Current Status on Stem Cells and Cancers of the Gastric Epithelium
Abstract
:1. Introduction
2. Gastric Self-Renewal and Gastric Stem Cells

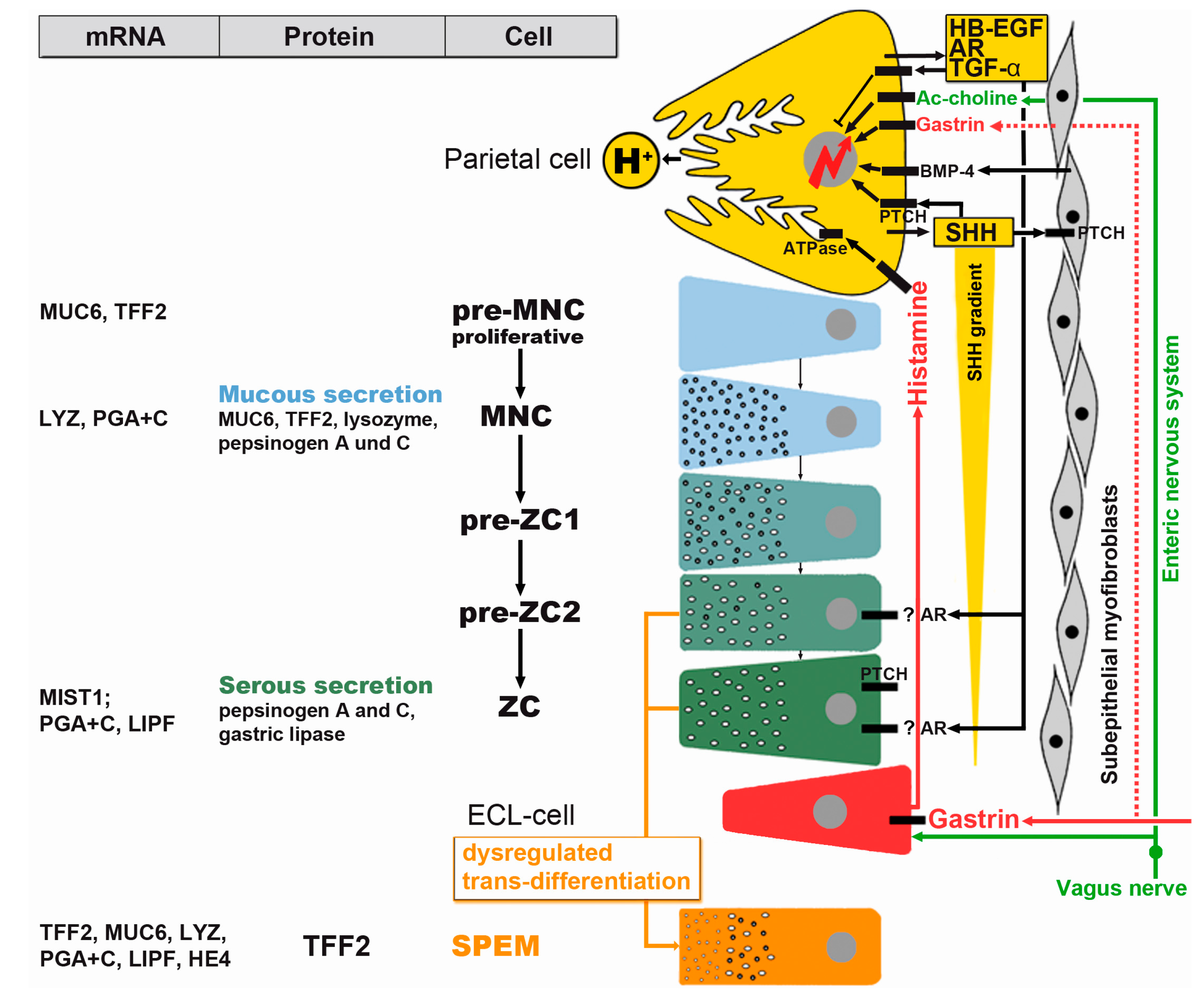
2.1. Bidirectional Self-Renewal of Gastric Units
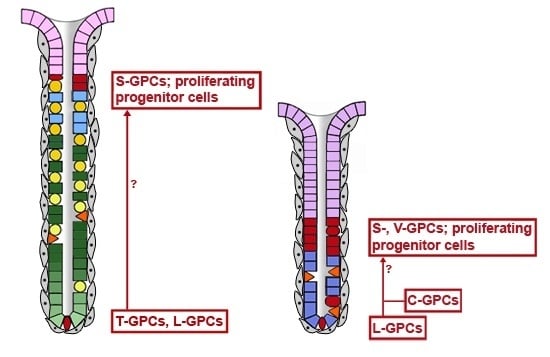
2.2. Gastric Units Contain Multiple Progenitor/Stem Cell Populations
2.3. Generation of the Anterior-Posterior Axis
3. Gastric Adenocarcinomas, Dysregulated Self-Renewal, and Gastric Cancer Stem Cells
3.1. Consequences of H. pylori Infection
3.2. Metaplasias
3.3. Cancer Stem Cells
4. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, J.; Leja, M.; Kupcinskas, J.; Link, A.; Weaver, J.; Rugge, M.; Malfertheiner, P. Molecular diagnostics in gastric cancer. Front. Biosci. 2014, 19, 312–338. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [PubMed]
- Nakamura, K.; Sugano, H.; Takagi, K. Carcinoma of the stomach in incipient phase: Its histogenesis and histological appearances. Gann 1968, 59, 251–258. [Google Scholar] [PubMed]
- Gomceli, I.; Demiriz, B.; Tez, M. Gastric carcinogenesis. World J. Gastroenterol. 2012, 18, 5164–5170. [Google Scholar] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar]
- Milne, A.N.; Carneiro, F.; O’morain, C.; Offerhaus, G. Nature meets nurture: Molecular genetics of gastric cancer. Hum. Genet. 2009, 126, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.M.; Morozov, A.; Smirnova, I.; Wang, T.C. Stem cells and cancer. Semin. Cancer Biol. 2007, 17, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, J.; Malfertheiner, P. Helicobacter pylori and gastric cancer. Dig. Dis. 2014, 32, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F. Hereditary gastric cancer. Pathologe 2012, 33 (Suppl. 2), 231–234. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bessede, E.; Dubus, P.; Megraud, F.; Varon, C. Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene 2014, 34, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Regeneration of the gastric mucosa and its glands from stem cells. Curr. Med. Chem. 2008, 15, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.; Greaves, L.C.; Gutierrez-Gonzalez, L.; Rodriguez-Justo, M.; Deheragoda, M.; Leedham, S.J.; Taylor, R.W.; Lee, C.Y.; Preston, S.L.; Lovell, M.; et al. Mechanisms of field cancerization in the human stomach: The expansion and spread of mutated gastric stem cells. Gastroenterology 2008, 134, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Self-renewal of the gastric epithelium from stem and progenitor cells. Front. Biosci. 2013, 5, 720–731. [Google Scholar] [CrossRef]
- Stevens, C.E.; Leblond, C.P. Renewal of the mucous cells in the gastric mucosa of the rat. Anat. Rec. 1953, 115, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 1999, 4, D286–D298. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, G.R. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol. Rev. 2007, 87, 1343–1375. [Google Scholar] [CrossRef] [PubMed]
- Goldenring, J.R.; Nam, K.T.; Mills, J.C. The origin of pre-neoplastic metaplasia in the stomach: Chief cells emerge from the Mist. Exp. Cell. Res. 2011, 317, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Merchant, J.L. Hedgehog signalling in gut development, physiology and cancer. J. Physiol. 2012, 90, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, W. Stem cells, self-renewal and cancer of the gastric epithelium. Curr. Med. Chem. 2012, 19, 5975–5983. [Google Scholar] [CrossRef] [PubMed]
- Bredemeyer, A.J.; Geahlen, J.H.; Weis, V.G.; Huh, W.J.; Zinselmeyer, B.H.; Srivatsan, S.; Miller, M.J.; Shaw, A.S.; Mills, J.C. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev. Biol. 2009, 325, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Marrache, F.; Goldenring, J.R.; Wang, T.C. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 2010, 139, 2018–2027.e2. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, I.; Kalinski, T.; Meyer, F.; Hoffmann, W. Self-renewal of the human gastric epithelium: New insights from expression profiling using laser microdissection. Mol. Biosyst. 2011, 7, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Kontinuierliche Regeneration des Magenepithels durch Zelldifferenzierung. Bedeutung für die Karzinogenese (Continual self-renewal of the gastric epithelium by cell differentiation:. Implications for carcinogenesis). Pathologe 2014, 35 (Suppl. 2), 202–206. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 1993, 236, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero, G. Über die schlauchförmigen Drüsen des Magendarmkanals und die Beziehungen ihres Epithels zu dem Oberflächenepithel der Schleimhaut Dritte Mittheilung Mittheilung (On tube-like glands in the gastrointestinal canal and the relations of their epithelium to the surface epithelium of the mucosa. Third communication). Arch. Mikrosk. Anat. 1893, 42, 82–152. [Google Scholar] [CrossRef]
- Lee, E.R.; Leblond, C.P. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am. J. Anat. 1985, 172, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Straiton, T.; Hassan, W.M.; Leblond, C.P. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells 2003, 21, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, M.; Stappenbeck, T.S.; Mills, J.C.; Leip, D.G.; Lovett, M.; Clifton, S.W.; Ippolito, J.E.; Glasscock, J.I.; Arumugam, M.; Brent, M.R.; et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J. Biol. Chem. 2006, 281, 11292–11300. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shivdasani, R.A. Notch signaling in stomach epithelial stem cell homeostasis. J. Exp. Med. 2011, 208, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G767–G677. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Shivdasani, R.A. Gastric epithelial stem cells. Gastroenterology 2011, 140, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.T.; Ziel, J.W.; McKimpson, W.; Madison, B.B.; Todisco, A.; Merchant, J.L.; Samuelson, L.C.; Gumucio, D.L. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 2007, 133, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Stange, D.E.; Koo, B.K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015, 148, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.G.; Lee, B.L.; Kim, W.H. Distribution of LGR5+ cells and associated implications during the early stage of gastric tumorigenesis. PLoS ONE 2013, 8, e82390. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Jin, G.; Wang, H.; Chen, X.; Westphalen, C.B.; Asfaha, S.; Renz, B.W.; Ariyama, H.; Dubeykovskaya, Z.A.; Takemoto, Y.; et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 2014. [CrossRef] [PubMed]
- Leushacke, M.; Ng, A.; Galle, J.; Loeffler, M.; Barker, N. Lgr5+ gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 2013, 5, 349–356. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.B.; Snel, B.; Clevers, H.C. The R-spondin protein family. Genome Biol. 2012, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Leushacke, M.; Barker, N. Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene 2012, 31, 3009–3022. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Bartfeld, S.; Clevers, H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Sem Cell 2010, 7, 656–670. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Cata, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Roland, J.T.; Barlow, B.J.; O’Neal, R.; Rich, A.E.; Nam, K.T.; Shi, C.; Goldenring, J.R. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014, 63, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Naumann, M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010, 18, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Donnelly, J.M.; Engevik, A.C.; Xiao, C.; Yang, L.; Kenny, S.; Varro, A.; Hollande, F.; Samuelson, L.C.; Zavros, Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 2012, 142, 1150–1159.e6. [Google Scholar] [CrossRef] [PubMed]
- Sigal, M.; Rothenberg, M.E.; Logan, C.Y.; Lee, J.Y.; Honaker, R.W.; Cooper, R.L.; Passarelli, B.; Camorlinga, M.; Bouley, D.M.; Alvarez, G.; et al. Helicobacter pylori activates and expands Lgr5+ stem cells through direct colonization of the gastric glands. Gastroenterology 2015, 148, 1392–1404.e21. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Ma, D.; Yao, Y.; Lynch, J.P.; Morales, K.; Ziober, A.H.; Feldmann, M.; Ota, H.; Sepulveda, A.R. H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig. Dis. Sci. 2013, 58, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Polarity proteins in migration and invasion. Oncogene 2008, 27, 6970–6980. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Cano, A. Tumorigenesis: Twist1 links EMT to self-renewal. Nat. Cell Biol. 2010, 12, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Baud, J.; Varon, C.; Chabas, S.; Chambonnier, L.; Darfeuille, F.; Staedel, C. Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS ONE 2013, 8, e60315. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, N.; Chang, H.; Lee, H.S.; Park, S.M.; Park, J.H.; Shin, C.M.; Kim, J.M.; Kim, J.S.; Lee, D.H.; et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015, 36, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.H.; Lee, J.R.; Joshi, V.; Playford, R.J.; Poulsom, R.; Wright, N.A.; Goldenring, J.R. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Investig. 1999, 79, 639–646. [Google Scholar] [PubMed]
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010, 138, 2207–2210.e1. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Weis, V.; Wang, T.C.; Falus, A.; Goldenring, J.R. Altered gastric chief cell lineage differentiation in histamine-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1211–G1220. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Lee, H.J.; Sousa, J.F.; Weis, V.G.; O’Neal, R.L.; Finke, P.E.; Romero-Gallo, J.; Shi, G.; Mills, J.C.; Peek, R.M., Jr.; et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010, 139, 2028–2037.e9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wodziak, D.; Tun, M.; Bouley, D.M.; Lowe, A.W. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J. Biol. Chem. 2013, 288, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gonzalez, L.; Graham, T.A.; Rodriguez-Justo, M.; Leedham, S.J.; Novelli, M.R.; Gay, L.J.; Ventayol-Garcia, T.; Green, A.; Mitchell, I.; Stoker, D.L.; et al. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology 2011, 140, 1251–1260.e1. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric cancer originating from bone marrow-derived cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Varon, C.; Dubus, P.; Mazurier, F.; Asencio, C.; Chambonnier, L.; Ferrand, J.; Giese, A.; Senant-Dugot, N.; Carlotti, M.; Megraud, F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology 2012, 142, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Stoicov, C.; Li, H.; Liu, J.H.; Houghton, J. Mesenchymal stem cells utilize CXCR4-SDF-1 signaling for acute, but not chronic, trafficking to gastric mucosal inflammation. Dig. Dis. Sci. 2013, 58, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Ariyama, H.; Westphalen, C.B.; Worthley, D.L.; Muthupalani, S.; Asfaha, S.; Dubeykovskaya, Z.; Quante, M.; Fox, J.G.; Wang, T.C. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut 2013, 62, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Poulsom, R.; Jeffery, R.; Dhillon, A.P.; Quaglia, A.; Jacob, J.; Novelli, M.; Prentice, G.; Williamson, J.; Wright, N.A. Hepatocytes from non-hepatic adult stem cells. Nature 2000, 406, 257. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Cells of origin in cancer. Nature 2011, 469, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shen, J.; Ou Yang, X.; Sasahara, M.; Su, X. Cancer stem cells: The “heartbeat” of gastric cancer. J. Gastroenterol. 2013, 48, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R. Gastric cancer stem cells: A novel therapeutic target. Cancer Lett. 2013, 338, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Oh, S.-O. Gastric stem cells and gastric cancer stem cells. Anat. Cell Biol. 2013, 46, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, L.P.; Zhang, N.; Zhao, C.H. Role of cancer stem cell marker CD44 in gastric cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 5059–5066. [Google Scholar] [PubMed]
- Zhan, Y.Y.; He, J.P.; Chen, H.Z.; Wang, W.J.; Cai, J.C. Orphan receptor TR3 is essential for the maintenance of stem-like properties in gastric cancer cells. Cancer Lett. 2013, 329, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, Z.; Wang, L.; Kong, X.; Li, Q.; Guo, K.; Tan, D.; Le, X.; Wei, D.; Huang, S.; et al. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology 2012, 142, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Chenard, M.P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, K.; Tomasetto, C.; Narko, K.; Rio, M.C.; Ristimaki, A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003, 63, 3032–3036. [Google Scholar] [PubMed]
- Ishimoto, T.; Sawayama, H.; Sugihara, H.; Baba, H. Interaction between gastric cancer stem cells and the tumor microenvironment. J. Gastroenterol. 2014, 49, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Stojnev, S.; Krstic, M.; Ristic-Petrovic, A.; Stefanovic, V.; Hattori, T. Gastric cancer stem cells: Therapeutic targets. Gastric Cancer 2014, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Han, M.E.; Baek, S.J.; Kim, S.Y.; Kang, C.D.; Oh, S.O. ATOH1 can regulate the tumorigenicity of gastric cancer cells by inducing the differentiation of cancer stem cells. PLoS ONE 2015, 10, e0126085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, K.E.; Zhao, E.; Li, W.; Shi, L.; Xie, G.; Jiang, B.; Wang, Y.; Li, R.; Zhang, P.; et al. B7-H1 enhances proliferation ability of gastric cancer stem-like cells as a receptor. Oncol. Lett. 2015, 9, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Samadani, A.A.; Akhavan-Niaki, H. Interaction of sonic hedgehog (SHH) pathway with cancer stem cell genes in gastric cancer. Med. Oncol. 2015, 32, 48. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Bhagat, G.; Abrams, J.A.; Marache, F.; Good, P.; Lee, M.D.; Lee, Y.; Friedman, R.; Asfaha, S.; Dubeykovskaya, Z.; et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model or Barrett-like metaplasia. Cancer Cell 2012, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, W. Current Status on Stem Cells and Cancers of the Gastric Epithelium. Int. J. Mol. Sci. 2015, 16, 19153-19169. https://doi.org/10.3390/ijms160819153
Hoffmann W. Current Status on Stem Cells and Cancers of the Gastric Epithelium. International Journal of Molecular Sciences. 2015; 16(8):19153-19169. https://doi.org/10.3390/ijms160819153
Chicago/Turabian StyleHoffmann, Werner. 2015. "Current Status on Stem Cells and Cancers of the Gastric Epithelium" International Journal of Molecular Sciences 16, no. 8: 19153-19169. https://doi.org/10.3390/ijms160819153