Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Astaxanthin for Cell Viability and Lipopolysaccharide (LPS) and Concanavalin A (Con A) Induced Lymphocyte Proliferation
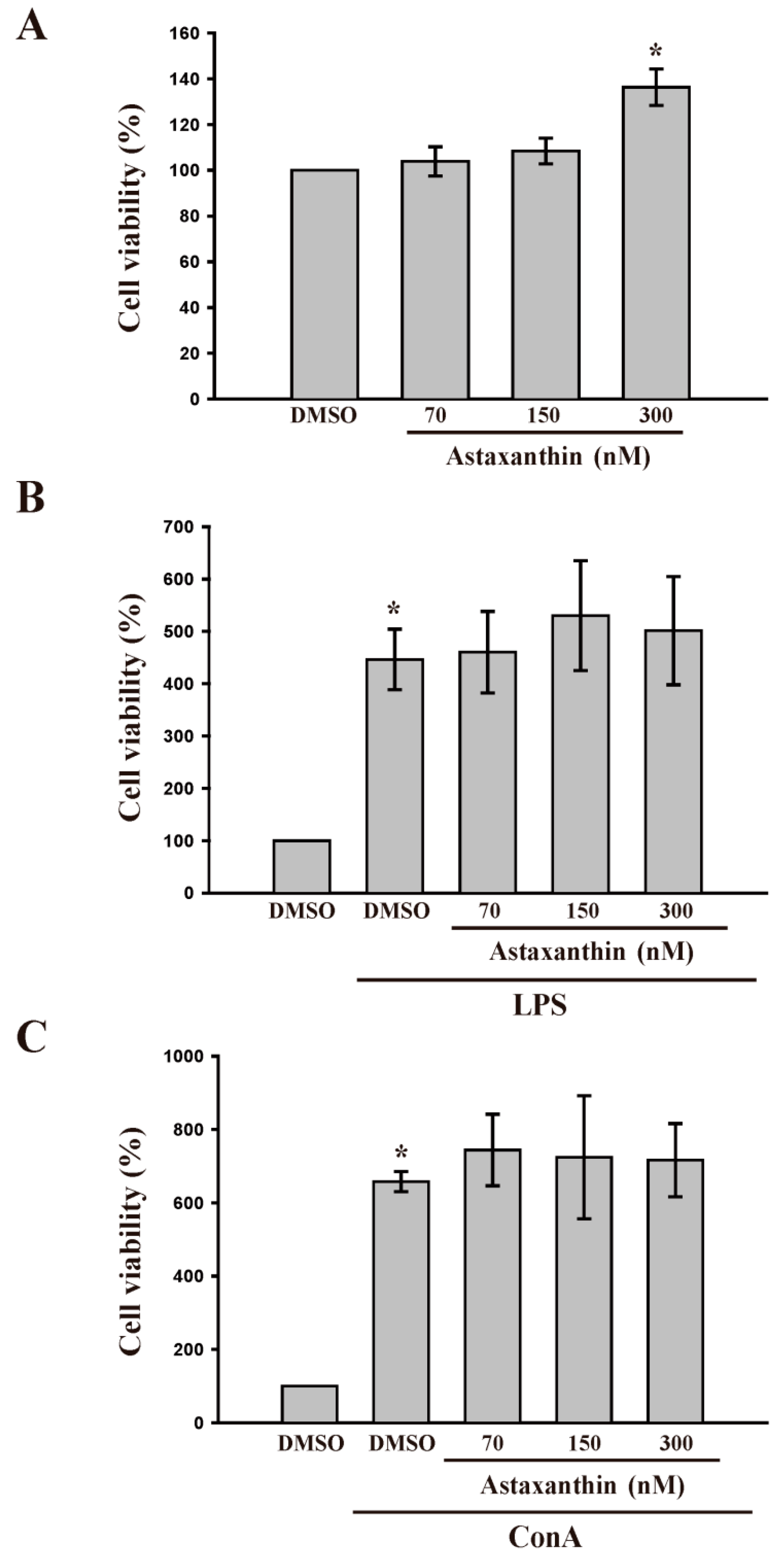
2.1.2. Astaxanthin and in Vitro LPS- and Con A Induced IFN-γ and IL-2 Production
| Astaxanthin Alone | LPS | Con A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines | DMSO | Astaxanthin (nM) | DMSO | DMSO | Astaxanthin (nM) | DMSO | DMSO | Astaxanthin (nM) | ||||||
| 70 | 150 | 300 | 70 | 150 | 300 | 70 | 150 | 300 | ||||||
| INF-γ (pg/mL) | 153.5 ± 2.6 | 153.9 ± 1.6 | 156.8 ± 6.4 | 152.5 ± 1.6 | 168.3 ± 29.2 | 840.1 ± 174.5 * | 1099.9 ± 70.7 # | 1037.3 ± 97.3 # | 1074.8 ± 111.7 # | 204.5 ± 54.0 | 1089.9 ± 22.9 * | 1074.5 ± 12.3 | 1119.0 ± 9.2 | 1113.0 ± 7.2 |
| IL-2 (pg/mL) | 18.9 ± 6.3 | 17.4 ± 5.1 | 18.5 ± 6.7 | 19.6 ± 7.4 | – | – | – | – | – | 21.1 ± 5.8 | 242.0 ± 42.1 * | 245.2 ± 39.9 | 242.2 ± 48.3 | 287.6 ± 32.3 |
| Astaxanthin Alone | LPS | Con A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines | NS | Astaxanthin (mg/kg) | NS | NS | Astaxanthin (mg/kg) | NS | NS | Astaxanthin (mg/kg) | ||||||
| 0.28 | 1.4 | 7 | 0.28 | 1.4 | 7 | 0.28 | 1.4 | 7 | ||||||
| INF-γ (pg/mL) | 281.2 ± 53.8 | 358.4 ± 70.3 | 455.5 ± 149.0 | 351.8 ± 93.4 | 281.2 ± 53.8 | 632.3 ± 172.1 * | 1027.7 ± 25.2 # | 865.7 ± 152.5 # | 900.6 ± 195.6 # | 281.2 ± 53.8 | 934.1 ± 181.8 * | 1107.9 ± 20.2 # | 1054.2 ± 20.4 # | 1084.2 ± 44.5 # |
| IL-2 (pg/mL) | 8.6 ± 6.0 | 37.3 ± 23.6 * | 47.9 ± 11.3 * | 56.9 ± 18.0 * | – | – | – | – | – | 21.7 ± 6.1 | 187.5 ± 19.2 * | 231.3 ± 34.9 # | 252.0 ± 7.9 # | 269.9 ± 45.6 # |
2.1.3. Astaxanthin and Cell Viability and LPS- and Con A-Induced Lymphocyte Proliferation ex Vivo
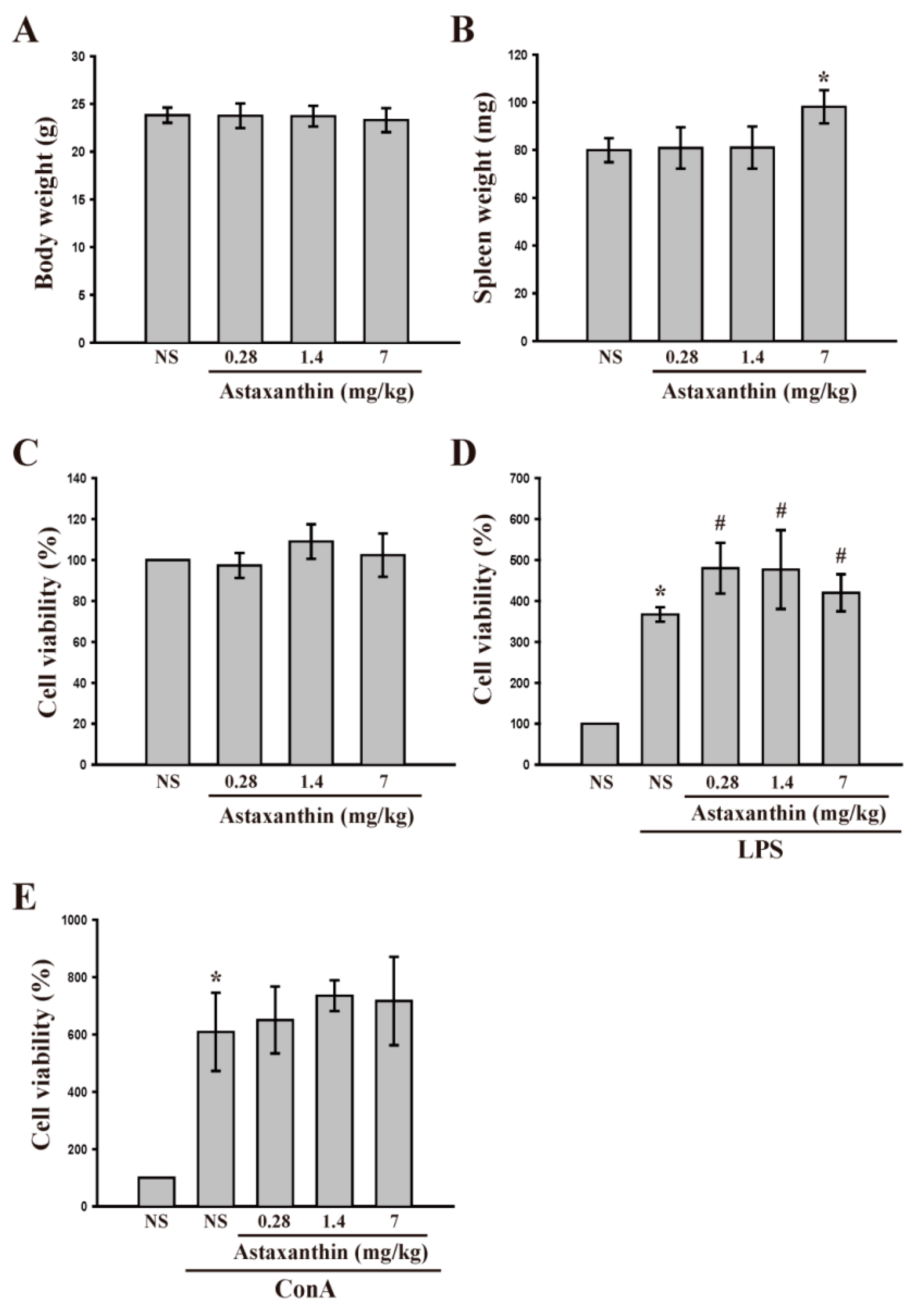
2.1.4. Astaxanthin and LPS- and Con A-Induced IL-2 and IFN-γ Production ex Vivo
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Mice
3.3. Lymphocyte Preparation
3.4. In Vitro Cell Viability
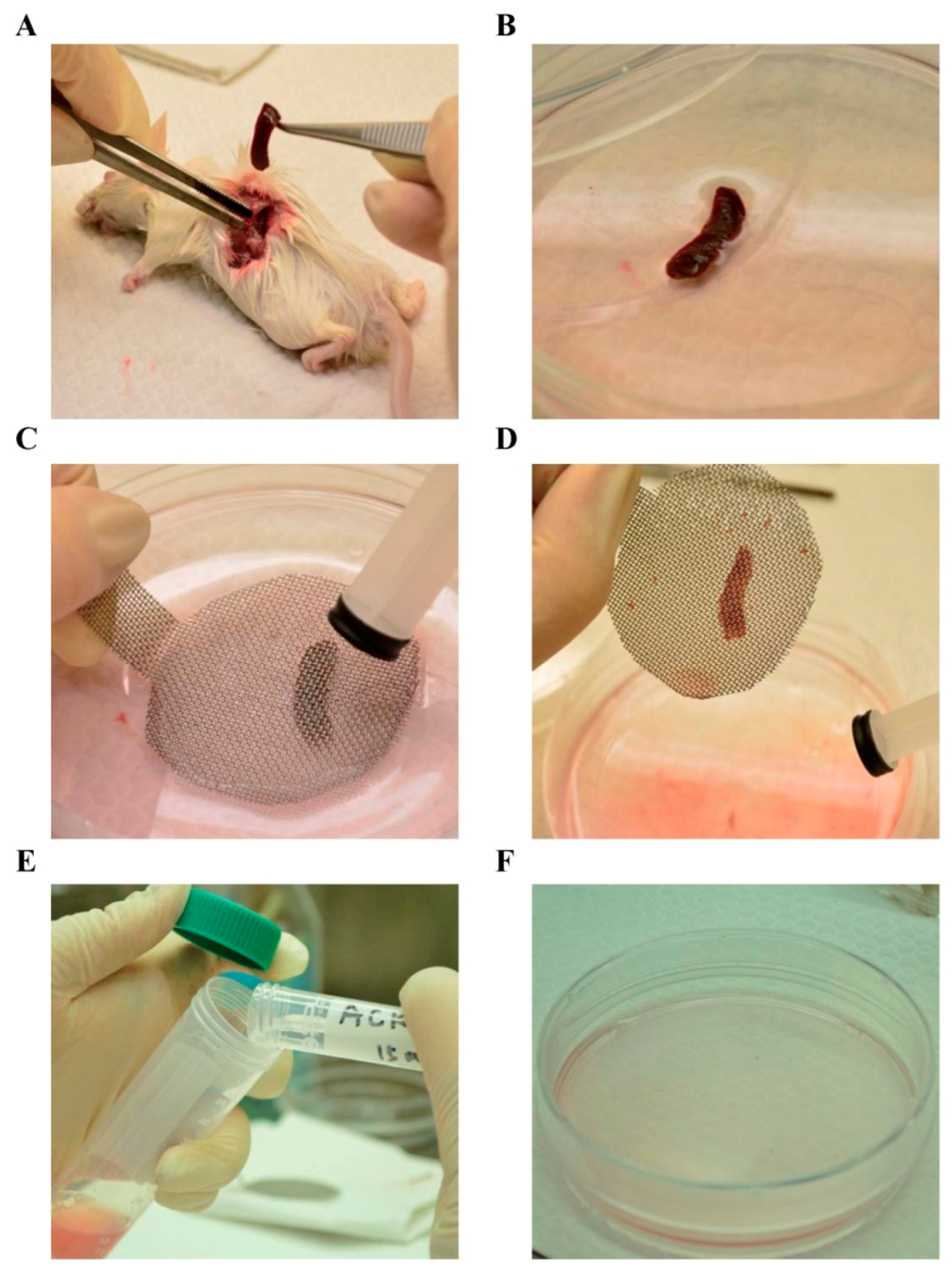
3.5. Ex Vivo Lymphocyte Proliferation Assay
3.6. Cytokine Evaluation
3.7. Data Analysis
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Mandal, S.C.; Lakshmi, S.M. Pharmacognosy and phytotherapy research. In Biodiversity and Environmental Biotechnology; Dwivedi, P.K., Dwivedi, S.K., Eds.; Scientific Publishers: Jodhpur, India, 2007; pp. 177–224. [Google Scholar]
- Bocci, V.; Paulesu, L.; Pessina, G.P.; Nicoletti, C. The physiological interferon response. IX. Interferon activity in rabbit lymph after intraduodenal administration of alimentary lectins. Lymphokine Res. 1988, 7, 49–59. [Google Scholar] [PubMed]
- Faber, J.; Vos, P.; Kegler, D.; van Norren, K.; Argile’s, J.M.; Laviano, A.; Garssen, J.; van Helvoort, A. Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br. J. Cancer 2008, 99, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Requena, P.; González, R.; López-Posadas, R.; Abadía-Molina, A.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. The intestinal antiinflammatory agent glycomacropeptide has immunomodulatory actions on rat splenocytes. Biochem. Pharmacol. 2010, 79, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, K.; Skibsted, L. Carotenoid scavenging radicals: Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm. Unters. Forsch. 1993, 196, 423–439. [Google Scholar] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. The immune system and disease. In Carotenoids: Nutrition and Health; Birkhauser Press: Basel, Switzerland, 2009; Volume 5, pp. 363–382. [Google Scholar]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, H.W.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin uptake in domestic dogs and cats. Nutr. Metab. 2010, 7, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Rishi, P.; Vohra, H.; Sharma, S.; Ganguly, N.K. Cellular immune response induced by Salmonella enterica serotype Typhiironregulated outer-membrane proteins at peripheral and mucosal levels. J. Med. Microbiol. 2005, 54, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Takano, F.; Yahagi, N.; Yahagi, R.; Takada, S.; Yamaguchi, M.; Shoda, S.; Murase, T.; Fushiya, S.; Ohta, T. The liquid culture filtrates of Paecilomycestenuipes (Peck) Samson (= Isaria japonica Yasuda) and Paecilomyces cicadae (Miquel) Samson (= Isariasinclairii (Berk.) Llond) regulate Th1 and Th2 cytokine response in murine Peyer’s patch cells in vitro and ex vivo. Int. J. Immunopharmacol. 2005, 5, 903–916. [Google Scholar]
- Bhat, B.A.; Dhar, K.L.; Puri, S.C.; Qurishi, M.A.; Khajuria, A.; Gupta, A.; Qazi, G.N. Isolation, characterization and biological evaluation of datura lactones as potential immunomodulators. Bioorg. Med. Chem. 2005, 13, 6672–6677. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Hill, R.; Tomita, Y.; Good, R. Studies of immunomodulating actions of carotenoids. I. Effects of β-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr. Cancer 1991, 16, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Zhang, L.; Gross, M.; Tomita, Y. Immunomodulating actions of carotenoids: Enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr. Cancer 1994, 21, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Gross, M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr. Cancer 1995, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.; Lundebye, A.K. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mookerjie, B.K.; Lee, T.P.; Logue, G.P.; Lippes, H.A.; Middleton, E. The effects of flavonoids on human lymphocyte proliferative responses. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological, and Structure Activity Relationships; Cody, V., Middleton, E., Harborne, J.E., Jr., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1986; pp. 511–520. [Google Scholar]
- Rajput, Z.I.; Hu, S.; Xiao, C.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.; Liu, F. Immunomodulation and anticancer activity of polysaccharide–protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Calcagni, E.; Elenkov, L. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. N. Y. Acad. Sci. 2006, 1069, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.; Julien, N.; Zacharie, B.; Penney, C.; Gagnon, L. Enhancement of Th1 type cytokine production and primary T cell activation by PBI-1393. Clin. Immunol. 2007, 12, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.P.; Fehniger, T.A.; Baiocchi, R.A.; Yu, F.; Shah, M.H.; Schiller, D.S.; Gould, M.; Gazzinelli, R.T.; Bernstein, Z.P.; Caligiuri, M.A. Ultralow dose interleukin-2 therapy promotes a type 1 cytokine profile in vivo in patients with AIDS and AIDS-associated malignancies. J. Clin. Investig. 1998, 101, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Newmark, H.; Miller, R.G. Oral β-carotene can increase cells in human blood. Immunol. Lett. 1985, 9, 221–224. [Google Scholar] [CrossRef]
- Watson, R.R.; Prabhala, R.H.; Plzia, P.M.; Alberts, D.S. Effects of β-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. Am. J. Clin. Nutr. 1991, 53, 90–94. [Google Scholar] [PubMed]
- Garewal, H.S.; Ampel, N.M.; Watson, R.R.; Prabhala, R.H.; Dols, C.L.A. Preliminary trial of β carotene in subjects infected with the human immunodeficiency virus. J. Nutr. 1992, 122, 728–732. [Google Scholar] [PubMed]
- Sato, Y.; Akiyama, H.; Suganuma, H.; Watanabe, T.; Nagaoka, M.H.; Inakuma, T.; Goda, Y.; Maitani, T. The feeding of β carotene down-regulates serum IgE levels and inhibits the type I allergic response in mice. Biol. Pharm. Bull. 2004, 27, 978–984. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-H.; Lin, K.-C.; Lu, W.-J.; Thomas, P.-A.; Jayakumar, T.; Sheu, J.-R. Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo. Int. J. Mol. Sci. 2016, 17, 44. https://doi.org/10.3390/ijms17010044
Lin K-H, Lin K-C, Lu W-J, Thomas P-A, Jayakumar T, Sheu J-R. Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo. International Journal of Molecular Sciences. 2016; 17(1):44. https://doi.org/10.3390/ijms17010044
Chicago/Turabian StyleLin, Kuan-Hung, Kao-Chang Lin, Wan-Jung Lu, Philip-Aloysius Thomas, Thanasekaran Jayakumar, and Joen-Rong Sheu. 2016. "Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo" International Journal of Molecular Sciences 17, no. 1: 44. https://doi.org/10.3390/ijms17010044





