Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression of Per2 mRNA in the LHb of LD Rats
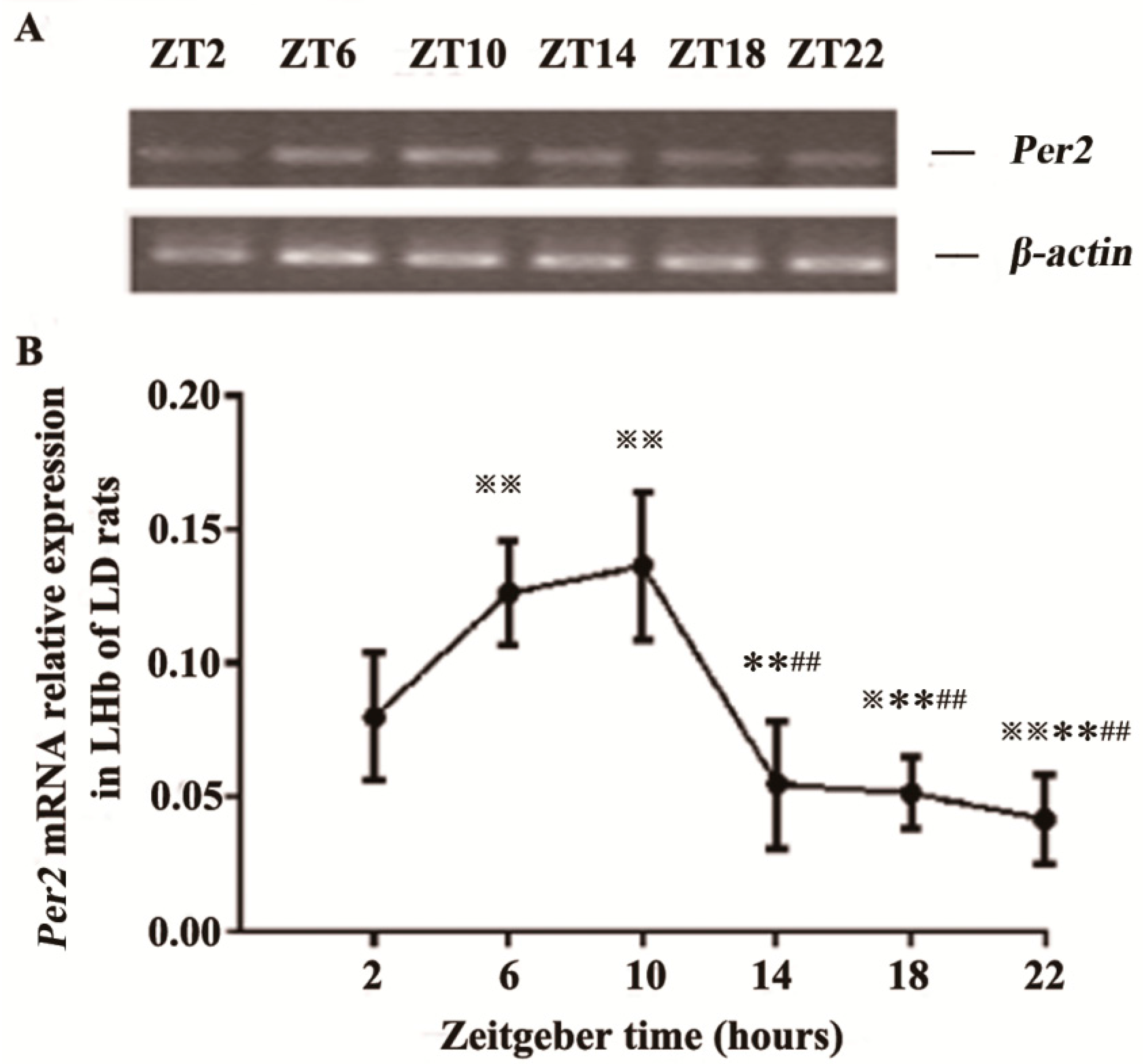
2.2. Expression of PER2 Protein in the LHb of LD Rats
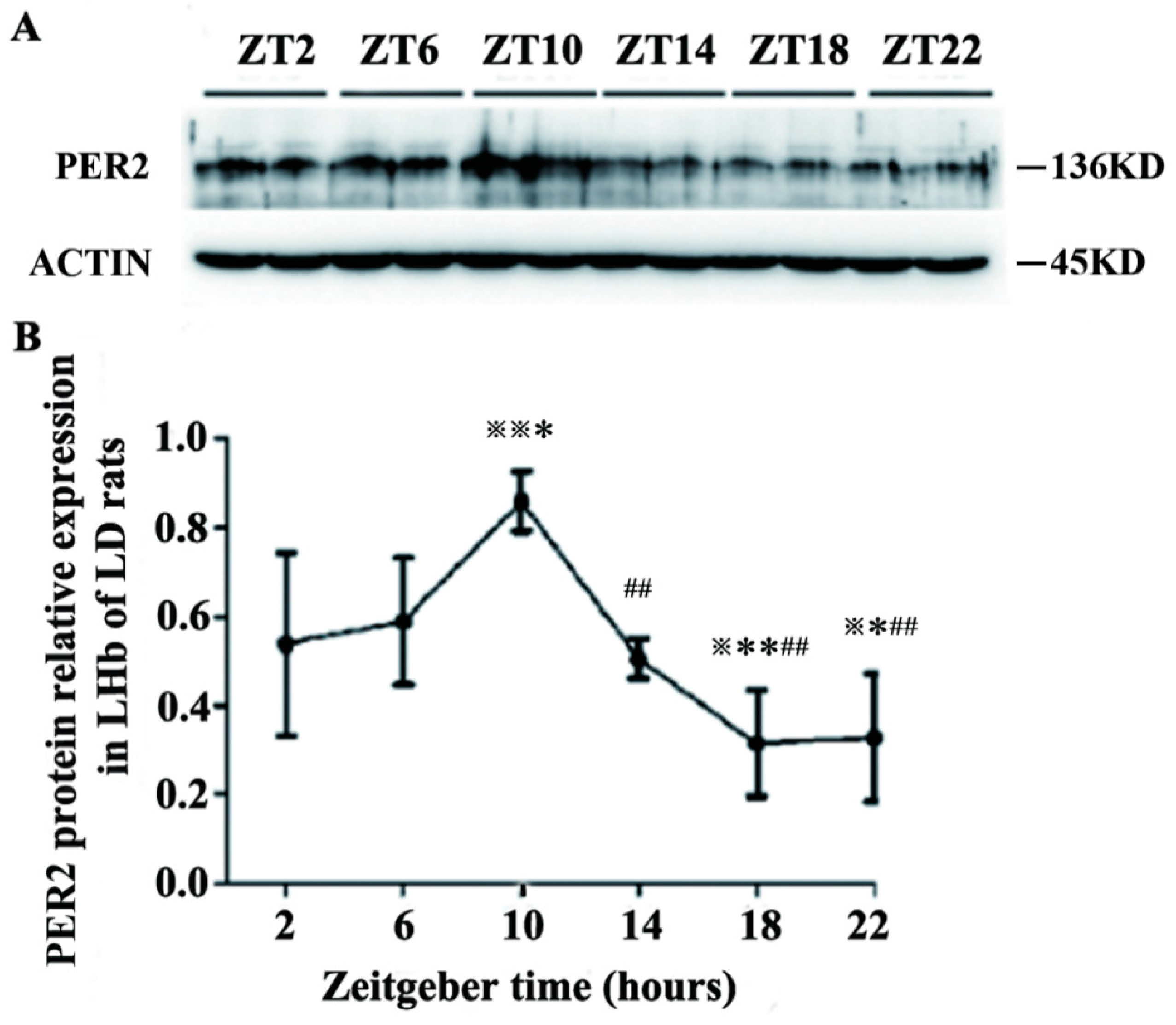
2.3. Discussion
3. Experimental Section
3.1. Animals
3.2. Detection of Real-Time PCR
3.3. Western Blotting Experiments
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Moore, R.Y. Organization and function of a central nervous system circadian oscillator: The suprachismatic hypothalamic nucleus. Fed. Proc. 1983, 42, 2783–2789. [Google Scholar] [PubMed]
- Hastings, M.H.; Brancaccio, M.; Maywood, E.S. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J. Neuroendocrinol. 2014, 26, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.F.; Moore, R.Y.; Morin, L.P. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988, 460, 297–313. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Moldavan, M.G.; Allen, C.N. Retinohypothalamic tract synapses in the rat suprachiasmatic nucleus demonstrate short-term synaptic plasticity. J. Neurophysiol. 2010, 103, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian rhythms in isolated brain regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [PubMed]
- Guilding, C.; Piggins, H.D. Challenging the omnipotence of the suprachiasmatic timekeeper: Are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007, 25, 3195–3216. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hughes, A.T.; Brown, T.M.; Namvar, S.; Piggins, H.D. A riot of rhythms: Neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol. Brain 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Lecourtier, L.; Kelly, P.H. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci. Biobehav. Rev. 2007, 31, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Trimble, M. The lateral habenula: No longer neglected. CNS Spectr. 2008, 13, 484–489. [Google Scholar] [PubMed]
- Hikosaka, O. The habenula: From stress evasion to value-based decision-making. Nat. Rev. Neurosci. 2010, 11, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.K.; Silverman, A.J.; Morrell, J.I. Evidence for estrogen receptor in cell nuclei and axon terminals within the lateral habenula of the rat: Regulation during pregnancy. J. Comp. Neurol. 1998, 392, 330–342. [Google Scholar] [CrossRef]
- Heldt, S.A.; Ressler, K.J. Lesions of the habenula produce stress-and dopamine-dependent alterations in prepulse inhibition and locomotion. Brain Res. 2006, 1073–1074, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Yanagihara, S.; Kobayashi, M.; Niisato, K.; Takekawa, T.; Harukuni, R. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J. Neurosci. 2013, 33, 8909–8921. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Hu, B.; Xia, Y.H.; Zhang, B.L.; Zhao, H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav. Brain Res. 2008, 188, 84–90. [Google Scholar] [CrossRef] [PubMed]
- McCathy, M.J.; Welsh, D.K. Cellular circadian clocks in mood disorders. J. Biol. Rhythm. 2012, 27, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli.-Nezhad, M.; Schwartz, W.J. Hamsters running on time: Is the lateral habenula a part of the clock? Chronobiol. Int. 2006, 23, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Indic, P.; Schwartz, W.J. A role for the habenula in the regulation of locomotor activity cycles. Eur. J. Neurosci. 2011, 34, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, B.L.; Yang, S.J.; Rusak, B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain Res. 2015, 277, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Rusak, B. Circadian firing-rate rhythms and light responses of rat habenula nucleus neurons in vivo and in vitro. Neuroscience 2005, 132, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli-Nezhad, M.; Schwartz, W.J. c-fos expression in the brains of behaviorally “split” hamsters in constant light: Calling attention to a dorsolateral region of the suprachiasmatic nucleus and the medial division of the lateral habenula. J. Biol. Rhythm. 2005, 20, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hughes, A.T.; Piggins, H.D. Circadian oscillators in the epithalamus. Neuroscience 2010, 169, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M. Intraand extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978, 192, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [PubMed]
- Zheng, B.; Larkin, D.W.; Albrecht, U.; Sun, Z.S.; Sage, M.; Eichele, G.; Lee, C.C.; Bradley, A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999, 400, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001, 30, 525–536. [Google Scholar] [CrossRef]
- Pendergast, J.S.; Friday, R.C.; Yamazaki, S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS ONE 2010, 5, e8552. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, K.; Wegner, S.; Belle, M.D.C.; Howarth, M.; Delagrange, P.; Brown, T.M.; Piggins, H.D. Intrinsic and extrinsic cues regulate the daily profile of mouse lateral habenula neuronal activity. J. Physiol. 2014, 592, 5025–5045. [Google Scholar] [CrossRef] [PubMed]
- Vasalou, C.; Henson, M.A. A Multiscale Model to Investigate Circadian Rhythmicity of Pacemaker Neurons in the Suprachiasmatic Nucleus. PLoS Comput. Biol. 2010, 6, e1000706. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Johnston, J.D.; Semikhodskii, A.G.; Nolan, T.; Cagampang, F.R.; Stirland, J.A.; Loudon, A.S. Photoperiod differentially regulates circadian oscillators in central and peripheral tissues of the syrian hamster. Curr. Biol. 2003, 13, 1543–1548. [Google Scholar] [CrossRef]
- Rocha, V.A.; Frazão, R.; Campos, L.M.; Mello, P.; Donato, J.J.; Cruz-Rizzolo, R.J.; Nogueira, M.I.; Pinato, L. Intrinsic organization of the suprachiasmatic nucleus in the capuchin monkey. Brain Res. 2014, 1543, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Swanson, L.W.; Sanchez-Watts, G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 1987, 258, 204–229. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Weindl, A. Projections from the parvocellular vasopressin and neurophysin-containing neurons of the suprachiasmatic nucleus. Am. J. Anat. 1978, 153, 391–429. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Dong, K.; Sugioka, K.; Yamadori, T. Demonstration of direct input from the retina to the lateral habenular nucleus in the albino rat. Brain Res. 1996, 709, 251–258. [Google Scholar] [CrossRef]
- Reuss, S.; Decker, K. Anterograde tracing of retinohypothalamic afferents with Fluoro-Gold. Brain Res. 1997, 745, 197–204. [Google Scholar] [CrossRef]
- Hannibal, J.; Kankipati, L.; Strang, C.E.; Peterson, B.B.; Dacey, D.; Gamlin, P.D. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J. Comp. Neurol. 2014, 522, 2231–2248. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Xu, H.; Liu, Y.; Mu, L.; Xiao, J.; Zhao, H. Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus. Int. J. Mol. Sci. 2015, 16, 16740-16749. https://doi.org/10.3390/ijms160816740
Zhao Z, Xu H, Liu Y, Mu L, Xiao J, Zhao H. Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus. International Journal of Molecular Sciences. 2015; 16(8):16740-16749. https://doi.org/10.3390/ijms160816740
Chicago/Turabian StyleZhao, Zhigong, Haiyan Xu, Yongmao Liu, Li Mu, Jinyu Xiao, and Hua Zhao. 2015. "Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus" International Journal of Molecular Sciences 16, no. 8: 16740-16749. https://doi.org/10.3390/ijms160816740




