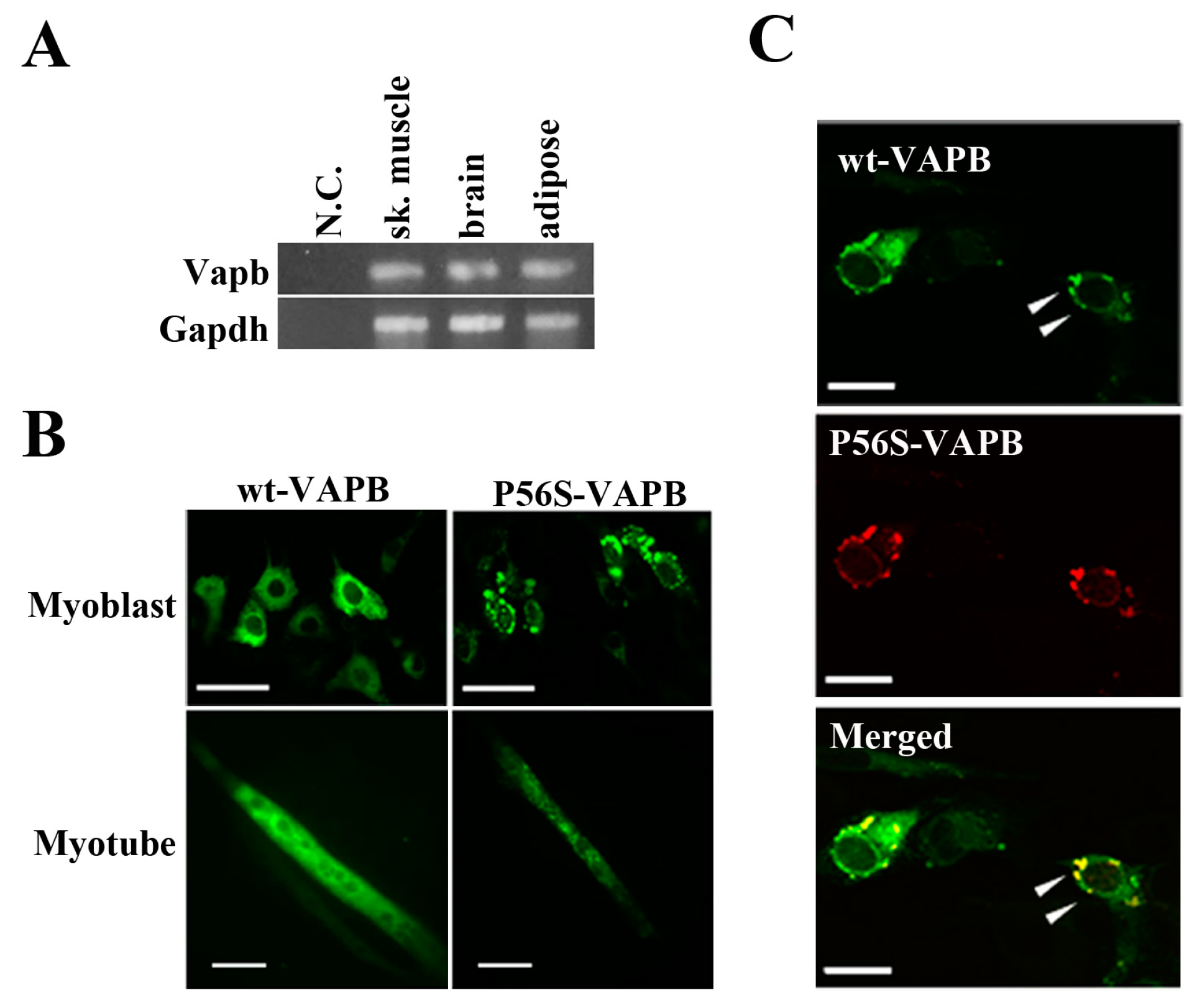
2.1. P56S Mutation Results in Aberrant Aggregation of VAPB in C2C12 Cells
First, we confirmed by RT-PCR experiments that VAPB mRNA was expressed in mouse skeletal muscle, brain, and adipose tissue (
Figure 1A). Further, we transfected expression vectors carrying a gene for either wt-VAPB or P56S-VAPB with GFP added at the C-terminus into the C2C12 cells and observed the intracellular localization of these proteins. wt-VAPB was distributed uniformly in the cells, whereas P56S-VAPB aggregated in the cells (
Figure 1B). Moreover, abnormal aggregation of P56S-VAPB was observed not only in undifferentiated cells, but also in myotubes, on the 6th day after the induction of differentiation. Furthermore, we co-transfected GFP-fused wt-VAPB and Ds-Red-fused P56S-VAPB genes into C2C12 cells, and we found that wt-VAPB and P56S-VAPB were co-localized as aggregates in the cells (
Figure 1C).
Figure 1.
P56S mutation leads to aberrant aggregation of VAPB in C2C12 cells. (A) VAPB mRNA expression was analyzed by RT-PCR. Total RNA was collected from the indicated tissues of mice. N.C. indicates negative control (sample in which no reverse transcriptase was added); (B) C2C12 cells were transfected with the indicated plasmids and fixed either before inducing differentiation (upper: Myoblast) or five days after differentiation (lower: Myotube). The distribution of the VAPB protein is indicated by GFP expression; (C) C2C12 cells were co-transfected with vectors encoding C-terminally GFP-fused wt-VAPB and Ds-Red-fused P56S-VAPB, followed by fixation 24 h after transfection. Scale bar = 20 μm. The merged image is shown on the bottom. Examples of co-localization are indicated with arrowheads. These images are representative of three similar experiments.
Figure 1.
P56S mutation leads to aberrant aggregation of VAPB in C2C12 cells. (A) VAPB mRNA expression was analyzed by RT-PCR. Total RNA was collected from the indicated tissues of mice. N.C. indicates negative control (sample in which no reverse transcriptase was added); (B) C2C12 cells were transfected with the indicated plasmids and fixed either before inducing differentiation (upper: Myoblast) or five days after differentiation (lower: Myotube). The distribution of the VAPB protein is indicated by GFP expression; (C) C2C12 cells were co-transfected with vectors encoding C-terminally GFP-fused wt-VAPB and Ds-Red-fused P56S-VAPB, followed by fixation 24 h after transfection. Scale bar = 20 μm. The merged image is shown on the bottom. Examples of co-localization are indicated with arrowheads. These images are representative of three similar experiments.
2.2. P56S Mutation Reduced the Myotube Elongation and Results in Aberrant Localization Pattern of Myonuclei
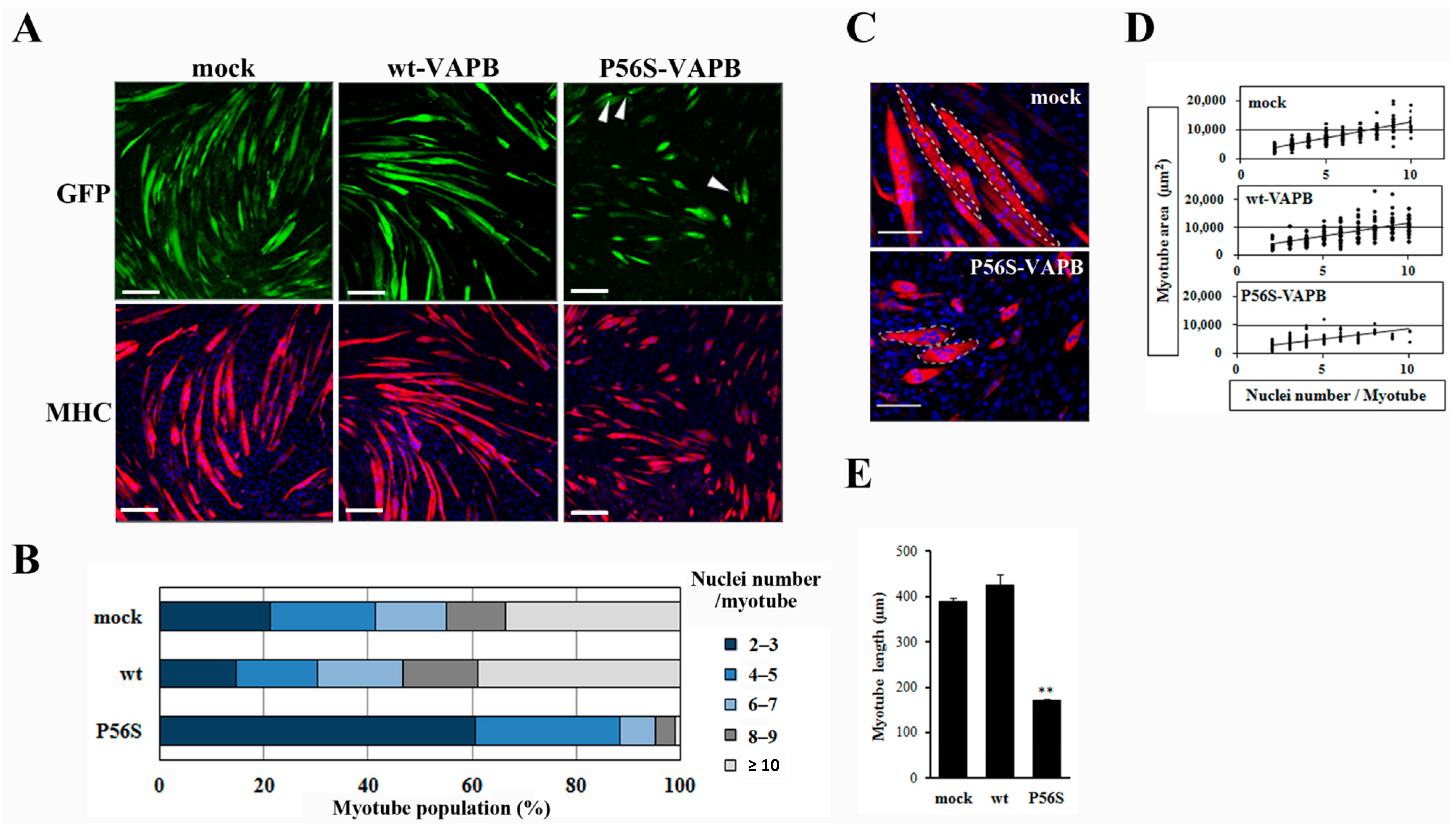
Next, we prepared cell lines stably expressing wt-VAPB, P56S-VAPB, or GFP (mock) to examine the influences of P56S mutation on differentiation of the skeletal muscles. Immunofluorescent staining of the cells with anti-myosin heavy chain (MHC) antibodies on the 5th day after the induction of differentiation showed that myotube formation was suppressed in the P56S-VAPB-expressing cell line, whereas no appreciable difference was found between the GFP-expressing cell line and the wt-VAPB-expressing cell line (
Figure 2A). The other P56S-VAPB-expressing cell line also showed reduced myotube formation (
Supplementary Figure S1). Nuclei were counted in each myotube, and the proportions of myotubes containing different numbers of nuclei were analyzed. For the P56S-VAPB-expressing cell line, we found that myotubes containing two or three nuclei accounted for 60.7% of the entire set of myotubes analyzed, myotubes containing six or more nuclei accounted for a smaller proportion than in the other cell lines, and myotubes containing more than 10 nuclei were not formed (
Figure 2B). The myotubes containing six or more nuclei in the P56S-VAPB-expressing cell line showed an aberrant localization pattern of nuclei and were smaller in size than the corresponding myotube subpopulations of other cell lines (
Figure 2C). An analysis of the relationship between the number of nuclei and the myotube area showed that the area of the myotube was smaller in the P56S-VABP-expressing cell line than in other cell lines, even when myotubes containing the same number of nuclei were compared across the cell lines (
Figure 2D). We next measured the myotube length of three cell lines. Quantitative analysis showed that myotube length of the P56S-VAPB-expressing cell line was significantly shorter than those of other cell lines (
Figure 2E). We did not observe any reduction of the numbers of P56S-VAPB-expressing cells during myogenic differentiation (
Supplementary Figure S2). The reduction of myotube formation was not correlated with any cell death. These results revealed that P56S-VAPB suppressed fusion and enlargement of C2C12 cells.
2.3. P56S Mutation Suppressed Fusion of C2C12 Cells for Late-Stage Differentiation
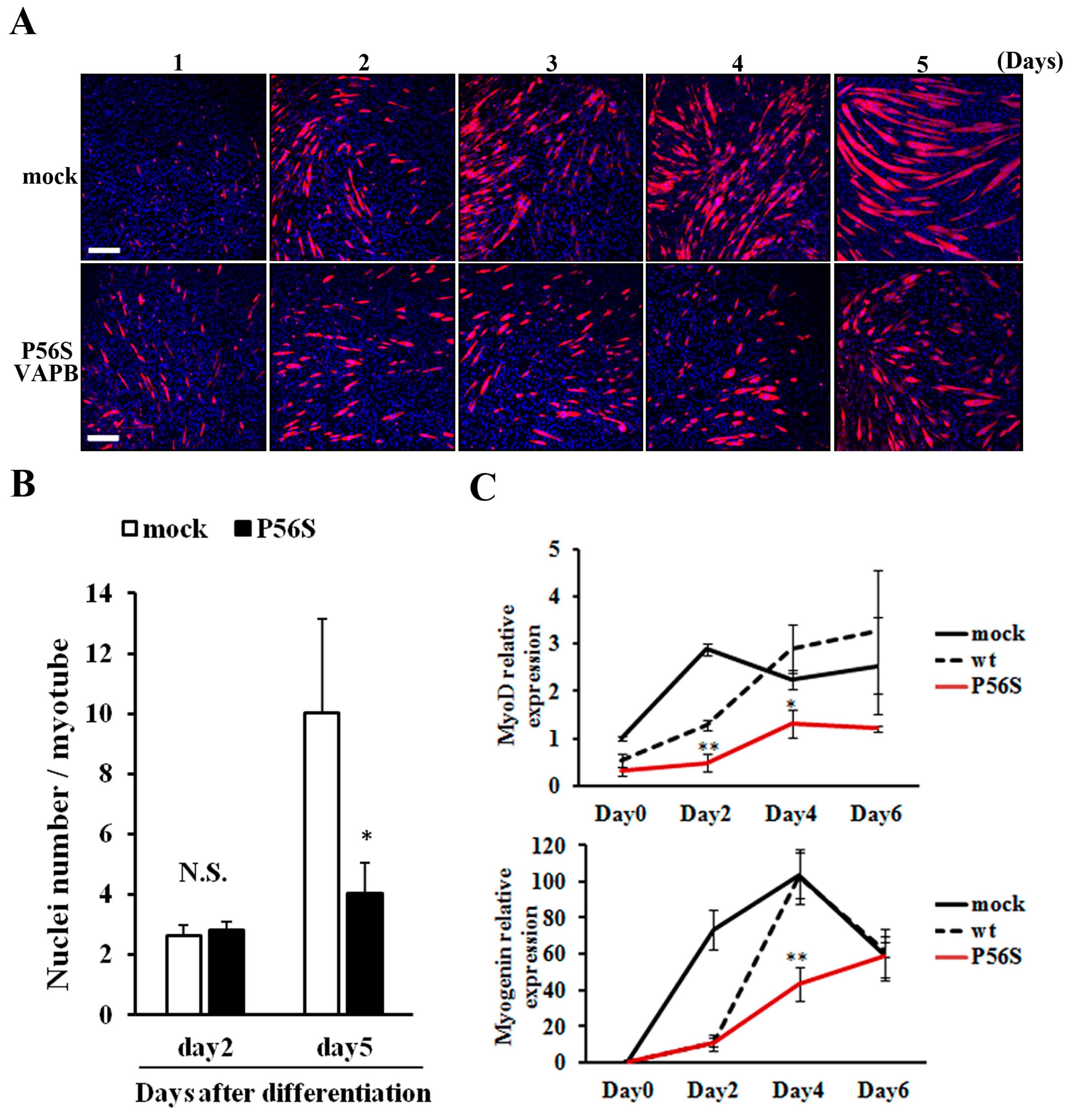
We compared changes in myotube formation over time between GFP-expressing and P56S-VAPB-expressing cell lines. No difference was observed between the two cell lines in the degree of myotube formation up to two days after differentiation induction; the difference appeared on the 3rd day and increased thereafter (
Figure 3A). Regarding the number of nuclei contained in a myotube, the two cell lines showed no difference on the 2nd day after differentiation induction, but on 5th day, myotubes of the P56S-VAPB-expressing cell line contained significantly fewer nuclei than those of the GFP-expressing cell line (
Figure 3B). We then analyzed the expression levels of myodifferentiation-related factors (MyoD and myogenin) over time in both cell lines using qRT-PCR. The results showed that the gene expression levels in the P56S-VAPB-expressing cell line were significantly lower than those in the GFP- and wt-VAPB-expressing cell line (
Figure 3C), indicating that gene expression of myogenic differentiation-related factors in the process of differentiation is markedly suppressed in P56S-VAPB-expressing cells.
Figure 2.
P56S mutation reduces myotube elongation and leads to an aberrant localization pattern of myonuclei. (A) C2C12 cells were transfected with the vectors encoding GFP (mock), GFP-fused wt-VAPB, or GFP-fused P56S-VAPB. Then, stable cell lines were generated by G418 selection. Stable cell lines were induced to differentiate until day five. Cells were immunostained using an anti-muscle heavy chain (MHC) antibody and secondary antibody conjugated to Alexa Fluor 568 (red). Nuclei were stained with DAPI (blue). Examples of P56S-VAPB aggregates are indicated with arrowheads. Scale bars = 200 µm; (B) The myotube population was segmented according to the number of nuclei per myotube. Each stable cell line was induced to differentiate until day five, and the number of nuclei contained in each myotube was counted. One hundred myotubes were included in each group; (C) Image comparison of the mock and P56S-VAPB expressing myotubes at 5 days after differentiation. The white dotted lines outline single myotubes. Mock myotubes contain six (left) and eight nuclei (right) each. P56S myotubes contain eight (top) and seven nuclei (bottom) each. Scale bars = 100 µm; (D) The myotube area was plotted according to the number of nuclei per myotube. Each stable cell line was induced to differentiate until day five, and the single myotube areas were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). n = 214 (mock myotubes), 211 (wt-VAPB myotubes), and 300 (P56S-VAPB myotubes); (E) Average myotube length. Each stable line was induced to differentiate until day five, and the single myotube length was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The values are the means ± standard deviation for 300 myotubes in each group. ** p < 0.01 compared with mock.
Figure 2.
P56S mutation reduces myotube elongation and leads to an aberrant localization pattern of myonuclei. (A) C2C12 cells were transfected with the vectors encoding GFP (mock), GFP-fused wt-VAPB, or GFP-fused P56S-VAPB. Then, stable cell lines were generated by G418 selection. Stable cell lines were induced to differentiate until day five. Cells were immunostained using an anti-muscle heavy chain (MHC) antibody and secondary antibody conjugated to Alexa Fluor 568 (red). Nuclei were stained with DAPI (blue). Examples of P56S-VAPB aggregates are indicated with arrowheads. Scale bars = 200 µm; (B) The myotube population was segmented according to the number of nuclei per myotube. Each stable cell line was induced to differentiate until day five, and the number of nuclei contained in each myotube was counted. One hundred myotubes were included in each group; (C) Image comparison of the mock and P56S-VAPB expressing myotubes at 5 days after differentiation. The white dotted lines outline single myotubes. Mock myotubes contain six (left) and eight nuclei (right) each. P56S myotubes contain eight (top) and seven nuclei (bottom) each. Scale bars = 100 µm; (D) The myotube area was plotted according to the number of nuclei per myotube. Each stable cell line was induced to differentiate until day five, and the single myotube areas were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). n = 214 (mock myotubes), 211 (wt-VAPB myotubes), and 300 (P56S-VAPB myotubes); (E) Average myotube length. Each stable line was induced to differentiate until day five, and the single myotube length was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The values are the means ± standard deviation for 300 myotubes in each group. ** p < 0.01 compared with mock.
![Ijms 16 18628 g002]()
Figure 3.
P56S mutation suppressed fusion of C2C12 cells for late-stage differentiation. (A) P56S mutation suppressed fusion of C2C12 cells for late-stage differentiation. GFP (mock) or P56S-VAPB stable expressing cell lines were induced to differentiate and detected MHC expression by immunostaining (red) on days 1–5 after differentiation. Nuclei were stained with DAPI (blue). Scale bars = 200 μm; (B) GFP (mock) or P56S-VAPB stable expressing cell lines were treated with differentiation medium and the number of nuclei per single myotube were counted at the indicated days after differentiation. The results are shown as means ± SEM (n = 3). * p < 0.05 compared with mock; (C) The mRNA expression of MyoD and Myogenin in GFP (mock), wt-VAPB-, or P56S-VAPB-expressing cells during differentiation induction. The level of mRNA was determined by real-time PCR and normalized to GAPDH. The results are expressed as means ± SEM for three independent experiments. * p < 0.05, ** p < 0.01 compared with mock and wt.
Figure 3.
P56S mutation suppressed fusion of C2C12 cells for late-stage differentiation. (A) P56S mutation suppressed fusion of C2C12 cells for late-stage differentiation. GFP (mock) or P56S-VAPB stable expressing cell lines were induced to differentiate and detected MHC expression by immunostaining (red) on days 1–5 after differentiation. Nuclei were stained with DAPI (blue). Scale bars = 200 μm; (B) GFP (mock) or P56S-VAPB stable expressing cell lines were treated with differentiation medium and the number of nuclei per single myotube were counted at the indicated days after differentiation. The results are shown as means ± SEM (n = 3). * p < 0.05 compared with mock; (C) The mRNA expression of MyoD and Myogenin in GFP (mock), wt-VAPB-, or P56S-VAPB-expressing cells during differentiation induction. The level of mRNA was determined by real-time PCR and normalized to GAPDH. The results are expressed as means ± SEM for three independent experiments. * p < 0.05, ** p < 0.01 compared with mock and wt.
![Ijms 16 18628 g003]()
2.4. P56S Mutation Disrupted IRE1-XBP1 Pathway in C2C12 Cells
The IRE1-XBP1 pathway, which is involved in the UPR, has been reported to be disrupted in neuronal cells expressing P56S-VAPB [
18,
19]. Therefore, we studied whether the IRE1-XBP1 pathway was disrupted in C2C12 cells expressing P56S-VAPB. In this experiment, we used the ER stress Activated Indicator (ERAI) system capable of visualizing activities of the IRE1-XBP1 pathway. Under ER stress conditions, IRE1 is activated and splicing of XBP1 occurs, followed by expression of XBP1-Venus fluorescent fusion proteins. Observation of the cells treated with the ER stress inducer tunicamycin revealed that Venus fluorescence in P56S-VAPB-expressing cells was noticeably weaker than in DsRed-expressing cells or wt-VAPB-expressing cells (
Figure 4A). In the absence of tunicamycin, we could not observe Venus fluorescence in all cells. (
Supplementary Figure S3). This indicates that the IRE1-XBP1 pathway is disrupted in P56S-VAPB-expressing C2C12 cells. We then examined the activity state of the IRE1-XBP1 pathway over the differentiation process of C2C12 cells using the ERAI system, and we found that the activity was undetectable before differentiation but enhanced after differentiation (
Figure 4B). Further, the mRNA expression level of XBP1(s) during the differentiation process was measured for GFP-, wt-VAPB-, and P56S-VAPB stable expressing cells using qRT-PCR. Although the expression level in GFP and wt-VAPB-expressing cells significantly increased with differentiation, no such increase was observed in P56S-VAPB-expressing cells (
Figure 4C). Rather, we found decreased the expression level in P56S-VAPB-expressing cells. We confirmed the specificity of our primer set for the spliced form of XBP1 mRNA (
Supplementary Figure S4).
2.5. Discussion
Previous studies on the motor neuron cell line NSC34 and the mouse adipocyte cell line 3T3-L1 have shown that P56S-VAPB aggregates in cells, whereas wt-VAPB distributes uniformly in cells [
13,
22]. In the present study, we co-transfected wt-VAPB and P56-VAPB into C2C12 cells, and found that these proteins were co-localized. This result is in agreement with the results of previous studies using NSC34 cells and 3T3-L1 cells [
14,
22], and suggests that P56S-VAPB recruits wt-VAPB, thereby inhibiting the function of wt-VAPB.
In the P56S-VAPB-expressing cells, myotube formation was suppressed, and nuclei localized in an abnormal manner in myotubes containing six or more nuclei. In addition, the number of nuclei contained in a myotube was not different between P56S-VAPB-expressing cells and the control cells on the 2nd day after differentiation induction, but was significantly lower in P56S-VAPB-expressing cells on the 5th day. In this experiment, we generated C-terminally GFP-fused wt-VAPB and P56S-VAPB, and examined the influences of P56S mutation on differentiation of the skeletal muscles. The extent of myotube formation is indistinguishable between GFP and GFP-fused wt-VAPB cells. As described above, the intracellular localization of both VAPB forms is consistent with previous studies. These results indicate that GFP at the C-terminus did not affect VAPB localization and function. Therefore, P56S mutation is caused for the reduced myotube formation. Aberrant localization of nuclei in myotubes has been reported to be found in amyotrophic diseases [
23]. The amount of cytoplasm controlled by a single myonucleus is called the myonuclear domain. Each myonucleus governs a limited region in the cytoplasm and supplies proteins thereto [
24]. Therefore, dysfunction of the myonuclear domain due to abnormal localization of nuclei is a likely cause of the suppressed myotube formation in P56S-VAPB-expressing cells.
Disruption to the IRE1-XBP1 pathway has been reported in P56S-VAPB-expressing neurons [
18,
19] and was also found in P56S-VAPB-expressing C2C12 cells in the present study. In addition, the IRE1-XBP1 pathway activity was enhanced in the differentiation process of normal C2C12 cells. Moreover, we found that the mRNA expression level of XBP1(s) was elevated in GFP and wt-VAPB-expressing cells after myogenic differentiation, but not in P56S-VAPB-expressing cells. IRE1 is an ER stress sensor, and when it is activated through accumulation of structurally abnormal proteins in the ER, the spliceosome-independent frame switch splicing is elicited and the active transcription factor XBP1(s) is produced from the spliced substrate XBP1 mRNA [
25]. XBP1(s) contains both DNA-binding and transcription activation domains and induces gene transcription of ER chaperones and factors of the ER-associated protein degradation pathway [
26]. In addition to the fact that the IRE1-XBP1 pathway plays a role in eliminating ER stress, XBP1 has been revealed to be an essential transcriptional factor for the terminal differentiation of B-cells into antibody-producing cells [
27]. Recently, XBP1 has also been reported to be involved in the differentiation of adipocytes and osteoblasts [
28,
29]. Therefore, it is conceivable that the IRE1-XBP1 pathway is also involved in the differentiation of myocytes, which are derived from mesenchymal stem cells, as are adipocytes and osteoblasts. Although further detailed studies are required, the disrupted IRE1-XBP1 pathway is a plausible cause of the reduced myotube formation in P56S-VAPB-expressing cells.
Approximately 20% of familial ALS is considered to be attributable to SOD mutations. The VAPB protein has been reported to be expressed at a reduced level in the spinal cords of mutant SOD transgenic mice [
14]. Moreover, the reduced VAPB protein expression has also been detected in the spinal cords of patients with sporadic ALS [
30]. Hence, it is currently thought that the loss of VAPB protein function causes ALS. So far, there has been no report of the relationship between VAPB level and UPR. VAPB is seen to play a significant role in the UPR [
16,
17]. Recent study suggested that VAPB is required for ER protein quality control (ERQC). Loss of VAPB in flies showed various ERQC associated defects, including protein accumulation, ER expansion, and ER stress [
31]. Although further investigations are required, loss of VAPB may lead to disruption of the UPR. Furthermore, previous report with the ALS mouse model suggested that XBP1(s) expression level was enhanced in SOD1
G93A motor neurons after adding thapsigargin, an inhibitor of the ER calcium pump, but the proportionate increase or slew rate of XBP1 activation after adding thapsigargin was higher in wild-type motor neurons [
32]. It is speculated that regulation of UPR after additional ER stress induction is disrupted in ALS.
Dysfunctions of myosatellite cells from mutant SOD transgenic mice and patients with sporadic ALS have been reported [
11,
33], but the underlying mechanism remains unknown. Our present study revealed that P56S-VAPB disrupted the IRE1-XBP1 pathway in C2C12 cells and, presumably, this pathway is also disrupted in myosatellite cells of patients with other types of familial ALS and sporadic ALS. Future studies on the association between the IRE1-XBP1 pathway disruption and myonuclei that are aberrantly localized and those in myosatellite cells from patients with SOD mutations or sporadic ALS with a particular focus on the IRE1-XBP1 pathway are expected to result in the identification of the cause of the disturbed muscular maintenance system in patients with ALS.
Figure 4.
P56S mutation disrupted the IRE1-XBP1 pathway in C2C12 cells. (A) C2C12 cells co-expressed DsRed (mock), DsRed-fused wt-VAPB, DsRed-fused P56S-VAPB, and the ERAI gene (Venus). To induce ER stress, the cells were exposed to tunicamycin for 8 h and then visualized. Under ER stress conditions, IRE1 is activated, and splicing of XBP1 occurs, followed by expression of XBP1-Venus fluorescent fusion proteins. Scale bars = 20 µm; (B) C2C12 cells were transfected with the ERAI gene. XBP1-Venus fluorescence was detected as an indicator of IRE1 activation at the indicated days after differentiation. Scale bar = 100 μm; (C) The mRNA expression of XBP1(s) in GFP- (mock) or wt-VAPB- or P56S-VAPB-expressing cells during differentiation induction. The level of mRNA was determined by real-time PCR and normalized to GAPDH. The results are expressed as means ± SEM for three independent experiments. ** p < 0.01 compared with Day 0.
Figure 4.
P56S mutation disrupted the IRE1-XBP1 pathway in C2C12 cells. (A) C2C12 cells co-expressed DsRed (mock), DsRed-fused wt-VAPB, DsRed-fused P56S-VAPB, and the ERAI gene (Venus). To induce ER stress, the cells were exposed to tunicamycin for 8 h and then visualized. Under ER stress conditions, IRE1 is activated, and splicing of XBP1 occurs, followed by expression of XBP1-Venus fluorescent fusion proteins. Scale bars = 20 µm; (B) C2C12 cells were transfected with the ERAI gene. XBP1-Venus fluorescence was detected as an indicator of IRE1 activation at the indicated days after differentiation. Scale bar = 100 μm; (C) The mRNA expression of XBP1(s) in GFP- (mock) or wt-VAPB- or P56S-VAPB-expressing cells during differentiation induction. The level of mRNA was determined by real-time PCR and normalized to GAPDH. The results are expressed as means ± SEM for three independent experiments. ** p < 0.01 compared with Day 0.