Anion Channel Inhibitor NPPB-Inhibited Fluoride Accumulation in Tea Plant (Camellia sinensis) Is Related to the Regulation of Ca2+, CaM and Depolarization of Plasma Membrane Potential
Abstract
:1. Introduction
2. Results
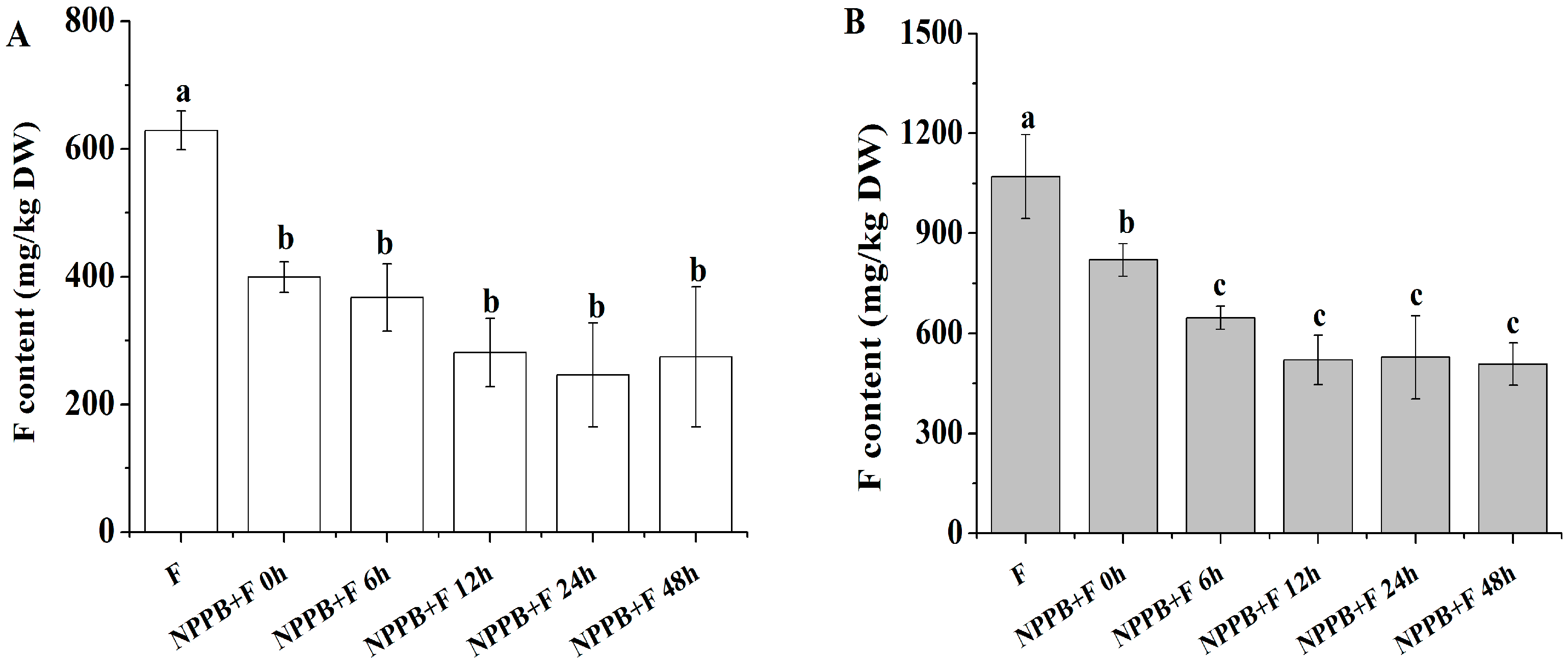
2.1. NPPB Significantly Inhibited F Accumulation in Tea Roots and Its Whole Plant
2.2. The Changes of Net Ca2+ Flux and Cytosolic Ca2+ Intensity in Tea Roots in Response to NPPB


2.3. The Pretreatment of EGTA on NPPB-Induced Change of Ca2+ Fluorescence Intensity

2.4. Effects of Ca2+ Chelator and CaM Antagonists on NPPB-Induced CaM Content

2.5. Endogenous Ca2+ and CaM Involved in NPPB-Inhibited F Accumulation in Tea Plants
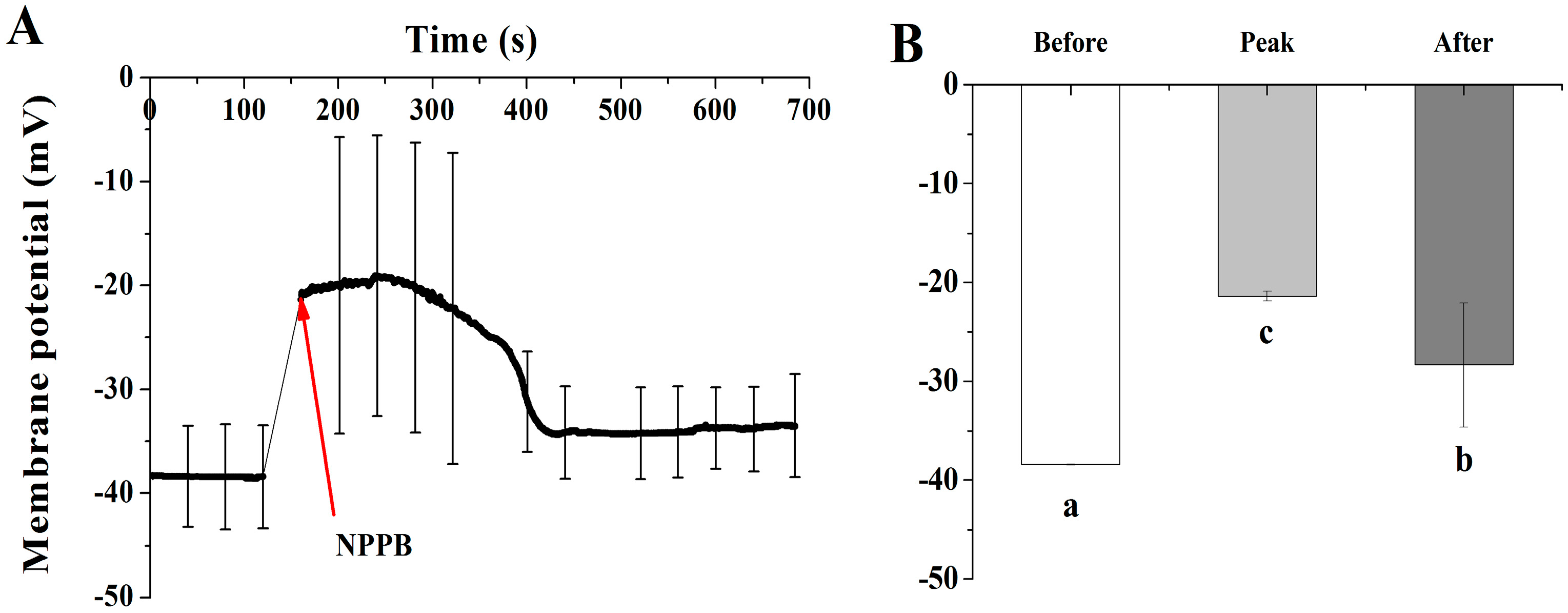
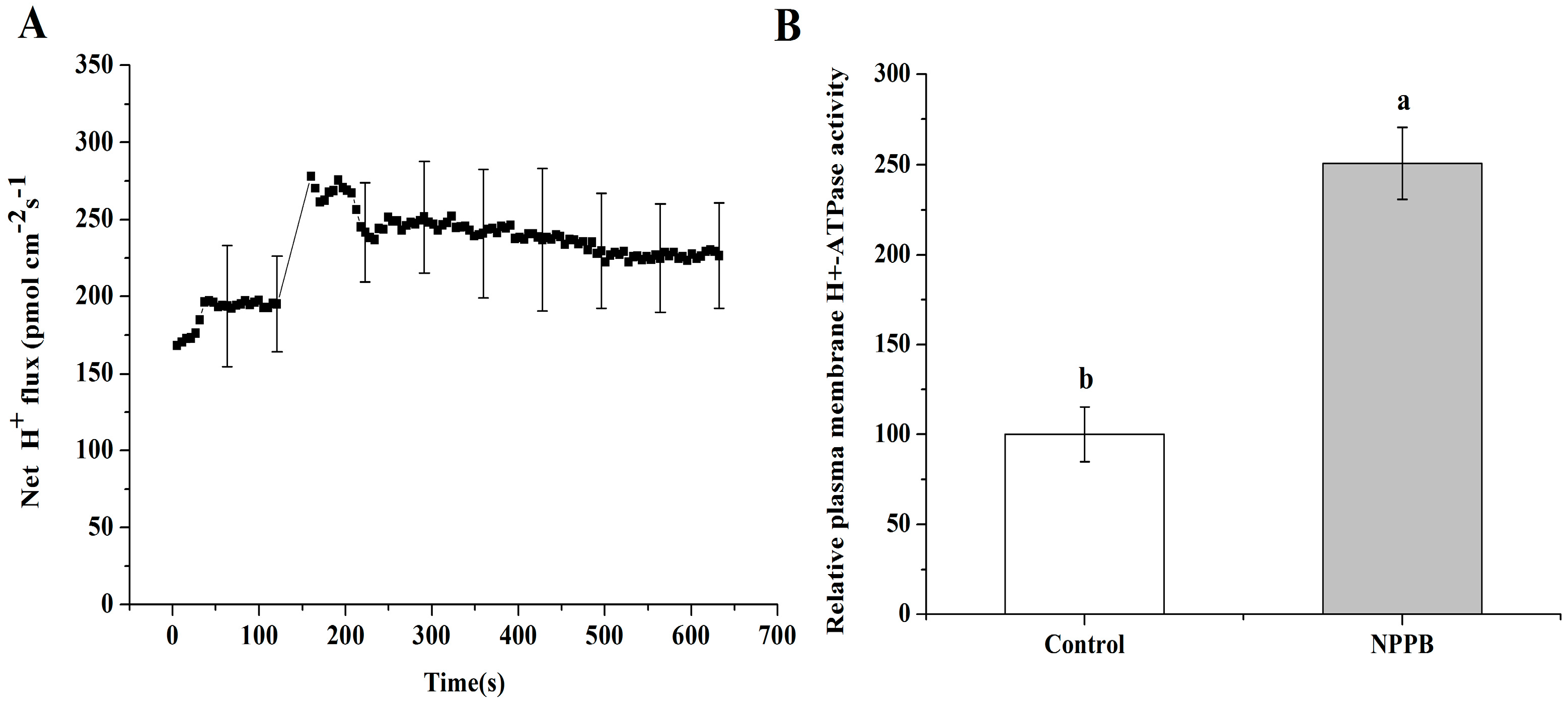
2.6. NPPB Depolarized Membrane Potential and Stimulated the Net H+ Effluxes in the Maturation Zone of Tea Roots

3. Discussion
3.1. Ca2+ and CaM Are Involved in NPPB-Inhibited F Accumulation in Tea Plants
3.2. A Possible Link between Regulation of Ca-CaM and Plasma Membrane Potential in NPPB-Inhibited F Accumulation in Tea Plants
4. Materials and Methods
4.1. Cultivation of Tea Plants
4.2. NPPB Treatment
4.3. Determination of F
4.4. Flux Measurement of Ca2+ and H+
4.5. Measurement of Cytosolic Ca2+ Intensity
4.6. Plasma Membrane H+-ATPase Assay
4.7. CaM Extraction and Analysis
4.8. Membrane Potential Measurements
4.9. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Ruan, J.Y.; Ma, L.F.; Shi, Y.Z.; Han, W.Y. Uptake of fluoride by tea plant (Camellia sinensis L.) and the impact of aluminium. J. Sci. Food Agric. 2003, 83, 1342–1348. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhao, Q.; Zhang, X.C.; Wan, X.C.; Mao, J.D. Localization of Fluoride and Aluminum in Subcellular Fractions of Tea Leaves and Roots. J. Agric. Food Chem. 2014, 62, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Shaw, L.; Smith, A.J. The bioavailability of fluoride from black tea. J. Dent. 2001, 29, 15–21. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Liu, J. Brick tea consumption as the cause of dental fluorosis among children from Mongol, Kazak and Yugu populations in China. Food Chem. Toxicol. 1997, 35, 827–833. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, W.F.; Yang, X.Q. Fluoride Content in Tea and Its Relationship with Tea Quality. J. Agric. Food Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Ma, L.F.; Ruan, J.Y. Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze). Plant Soil 2013, 366, 659–669. [Google Scholar] [CrossRef]
- Zhang, X.C.; Gao, H.J.; Zhang, Z.Z.; Wan, X.C. Influences of different ion channel inhibitors on the absorption of fluoride in tea plants. Plant Growth Regul. 2013, 69, 99–106. [Google Scholar]
- Angeli, A.D.; Thominea, S.; Frachissea, J.M.; Ephritikhinea, G.; Gambale, F.; Barbier-Brygooa, H. Anion channels and transporters in plant cell membranes. FEBS Lett. 2007, 581, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Barbier-Brygoo, H.; Vinauger, M.; Colcombet, J.; Ephritikhine, G.; Frachisse, J.M.; Maurel, C. Anion channels in higher plants: Functional characterization, molecular structure and physiological role. Biochim. Biophy. Acta 2000, 1465, 199–218. [Google Scholar] [CrossRef]
- Ryan, P.R.; Skerrett, M.; Findlay, G.P.; Delhaize, E.; Tyerman, S.D. Aluminum activates an anion channel in the apical cells of wheat roots. Proc. Natl. Acad. Sci. USA 1997, 94, 6547–6552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Ryan, P.R.; Tyerman, S.D. Malate-Permeable Channels and Cation Channels Activated by Aluminum in the Apical Cells of Wheat Roots. Plant Physiol. 2001, 125, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Yang, L.; Luan, M.D.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.S.; Zhao, F.G.; Lan, W.Z.; Luan, S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 6571–6578. [Google Scholar]
- Takahashi, Y.; Tateda, C. The functions of voltage-dependent anion channels in plants. Apoptosis 2013, 18, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Pei, Z.M.; Schroeder, J.I. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 1995, 7, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Luan, S. CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Pascal Maser, P.; Julian, I.; Schroeder, J.I. Plant Ion Channels: Gene Families, Physiology, and Functional Genomics Analyses. Annu. Rev. Physiol. 2009, 71, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Brownlee, C. The generation of Ca2+ signals in plants. Annu. Rev. Plant Biol. 2004, 55, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Marten, H.; Konrad, K.R.; Dietrich, P.; Roelfsema, M.R.G.; Hedrich, R. Ca2+-Dependent and-Independent Abscisic Acid Activation of Plasma Membrane Anion Channels in Guard Cells of Nicotiana tabacum. Plant Physiol. 2007, 143, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Hills, A.; Lim, C.K.; Blatt, M.R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 2012, 61, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Chung, W.S.; Yun, D.J.; Cho, M.J. Calcium and Calmodulin-Mediated Regulation of Gene Expression in Plants. Mol. Plant 2009, 2, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.B.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Adelman, J.P. SK Channels and Calmodulin. Channels 2015. [Google Scholar] [CrossRef] [PubMed]
- Halling, D.B.; Aracena-Parks, P.; Hamilton, S.L. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci. Signal. 2006, 318, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Chung, B.C.; Yan, H.D.; Wang, H.G.; Lee, S.Y.; Pitt, G.S. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Jossier, M.; Laanemets, K.; Thomine, S. Anion channel in plant cells. FEBS J. 2011, 278, 4277–4292. [Google Scholar] [CrossRef] [PubMed]
- Michelet, B.; Boutr, M. The Plasma Membrane H+-ATPase. Plant Physiol. 1995, 108, 1–6. [Google Scholar] [PubMed]
- Palmgren, M.G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Tester, M. The regulation of anion loading to the maize root xylem. Plant Physiol. 2005, 137, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000. [Google Scholar]
- Zhang, X.C.; Gao, H.J.; Wu, H.H.; Yang, T.Y.; Zhang, Z.Z.; Mao, J.D.; Wan, X.C. Ca2+ and CaM are involved in Al3+ pretreatment-promoted fluoride accumulation in tea plants (Camellia sinesis L.). Plant Physiol. Biochem. 2015, 96, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef]
- Liao, W.B.; Zhang, M.L.; Huang, G.B.; Yu, G.H. Ca2+ and CaM are involved in NO and H2O2-induced Adventitious Root Development in Marigold. J. Plant Growth Regul. 2012, 31, 253–264. [Google Scholar] [CrossRef]
- Hu, X.L.; Jiang, M.Y.; Zhang, J.H.; Zhang, A.Y.; Lin, F.; Tan, M.P. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 2007, 173, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, X.Q.; Chen, Y.M.; Li, X.; Huang, M.; Zheng, M.; Baluska, F.; Samaj, J.; Lin, J. Combined proteomic and cytological analysis of Ca2+-calmodulin regulation in picea meyeri pollen tube growth. Plant Physiol. 2009, 149, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Antoine, A.F.; Faure, J.E.; Dumas, C.; Feijó, J.A. Differential contribution of cytoplasmic Ca2+ and Ca2+ influx to gamete fusion and egg activation in maize. Nat. Cell Biol. 2001, 3, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Wang, M.Q.; Yu, B.; Zeng, Z.H.; Guo, F.; Han, N.; Bian, H.; Wang, J.; Pan, J.; Zhu, M. Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice. Plant Physiol. Biochem. 2014, 80, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.L.; Wang, M.J.; Ding, M.; Rui, D.; Xin, S.; Zhou, X.Y.; Lu, C.F.; et al. Extracellular ATP Signaling Is Mediated by H2O2 and Cytosolic Ca2+ in the Salt Response of Populus euphratica Cells. PLoS ONE 2012, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K. Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol. 2006, 169, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Johannes, E.; Crofts, A.; Sanders, D. Control of Cl− Efflux in Chara corallina by Cytosolic pH, Free Ca2+, and Phosphorylation Indicates a Role of Plasma Membrane Anion Channels in Cytosolic pH Regulation. Plant Physiol. 1998, 118, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Chen, Q.; Liu, Y.L. Relationship between tonoplast H+-ATPase activity, ion uptake and calcium in barley roots under NaCl stress. Acta Bot. Sin. 2002, 44, 667–672. [Google Scholar]
- Bouche, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.M.; Ward, J.M.; Harper, J.F.; Schroeder, J.I. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996, 15, 6564–6574. [Google Scholar] [PubMed]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Li, L.G.; Kim, B.G.; Lee, S.C.; Kudla, J.; et al. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A.; Pang, J.Y.; Percey, W.; Chen, Z.H.; Conn, S.; Eing, C.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 61, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Cuin, T.A.; Shabala, S. Exogenously Supplied Compatible Solutes Rapidly Ameliorate NaCl-induced Potassium Efflux from Barley Roots. Plant Cell Physiol. 2005, 46, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Isaac, Z.J.; Zhou, M.; Michael, G.P.; Newman, I.A. Root Plasma Membrane Transporters Controlling K+/Na+ Homeostasis in Salt-Stressed Barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, M.J.; Ding, M.Q.; Deng, S.R.; Liu, M.Q.; Lu, C.F.; Zhou, X.Y.; Shen, X.; Zheng, X.J.; Zhang, Z.K.; et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010, 33, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Lamottea, O.; Courtoisa, C.; Dobrowolsk, G.; Bessona, A.; Pugina, A.; Wendehennea, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Biol. Med. 2006, 40, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Droogmans, G. Amazing chloride channels: An overview. Acta Physiol. Scand. 2003, 177, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.N.; Newman, I.A.; Morris, J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 2000, 113, 111–118. [Google Scholar]
- Zhang, W.H.; Rengel, Z.; Kuo, J. Determination of intracellular Ca2+ in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo-3 under low temperature. Plant J. 1998, 15, 147–151. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guo, J.K.; Zhang, F.; Zhao, L.Q.; Zhang, L.X. NaCl induced changes of the H+-ATPase in root plasma membrane of two wheat cultivars. Plant Sci. 2004, 166, 913–938. [Google Scholar] [CrossRef]
- Sun, D.Y.; Bian, Y.Q.; Zhao, B.H.; Zhao, L.Y.; Yu, X.M.; Duan, S.J. The effects of extracellular calmodulin on cell wall regeneration of protoplasts and cell division. Plant Cell Physiol. 1995, 36, 133–138. [Google Scholar]
- Bradford, M.M. A rapid and sensitve method for the quantitation of microgram quantities of protein utilizing the principle of potein-dye binging. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-C.; Gao, H.-J.; Yang, T.-Y.; Wu, H.-H.; Wang, Y.-M.; Zhang, Z.-Z.; Wan, X.-C. Anion Channel Inhibitor NPPB-Inhibited Fluoride Accumulation in Tea Plant (Camellia sinensis) Is Related to the Regulation of Ca2+, CaM and Depolarization of Plasma Membrane Potential. Int. J. Mol. Sci. 2016, 17, 57. https://doi.org/10.3390/ijms17010057
Zhang X-C, Gao H-J, Yang T-Y, Wu H-H, Wang Y-M, Zhang Z-Z, Wan X-C. Anion Channel Inhibitor NPPB-Inhibited Fluoride Accumulation in Tea Plant (Camellia sinensis) Is Related to the Regulation of Ca2+, CaM and Depolarization of Plasma Membrane Potential. International Journal of Molecular Sciences. 2016; 17(1):57. https://doi.org/10.3390/ijms17010057
Chicago/Turabian StyleZhang, Xian-Chen, Hong-Jian Gao, Tian-Yuan Yang, Hong-Hong Wu, Yu-Mei Wang, Zheng-Zhu Zhang, and Xiao-Chun Wan. 2016. "Anion Channel Inhibitor NPPB-Inhibited Fluoride Accumulation in Tea Plant (Camellia sinensis) Is Related to the Regulation of Ca2+, CaM and Depolarization of Plasma Membrane Potential" International Journal of Molecular Sciences 17, no. 1: 57. https://doi.org/10.3390/ijms17010057





