Effects of Oral Administration of Chitin Nanofiber on Plasma Metabolites and Gut Microorganisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Oral Administration of CNFs and SDACNFs on Plasma Metabolites

| Compound | Comparative Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| CNF/Control | SDACNF/Control | Chitin/Control | CLNF/Control | |||||
| Ratio | p-Value | Ratio | p-Value | Ratio | p-Value | Ratio | p-Value | |
| ATP | 2.9 | 0.003 | 1.2 | 0.516 | 1.5 | 0.070 | 1.5 | 0.122 |
| 2,3-Diphosphoglyceric acid | 2.7 | 0.003 | 1.1 | 0.805 | 1.5 | 0.238 | 1.5 | 0.277 |
| ADP | 2.2 | 0.003 | 1.1 | 0.678 | 1.4 | 0.201 | 1.3 | 0.346 |
| 5-Hydroxytryptophan | 2.0 | 0.003 | 1.8 | 0.151 | 2.1 | 0.065 | 2.0 | 0.051 |
| ADP-ribose | 1.8 | 0.003 | 1.2 | 0.429 | 1.1 | 0.689 | 1.3 | 0.447 |
| UTP | 1.7 | 0.028 | 1.2 | 0.161 | 1.0 | 0.986 | 1.4 | N.A. |
| Cystine | 1.7 | 0.003 | 1.6 | 0.009 | 3.4 | 1.1 × 10−6 | 2.3 | 5.2 × 10−4 |
| Serotonin | 1.6 | 0.014 | 1.4 | 0.238 | 1.6 | 0.056 | 1.3 | 0.240 |
| Urocanic acid | 1.6 | 0.058 | 0.8 | 0.013 | 1.0 | 0.750 | 1.2 | 0.202 |
| 2,6-Diaminopimelic acid | 1.5 | 0.043 | 1.1 | N.A. | 1.7 | 0.040 | 2.4 | 0.253 |
| 1-Methylhistamine | 1.5 | 0.031 | 1.1 | 0.301 | 1.0 | 0.764 | 1.2 | 0.610 |
| 2-Aminoisobutyric acid | 1.4 | 0.023 | 0.9 | 0.470 | 1.0 | 0.723 | 1.3 | 0.041 |
| 2-Hydroxyisobutyric acid | 1.4 | 0.017 | 1.0 | 0.618 | 1.1 | 0.208 | 1.2 | 0.353 |
| N5-Ethylglutamine | 1.3 | 0.041 | 0.9 | 0.566 | 0.8 | 0.104 | 0.7 | 0.080 |
| Thiamine | 1.3 | 0.018 | 1.0 | 0.950 | 0.7 | 0.095 | 0.9 | 0.617 |
| Putrescine | 1.2 | 0.002 | 1.0 | 0.707 | 1.1 | 0.469 | 1.3 | 0.305 |
| Trimethylamine N-oxide | 1.1 | 0.696 | 1.6 | 0.013 | 1.4 | 0.238 | 2.2 | 0.109 |
| N-Acetylglycine | 1.1 | 0.324 | 0.9 | 0.040 | 1.0 | 0.721 | 1.2 | 0.054 |
| FA(17:1) | 0.9 | 0.544 | 0.7 | 0.022 | 0.7 | 0.013 | 0.9 | 0.253 |
| Cystathionine | 0.9 | 0.223 | 0.8 | 0.028 | 1.1 | 0.485 | 0.9 | 0.633 |
| Stearic acid | 0.9 | 0.349 | 0.8 | 0.033 | 0.8 | 0.016 | 0.9 | 0.353 |
| Glycolic acid | 0.8 | 0.026 | 1.0 | 0.864 | 1.0 | 0.826 | 1.0 | 0.934 |
| Glu | 0.8 | 0.038 | 0.8 | 0.101 | 0.8 | 0.031 | 0.8 | 0.042 |
| Betaine | 0.8 | 0.046 | 0.9 | 0.220 | 1.1 | 0.372 | 0.9 | 0.184 |
| Argininosuccinic acid | 0.7 | 0.027 | 0.7 | 0.024 | 0.6 | 0.005 | 0.8 | 0.429 |
| AC(18:0) | 0.7 | 0.007 | 0.6 | 0.002 | 0.5 | 4.9 × 10−4 | 0.8 | 0.140 |
| Palmitoylcarnitine | 0.7 | 0.138 | 0.4 | 0.019 | 0.4 | 0.020 | 0.7 | 0.221 |
| AC(18:1) | 0.6 | 0.080 | 0.4 | 0.019 | 0.5 | 0.029 | 0.8 | 0.525 |
| AC(18:2) | 0.5 | 0.015 | 0.4 | 0.008 | 0.5 | 0.013 | 0.8 | 0.431 |
| Taurodeoxycholic acid | 0.4 | 0.028 | 0.4 | 0.025 | 0.5 | 0.056 | 0.5 | 0.083 |
| 20α-Hydroxyprogesterone | 0.4 | 0.013 | 0.7 | 0.176 | 0.8 | 0.375 | 0.9 | 0.734 |
| Taurocholic acid | 0.3 | 0.028 | 0.9 | 0.872 | 0.4 | 0.042 | 0.6 | 0.122 |
| Compound Name | Control | CNF | SDACNF | Chitin | CLNF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| ATP | 8.0 × 10−4 | 3.1 × 10−4 | 2.3 × 10−3 | 7.5 × 10−4 | 9.6 × 10−4 | 4.3 × 10−4 | 1.2 × 10−3 | 3.0 × 10−4 | 1.2 × 10−3 | 4.5 × 10−4 |
| 2,3-Diphosphoglyceric acid | 6.4 × 10−4 | 1.5 × 10−4 | 1.7 × 10−3 | 5.2 × 10−4 | 6.7 × 10−4 | 2.6 × 10−4 | 9.6 × 10−4 | 5.2 × 10−4 | 9.8 × 10−4 | 5.9 × 10−4 |
| ADP | 7.8 × 10−4 | 3.0 × 10−4 | 1.7 × 10−3 | 4.4 × 10−4 | 8.8 × 10−4 | 4.3 × 10−4 | 1.1 × 10−3 | 3.5 × 10−4 | 1.1 × 10−3 | 5.1 × 10−4 |
| 5-Hydroxytryptophan | 8.4 × 10−6 | 1.9 × 10−6 | 1.7 × 10−5 | 4.0 × 10−6 | 1.5 × 10−5 | 6.9 × 10−6 | 1.8 × 10−5 | 8.5 × 10−6 | 1.7 × 10−5 | 5.6 × 10−6 |
| ADP-ribose | 1.2 × 10−4 | 4.0 × 10−5 | 2.2 × 10−4 | 3.6 × 10−5 | 1.5 × 10−4 | 5.9 × 10−5 | 1.3 × 10−4 | 2.9 × 10−5 | 1.7 × 10−4 | 1.1 × 10−4 |
| UTP | 9.4 × 10−5 | 1.8 × 10−5 | 1.6 × 10−4 | 5.3 × 10−5 | 1.2 × 10−4 | 1.5 × 10−6 | 9.4 × 10−5 | 2.1 × 10−5 | 1.3 × 10−4 | N.A. |
| Cystine | 7.4 × 10−4 | 2.1× 10−4 | 1.2 × 10−4 | 1.2 × 10−4 | 1.2 × 10−4 | 1.4 × 10−4 | 2.5 × 10−3 | 2.1 × 10−4 | 1.7 × 10−3 | 2.9 × 10−4 |
| Serotonin | 1.3 × 10−4 | 4.7 × 10−5 | 2.1 × 10−4 | 3.1 × 10−5 | 1.8 × 10−4 | 7.5 × 10−5 | 2.1 × 10−4 | 6.3 × 10−5 | 1.7 × 10−4 | 5.2 × 10−5 |
| Urocanic acid | 2.1 × 10−4 | 2.3 × 10−5 | 3.3 × 10−4 | 1.2 × 10−4 | 1.7 × 10−4 | 1.7 × 10−5 | 2.1 × 10−4 | 3.4 × 10−5 | 2.5 × 10−4 | 6.5 × 10−5 |
| 32,6-Diaminopimelic acid | 5.8 × 10−5 | 8.4 × 10−6 | 8.6 × 10−5 | 1.2 × 10−5 | 6.6 × 10−5 | N.A. | 9.8 × 10−5 | 8.2 × 10−6 | 1.4 × 10−4 | 8.6 × 10−5 |
| 1-Methylhistamine | 1.2 × 10−4 | 1.1 × 10−5 | 1.8 × 10−4 | 4.8 × 10−5 | 1.4 × 10−4 | 2.4 × 10−5 | 1.2 × 10−4 | 1.6 × 10−5 | 1.4 × 10−4 | 7.8 × 10−5 |
| 2-Aminoisobutyric acid | 2.4 × 10−3 | 5.2 × 10−4 | 3.4 × 10−3 | 6.7 × 10−4 | 2.2 × 10−3 | 4.5 × 10−4 | 2.3 × 10−3 | 3.2 × 10−4 | 3.2 × 10−3 | 4.8 × 10−4 |
| 2-Hydroxyisobutyric acid | 5.2 × 10−4 | 9.8 × 10−5 | 7.3 × 10−4 | 1.3 × 10−4 | 5.0 × 10−4 | 5.0 × 10−4 | 6.0 × 10−4 | 7.4 × 10−5 | 6.0 × 10−4 | 1.5 × 10−4 |
| N5-Ethylglutamine | 1.3 × 10−3 | 3.4 × 10−4 | 1.7 × 10−3 | 2.2 × 10−4 | 1.2 × 10−3 | 2.1 × 10−4 | 9.8 × 10−4 | 1.2 × 10−4 | 9.4 × 10−4 | 1.7 × 10−4 |
| Thiamine | 1.2 × 10−4 | 2.1 × 10−5 | 1.6 × 10−4 | 1.6 × 10−5 | 1.3 × 10−4 | 7.9 × 10−5 | 9.3 × 10−5 | 2.9 × 10−5 | 1.2 × 10−4 | 2.9 × 10−5 |
| Putrescine | 1.5 × 10−4 | 1.1 × 10−5 | 1.8 × 10−4 | 1.0 × 10−5 | 1.5 × 10−4 | 2.4 × 10−5 | 1.6 × 10−4 | 1.8 × 10−5 | 1.9 × 10−4 | 7.5 × 10−5 |
| Trimethylamine N-oxide | 4.6 × 10−3 | 7.7 × 10−4 | 5.0 × 10−3 | 2.6 × 10−3 | 7.4 × 10−3 | 1.6 × 10−3 | 6.6 × 10−3 | 3.2 × 10−3 | 9.9 × 10−3 | 5.8 × 10−3 |
| N-Acetylglycine | 1.7 × 10−4 | 1.5 × 10−5 | 1.8 × 10−4 | 1.7 × 10−5 | 1.5 × 10−4 | 1.5 × 10−5 | 1.7 × 10−4 | 2.7 × 10−5 | 2.2 × 10−4 | 3.5 × 10−5 |
| FA(17:1) | 3.2 × 10−5 | 4.6 × 10−6 | 3.0 × 10−5 | 7.8 × 10−6 | 2.3 × 10−5 | 5.1 × 10−6 | 2.2 × 10−5 | 5.2 × 10−6 | 2.8 × 10−5 | 5.4 × 10−6 |
| Ser | 4.3 × 10−2 | 9.7 × 10−3 | 4.0 × 10−2 | 5.6 × 10−3 | 4.0 × 10−2 | 4.3 × 10−3 | 3.7 × 10−2 | 4.6 × 10−3 | 3.7 × 10−2 | 6.1 × 10−3 |
| Cytidine | 5.9 × 10−4 | 1.1 × 10−4 | 5.4 × 10−4 | 1.6 × 10−4 | 5.4 × 10−4 | 9.3 × 10−5 | 6.2 × 10−4 | 5.3 × 10−5 | 7.0 × 10−4 | 1.3 × 10−4 |
| Cystathionine | 2.6 × 10−4 | 2.1 × 10−5 | 2.4 × 10−4 | 2.7 × 10−5 | 2.2 × 10−4 | 2.6 × 10−4 | 2.8 × 10−4 | 5.7 × 10−5 | 2.5 × 10−4 | 4.0 × 10−5 |
| Stearic acid | 1.1 × 10−3 | 1.2 × 10−4 | 9.7 × 10−4 | 2.0 × 10−4 | 8.7 × 10−4 | 1.2 × 10−4 | 8.1 × 10−4 | 1.4 × 10−4 | 9.7 × 10−4 | 1.9 × 10−4 |
| Glyoxylic acid | 1.4 × 10−4 | 4.0 × 10−4 | 1.3 × 10−4 | 2.0 × 10−5 | 1.4 × 10−4 | 2.4 × 10−4 | 1.4 × 10−4 | 2.2 × 10−5 | 1.9 × 10−4 | 5.8 × 10−5 |
| Glu | 1.4 × 10−2 | 2.4 × 10−3 | 1.1 × 10−2 | 8.0 × 10−4 | 1.1 × 10−2 | 2.3 × 10−3 | 1.1 × 10−2 | 1.1 × 10−3 | 1.1 × 10−2 | 9.1 × 10−4 |
| Betaine | 5.1 × 10−2 | 9.3 × 10−3 | 3.9 × 10−2 | 5.9 × 10−3 | 4.4 × 10−2 | 4.7 × 10−3 | 5.6 × 10−2 | 1.0 × 10−2 | 4.4 × 10−2 | 4.5 × 10−3 |
| Argininosuccinic acid | 1.7 × 10−3 | 2.9 × 10−5 | 1.3 × 10−4 | 2.5 × 10−5 | 1.3 × 10−4 | 2.7 × 10−5 | 1.1 × 10−4 | 2.6 × 10−5 | 1.4 × 10−4 | 7.5 × 10−5 |
| AC(18:0) | 5.3 × 10−5 | 7.2 × 10−6 | 3.7 × 10−5 | 8.0 × 10−6 | 3.2 × 10−5 | 2.8 × 10−6 | 2.8 × 10−5 | 7.0 × 10−6 | 4.3 × 10−5 | 1.1 × 10−5 |
| Palmitoylcarnitine | 3.5 × 10−4 | 1.3 × 10−4 | 2.4 × 10−4 | 8.7 × 10−5 | 1.3 × 10−4 | 1.9 × 10−5 | 1.5 × 10−4 | 4.7 × 10−5 | 2.4 × 10−4 | 1.2 × 10−4 |
| AC(18:1) | 2.6 × 10−4 | 9.7 × 10−5 | 1.6 × 10−4 | 5.5 × 10−5 | 9.8 × 10−5 | 1.9 × 10−5 | 1.2 × 10−4 | 5.4 × 10−5 | 2.2 × 10−4 | 1.2 × 10−4 |
| AC(18:2) | 1.2 × 10−4 | 3.5 × 10−5 | 6.3 × 10−5 | 2.1 × 10−5 | 5.0 × 10−5 | 1.1 × 10−5 | 5.9 × 10−5 | 2.7 × 10−5 | 9.9 × 10−5 | 5.5 × 10−5 |
| Taurodeoxycholic acid | 6.2 × 10−5 | 2.5 × 10−5 | 2.5 × 10−5 | 6.2 × 10−6 | 2.5 × 10−5 | 9.1 × 10−6 | 3.1 × 10−5 | 1.7 × 10−5 | 3.4 × 10−5 | 1.9 × 10−5 |
| 20α-Hydroxyprogesterone | 1.3 × 10−5 | 4.8 × 10−5 | 4.9 × 10−5 | 2.2 × 10−5 | 9.6 × 10−5 | 2.9 × 10−5 | 1.1 × 10−4 | 3.1 × 10−5 | 1.2 × 10−4 | 8.6 × 10−5 |
| Taurocholic acid | 4.0 × 10−4 | 1.8 × 10−4 | 1.4 × 10−4 | 3.1 × 10−5 | 3.7 × 10−4 | 3.3 × 10−4 | 1.7 × 10−4 | 1.1 × 10−4 | 2.4 × 10−4 | 7.6 × 10−5 |
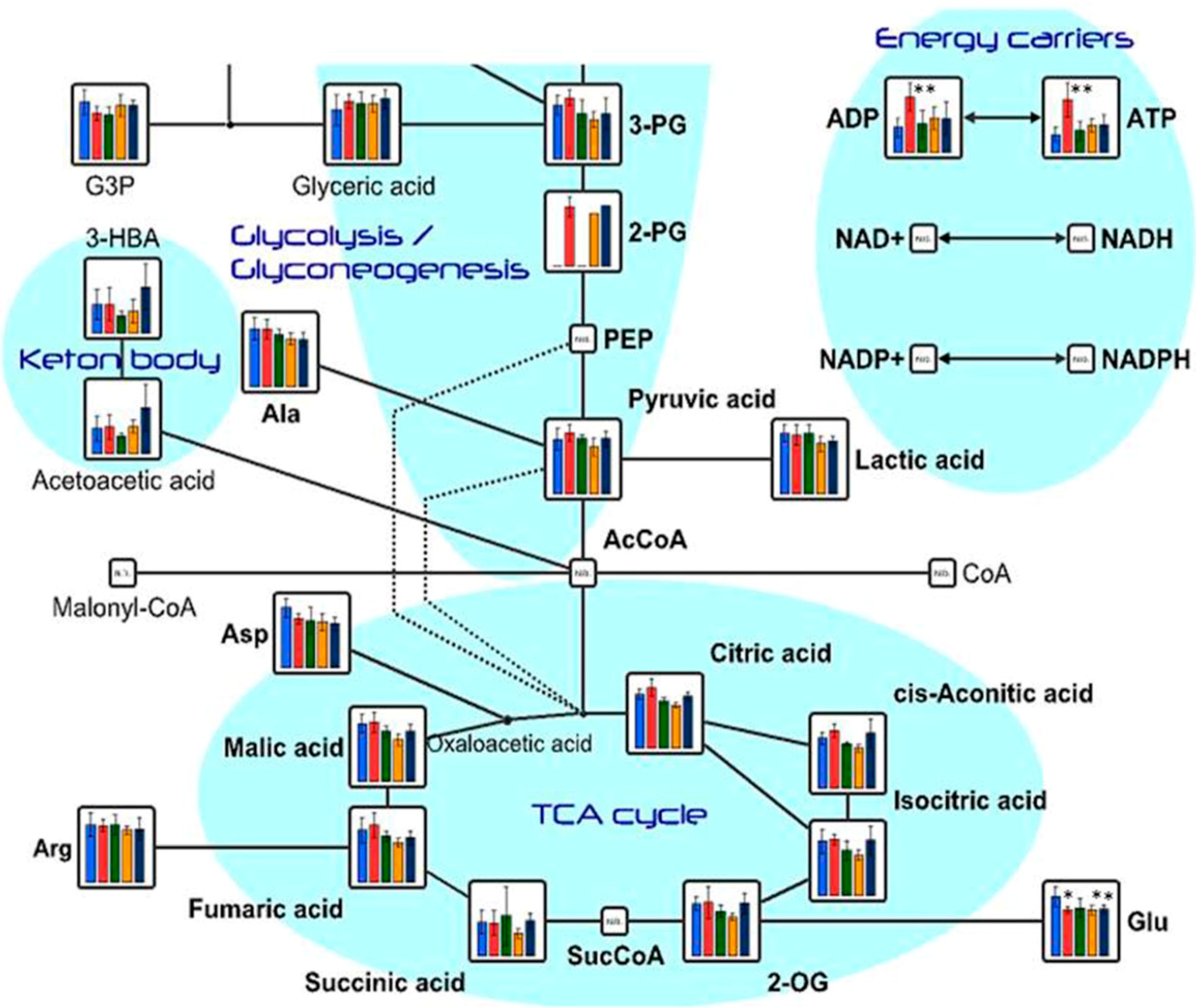

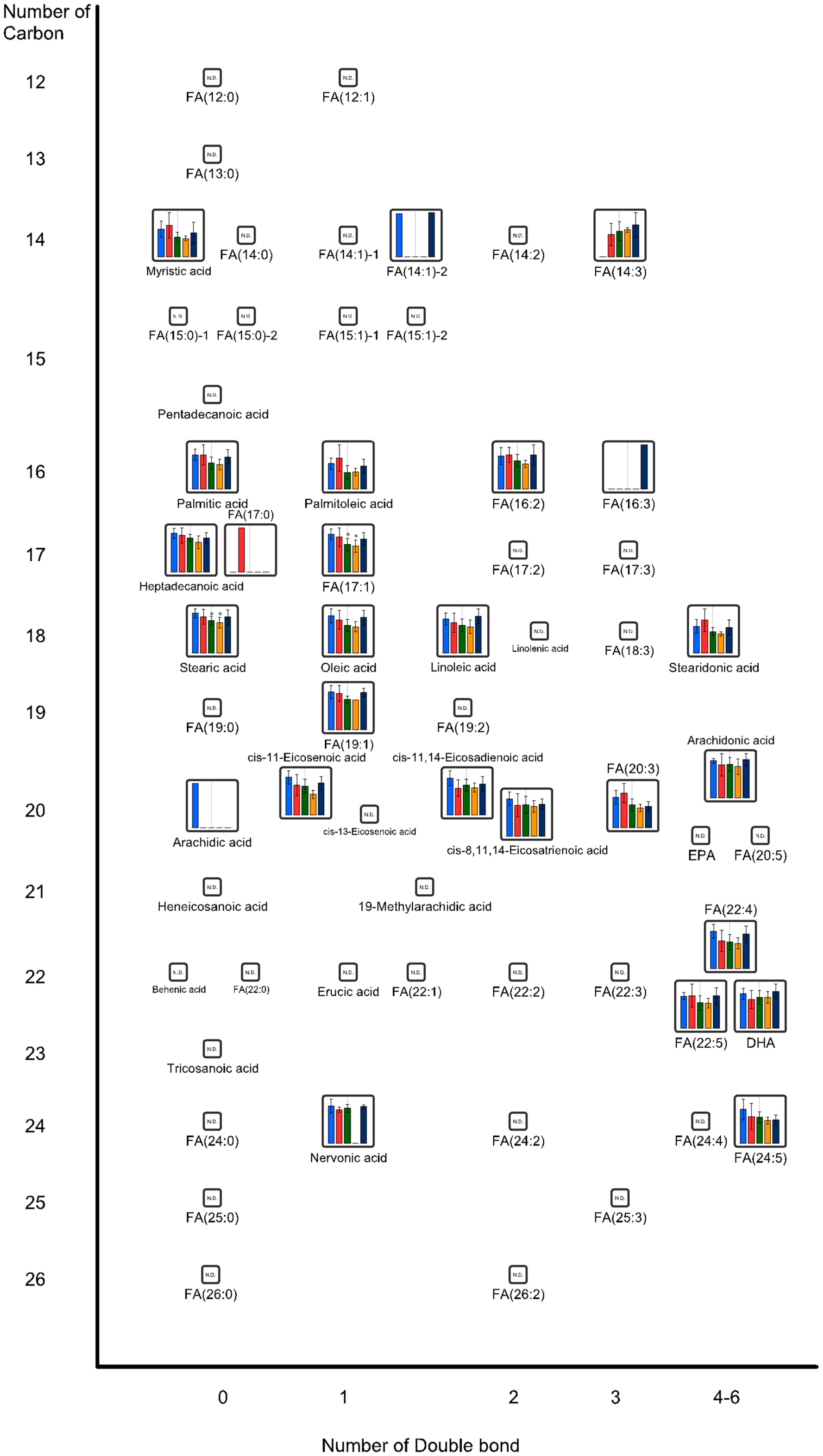
2.2. Effects of Oral Administrations of CNFs and SDACNFs on Gut Microbiota and Fecal Organic Acid Levels
| % Peak Area | Control | CNF | SDACNF | Chitin | CLNF |
|---|---|---|---|---|---|
| Bacteroidales | 36.8 ± 3.5 | 38.1 ± 6.5 | 52.0 ± 6.6 * | 42.3 ± 3.4 | 41.1 ± 5.2 |
| Lactobacillus | 28.9 ± 14.2 | 27.6 ± 5.2 | 18.1 ± 12.9 | 27.7 ± 2.8 | 21.4 ± 6.6 |
| Clostridiales | 18.5 ± 12.4 | 14.0 ± 3.9 | 15.4 ± 12.6 | 15.1 ± 7.0 | 21.4 ± 6.1 |
| Erysipelotrichaceae | 1.5 ± 0.7 | 1.6 ± 0.4 | 1.0 ± 0.3 | 2.1 ± 0.7 | 2.1 ± 0.5 |
| Akkermansia | 0.3 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.0 ± 0.0 |
| Anaeroplasma | 0.3 ± 0.2 | 0.4 ± 0.7 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Corinebacteriales | 0.6 ± 0.4 | 0.5 ± 0.1 | 0.5 ± 0.4 | 0.5 ± 0.3 | 0.7 ± 0.2 |
| Mucispirillum | 2.5 ± 1.7 | 1.7 ± 0.2 | 1.4 ± 1.2 | 2.4 ± 0.3 | 1.3 ± 0.4 |
| Parasutterella | 2.1 ± 1.5 | 4.1 ± 1.3 | 1.9 ± 1.0 | 2.0 ± 0.8 | 3.2 ± 1.2 |
| mg/g | Control | CNF | SDACNF | Chitin | CLNF |
|---|---|---|---|---|---|
| Lactic acid | 2.1 ± 0.8 | 4.2 ± 1.2 * | 1.6 ± 1.3 | 1.6 ± 0.5 | 2.3 ± 0.6 |
| Acetic acid | 3.3 ± 0.7 | 4.1 ± 0.7 † | 3.8 ± 0.8 | 3.3 ± 0.4 | 2.7 ± 0.3 |
| Propionic acid | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 * | 0.5 ± 0.1 | 0.3 ± 0.1 |
| n-Butyric acid | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
3. Experimental Section
3.1. Animals and Reagents
3.2. Preparations of CNF, SDACNF, and CLNF
3.3. Study Design
3.4. Measurement of Metabolites
3.4.1. CE-TOFMS Measurement
3.4.2. LC-TOFMS Measurement
3.5. Measurement of Gut Microbiota
3.6. Measurements of Fecal SCFAs
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muzzarelli, R.A.A. Chitin nanostructures in living organisms. In Chitin: Formation and Diagenesis; Gupta, N., Ed.; Springer: Dordrecht, Dutch, 2011; Volume 34, pp. 1–34. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [PubMed]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Wakuda, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model. Carbohydr. Polym. 2012, 87, 1399–1403. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. α-Chitin nanofibrils improve inflammatory and fibrosis responses in mice with inflammatory bowel disease. Carbohydr. Polym. 2012, 90, 197–200. [Google Scholar] [PubMed]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. A comparative study analysis of α-chitin and β-chitin nanofibrils by using an inflammatory-bowel disease mouse model. J. Chitin Chitosan Sci. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Azuma, K.; Nagae, T.; Nagai, T.; Izawa, H.; Morimoto, M.; Murahata, Y.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; et al. Effects of surface-deacetylated chitin nanofibers in an experimental model of hypercholesterolemia. Int. J. Mol. Sci. 2015, 16, 17445–17455. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Tokuda, K.; Asami, T.; Takamori, Y.; Kurozumi, S.; Ifuku, S.; Saimoto, H.; Imagawa, T.; Azuma, K.; et al. Effect of chitin nanofibril combined in rayon animal bedding on hairless mouse skin and on a three dimensional culture human skin model. J. Chitin Chitosan Sci. 2014, 2, 82–88. [Google Scholar] [CrossRef]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Nishihara, M.; Shimizu, H.; Itoh, Y.; Takashima, O.; Osaki, T.; Itoh, N.; Imagawa, T.; Murahata, Y.; Tsuka, T.; et al. Biological adhesive based on carboxymethyl chitin derivatives and chitin nanofibers. Biomaterials 2015, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Suzuki, M.; Kobayashi, T.; Matsubara, A.; Azuma, T.; Yoshida, M. Metabolomics for biomarker discovery in gastroenterological cancer. Metabolites 2014, 4, 547–571. [Google Scholar] [PubMed]
- Nambiar, P.R.; Gupta, R.R.; Misra, V. An “Omics” based survey of human colon cancer. Mutat. Res. 2010, 693, 3–18. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics reviewed: A new “Omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. Genomics: The tale of our other genome. Nature 2010, 465, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P.; Tsang, T.M.; Wang, Y.; Cloarec, O.; Skordi, E.; Martin, F.P.; Rezzi, S.; Ross, A.; Kochhar, S.; Holmes, E.; et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 2008, 4, 219. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Dumas, M.E.; Wang, Y.; Legido-Quigley, C.; Yap, I.K.; Tang, H.; Zirah, S.; Murphy, G.M.; Cloarec, O.; Lindon, J.C.; et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 2007, 3, 112. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Boren, J.; Oresic, M.; et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010, 51, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 1992, 284, 1–13. [Google Scholar]
- Burnstock, G.; Campbell, G.; Satchell, D.; Smythe, A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970, 40, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Jouaville, L.S.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Thore, A. Comparison of methods for extract of bacterial adenine nucleotides determined by firefly assay. Appl. Microbiol. 1975, 30, 713–721. [Google Scholar] [PubMed]
- Perriman, R.; Barta, I.; Voeltz, G.K.; Abelson, J.; Ares, M., Jr. ATP requirement for Prp5p function is determined by Cus2p and the structure of U2 small nuclear RNA. Proc. Natl. Acad. Sci. USA 2003, 100, 13857–13862. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 1996, 49, 589–618. [Google Scholar] [CrossRef]
- Helenius, M.; Jalkanen, S.; Yegutkin, G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim. Biophys. Acta 2012, 1823, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 15, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 9, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.; Wanders, R. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.W.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.J.; Tubbs, P.K. Carnitine and derivatives in rat tissues. Biochem. J. 1967, 105, 953–963. [Google Scholar] [PubMed]
- Murakami, R.; Tanaka, A.; Nakamura, H. The effect of starvation on brain carnitine concentration in neonatal rats. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.C.G.; de Almeida, I.T.; Jakobs, C.; Poll-The, B.T.; Duran, M. Dynamic changes of plasma acylcarnitine levels induced by fasting and sunflower oil challenge test in children. Pediatr. Res. 1999, 46, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.S. Non-esterified fatty acids in blood of obese and lean subjects. Am. J. Clin. Nutr. 1960, 8, 740–747. [Google Scholar]
- Jensen, M.D.; Haymond, M.W.; Rizza, R.A.; Cryer, P.E.; Miles, J.M. Influence of body fat distribution on free fatty acid metabolism in obesity. J. Clin. Investig. 1989, 83, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P.; Bergman, H.; Varnauskas, E. Plasma free fatty acid turnover rate in obesity. Acta Med. Scand. 1969, 185, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K. Effects of diet on gut microbiota profile and the implication for health and disease. Biosci. Microbiota Food Health 2003, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 2010, 15, 25–34. [Google Scholar] [CrossRef]
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J. Gastroenterol. 2011, 17, 557–566. [Google Scholar] [PubMed]
- Willing, B.; Halfvarson, J.; Dicksved, J.; Rosenquist, M.; Jarnerot, G.; Engstrand, L.; Tysk, C.; Jansson, J.K. Twin studies reveal specific imbalances in the mucosa associated microbiota of patients with ileal Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Vaahtovuo, J.; Munukka, E.; Korkeamaki, M.; Luukkainen, R.; Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 1500–1505. [Google Scholar] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Brighenti, F.; Royall, D.; Jenkins, A.L.; Jenkins, D.J. Effect of rectal infusion of short chain fatty acids in human subjects. Am. J. Gastroenterol. 1989, 84, 1027–1033. [Google Scholar] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 14, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Heiger, D.N. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000, 72, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ueno, Y.; Naraoka, H.; Ohashi, Y.; Tomita, M.; Nishioka, T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2002, 74, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2009, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Junker, B.H.; Klukas, C.; Schreiber, F. VANTED: A system for advanced data analysis and visualization in the context of biological networks. BMC Bioinform. 2006, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kuwayama, E.; Nozu, R.; Ueno, M.; Hashimoto, N. Effect of hypochlorous acid solution on the eradication and prevention of Pseudomonas aeruginosa infection, serum biochemical variables, and cecum microbiota in rats. Exp. Anim. 2015, 64, 191–197. [Google Scholar] [PubMed]
- Kibe, R.; Sakamoto, M.; Hayashi, H.; Yokota, H.; Benno, Y. Maturation of the murine cecal microbiota as revealed by terminal restriction fragment length polymorphism and 16S rRNA gene clone libraries. FEMS Microbiol. Lett. 2004, 235, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Hisada, T.; Sato, M.; Mochizuki, J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol. 2003, 69, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, K.; Izumi, R.; Kawata, M.; Nagae, T.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Ito, N.; Okamoto, Y.; et al. Effects of Oral Administration of Chitin Nanofiber on Plasma Metabolites and Gut Microorganisms. Int. J. Mol. Sci. 2015, 16, 21931-21949. https://doi.org/10.3390/ijms160921931
Azuma K, Izumi R, Kawata M, Nagae T, Osaki T, Murahata Y, Tsuka T, Imagawa T, Ito N, Okamoto Y, et al. Effects of Oral Administration of Chitin Nanofiber on Plasma Metabolites and Gut Microorganisms. International Journal of Molecular Sciences. 2015; 16(9):21931-21949. https://doi.org/10.3390/ijms160921931
Chicago/Turabian StyleAzuma, Kazuo, Ryotaro Izumi, Mari Kawata, Tomone Nagae, Tomohiro Osaki, Yusuke Murahata, Takeshi Tsuka, Tomohiro Imagawa, Norihiko Ito, Yoshiharu Okamoto, and et al. 2015. "Effects of Oral Administration of Chitin Nanofiber on Plasma Metabolites and Gut Microorganisms" International Journal of Molecular Sciences 16, no. 9: 21931-21949. https://doi.org/10.3390/ijms160921931







