, T.; Zhu, L.; Zhao, W.; Zhao, F.; Wang, H. Adenovirus-mediated expression of keratinocyte growth factor promotes secondary flap necrotic wound healing in an extended animal model. Aesthet. Plast. Surg. 2013, 37, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.B.; Dallan, L.A.; Campana-filho, S.P.; Lisboa, L.A.; Gutierrez, P.S.; Moreira, L.F.; Oliveira, S.A.; Stolf, N.A. Keratinocyte growth factor: A new mesothelial targeted therapy to reduce postoperative pericardial adhesions. Eur. J. Cardiothorac. Surg. 2009, 35, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, Z.W. Combined application of acellular bovine pericardium and hyaluronic acid in prevention of postoperative pericardial adhesion. Artif. Organs 2014, 38, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chen, X.; Wang, G.; Jia, P.; Xu, Q.; Ping, G.; Wang, K.; Li, X. Inhibition of cyclooxygenase-2 prevents intra-abdominal adhesions by decreasing activity of peritoneal fibroblasts. Drug Des. Dev. Ther. 2015, 9, 3083–3098. [Google Scholar]
- Wei, G.; Chen, X.; Wang, G.; Fan, L.; Wang, K.; Li, X. Effect of resveratrol on the prevention of intra-abdominal adhesion formation in a rat model. Cell. Physiol. Biochem. 2016, 39, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Robb, W.B.; Mariette, C. Strategies in the prevention of the formation of postoperative adhesions in digestive surgery: A systematic review of the literature. Dis. Colon Rectum 2014, 57, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Fayez, J.A.; Schneider, P.J. Prevention of pelvic adhesion formation by different modalities of treatment. Am. J. Obstet. Gynecol. 1987, 157, 1184–1188. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Mesothelial cells. Perit. Dial. Int. 2007, 27, S110–S115. [Google Scholar] [PubMed]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Haney, A.F.; Doty, E. The formation of coalescing peritoneal adhesions requires injury to both contacting peritoneal surfaces. Fertil. Steril. 1994, 61, 767–775. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Awonuga, A.O.; Abusamaan, M.S.; Saed, M.G.; Diamond, M.P.; Saed, G.M. Adhesion phenotype manifests an altered metabolic profile favoring glycolysis. Fertil. Steril. 2016, 105, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Fletcher, N.M.; Diamond, M.P. The creation of a model for ex vivo development of postoperative adhesions. Reprod. Sci. 2016, 23, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Kruger, M.; Diamond, M.P. Expression of transforming growth factor-β and extracellular matrix by human peritoneal mesothelial cells and by fibroblasts from normal peritoneum and adhesions: Effect of Tisseel. Wound Repair Regen. 2004, 12, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. Peritoneal molecular environment, adhesion formation and clinical implication. Front. Biosci. 2002, 7, e91–e115. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Nitta, K. Cell sheet-based tissue engineering for mesothelial cell injury. Contrib. Nephrol. 2015, 185, 66–75. [Google Scholar] [PubMed]
- Bertram, P.; Tietze, L.; Hoopmann, M.; Treutner, K.H.; Mittermayer, C.; Schumpelick, V. Intraperitoneal transplantation of isologous mesothelial cells for prevention of adhesions. Eur. J. Surg. 1999, 165, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Q.F.; Liu, H.J.; Li, R.; Wu, C.T.; Wang, L.S. Sphingosine kinase 1 gene transfer reduces postoperative peritoneal adhesion in an experimental model. Br. J. Surg. 2008, 95, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Finch, P.W.; Rubin, J.S. Keratinocyte growth factor expression and activity in cancer: Implications for use in patients with solid tumors. J. Natl. Cancer Inst. 2006, 98, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Raffa, S.; Flori, E.; Aspite, N.; Briganti, S.; Cardinali, G.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J. Dermatol. Sci. 2009, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Abo, T.; Nagayasu, T.; Hishikawa, Y.; Tagawa, T.; Nanashima, A.; Yamayoshi, T.; Matsumoto, K.; An, S.; Koji, T. Expression of keratinocyte growth factor and its receptor in rat tracheal cartilage: Possible involvement in wound healing of the damaged cartilage. Acta Histochem. Cytochem. 2010, 43, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, F.; Scrofani, C.; Torrisi, M.R.; Mancini, P. Polarized endocytosis of the keratinocyte growth factor receptor in migrating cells: Role of SRC-signaling and cortactin. PLoS ONE 2011, 6, e29159. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.C.; Laird, S.M.; Li, T.C.; Shelton, J.B.; Ledger, W.L.; Cooke, I.D. Peritoneal healing and adhesion formation/reformation. Hum. Reprod. Update 2001, 7, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Szabó, A.; Aigner, Z.; Balogh, E.; Sebe, I.; Zelkó, R.; Antal, I. Microstructural analysis of the fast gelling freeze-dried sodium hyaluronate. J. Pharm. Biomed. Anal. 2015, 104, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.C.; Stewart, A.A.; Byron, C.R.; Pondenis, H.C.; Kaufmann, K.M.; Constable, P.D. Effects of sodium hyaluronate and methylprednisolone acetate on proteoglycan metabolism in equine articular chondrocytes treated with interleukin-1. Am. J. Vet. Res. 2006, 67, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, S.; Ceccarelli, S.; Romano, F.; Frati, L.; Marchese, C.; Angeloni, A. Silencing of keratinocyte growth factor receptor restores 5-fluorouracil and tamoxifen efficacy on responsive cancer cells. PLoS ONE 2008, 3, e2528. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, E.; Haghgu, S.; Kulimova, E.; Mesters, R.M.; Kienast, J.; Herbst, H.; Schmitmann, C.; Kolkmeyer, A.; Serve, H.; Berdel, W.E. Influence of keratinocyte growth factor on clonal growth of epithelial tumor cells, lymphoma and leukemia cells and on sensitivity of tumor cells towards 5-fluorouracil in vitro. Int. J. Oncol. 2004, 25, 1001–1012. [Google Scholar] [PubMed]
- Kanuga, S. Cryotherapy and keratinocyte growth factor may be beneficial in preventing oral mucositis in patients with cancer, and sucralfate is effective in reducing its severity. J. Am. Dent. Assoc. 2013, 144, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Peyton, C.C.; Keys, T.; Tomblyn, S.; Burmeister, D.; Beumer, J.H.; Holleran, J.L.; Sirintrapun, J.; Washburn, S.; Hodges, S.J. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. J. Surg. Res. 2012, 178, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Bhat, I.K.; Aurora, A.L. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch. Surg. 1974, 108, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Leach, R.E.; Burns, J.W.; Dawe, E.J.; SmithBarbour, M.D.; Diamond, M.P. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil. Steril. 1998, 69, 415–418. [Google Scholar] [CrossRef]
- Mahdy, T.; Mohamed, G.; Elhawary, A. Effect of methylene blue on intra-abdominal adhesion formation in rats. Int. J. Surg. 2008, 6, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ma, J.; Ma, Q.; Li, X.; Liu, H.; Xu, Q.; Duan, W.; Sun, Q.; Xu, J.; Wu, Z.; et al. Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol. Cancer 2013, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Zhou, C.; Wang, G.; Fan, L.; Wang, K.; Li, X. Keratinocyte Growth Factor Combined with a Sodium Hyaluronate Gel Inhibits Postoperative Intra-Abdominal Adhesions. Int. J. Mol. Sci. 2016, 17, 1611. https://doi.org/10.3390/ijms17101611
Wei G, Zhou C, Wang G, Fan L, Wang K, Li X. Keratinocyte Growth Factor Combined with a Sodium Hyaluronate Gel Inhibits Postoperative Intra-Abdominal Adhesions. International Journal of Molecular Sciences. 2016; 17(10):1611. https://doi.org/10.3390/ijms17101611
Chicago/Turabian StyleWei, Guangbing, Cancan Zhou, Guanghui Wang, Lin Fan, Kang Wang, and Xuqi Li. 2016. "Keratinocyte Growth Factor Combined with a Sodium Hyaluronate Gel Inhibits Postoperative Intra-Abdominal Adhesions" International Journal of Molecular Sciences 17, no. 10: 1611. https://doi.org/10.3390/ijms17101611