Purification and Structural Identification of Polysaccharides from Bamboo Shoots (Dendrocalamus latiflorus)
Abstract
:1. Introduction
2. Results and Discussion
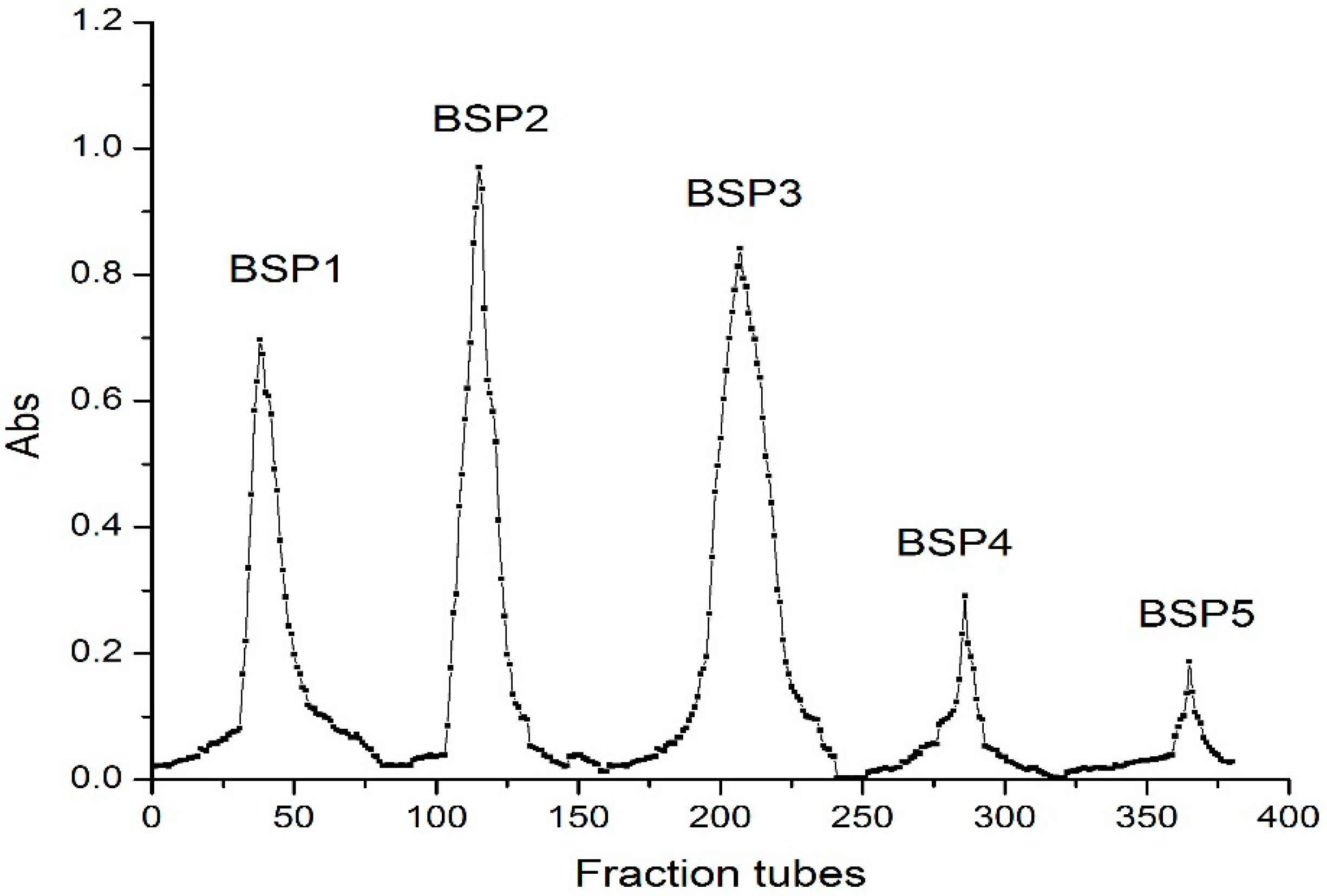
2.1. Isolation and Purification of Bamboo Shoot Polysaccharides
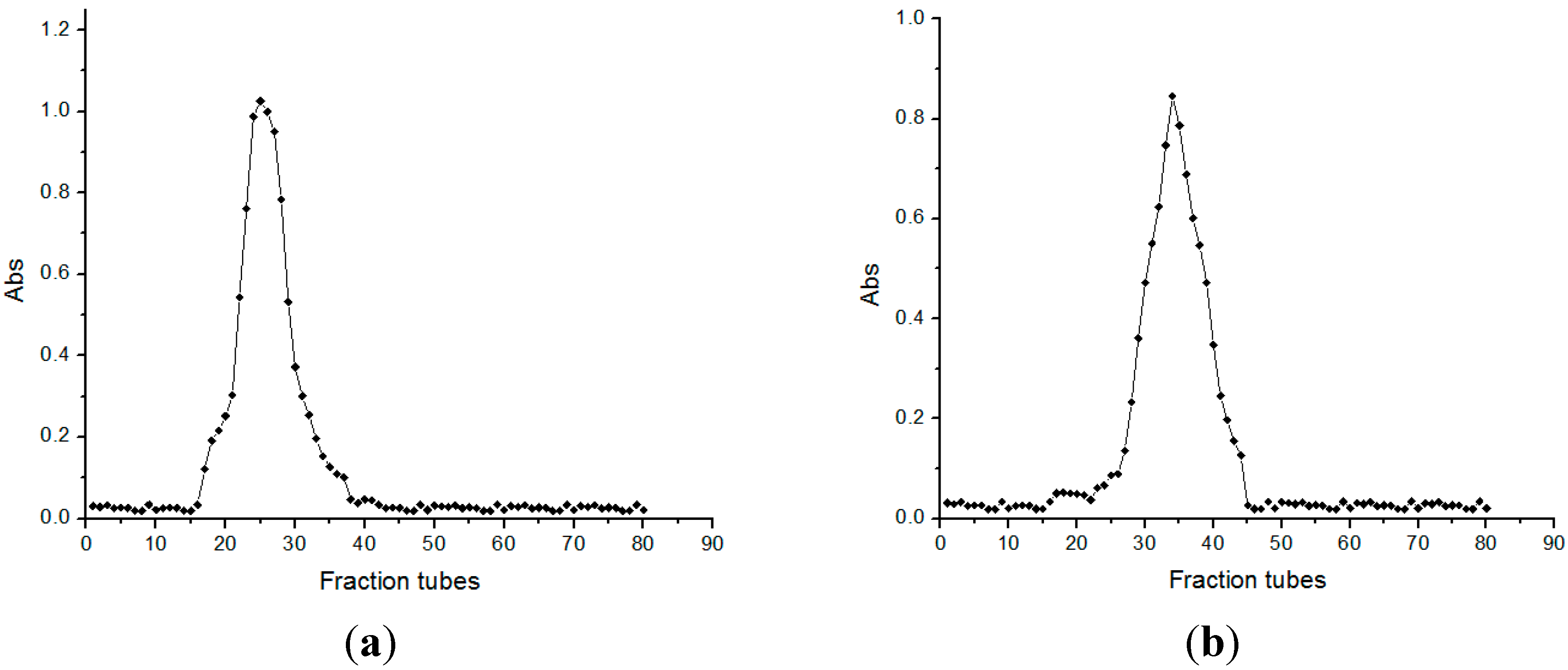
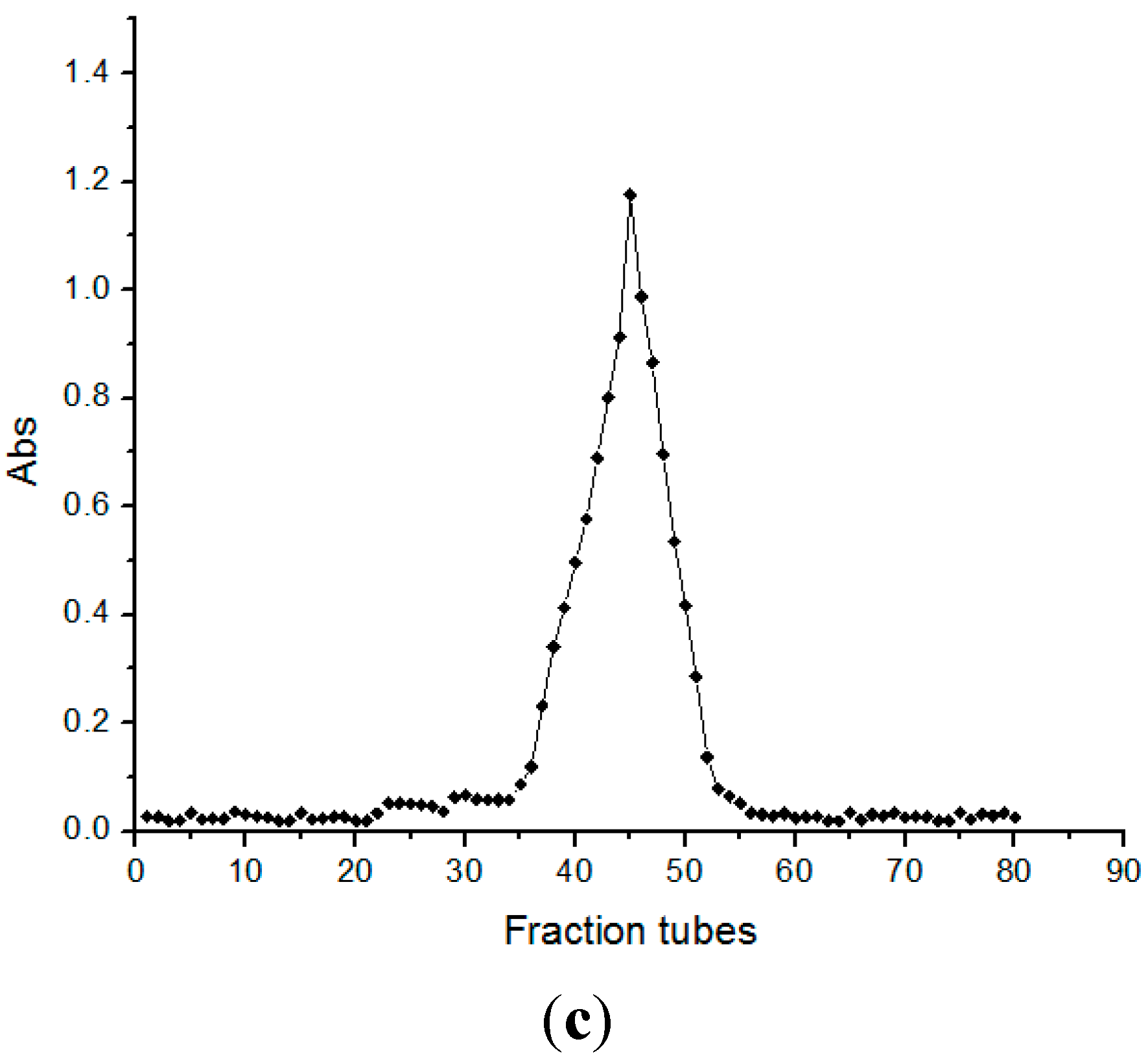
2.2. Molecular Weight Determination
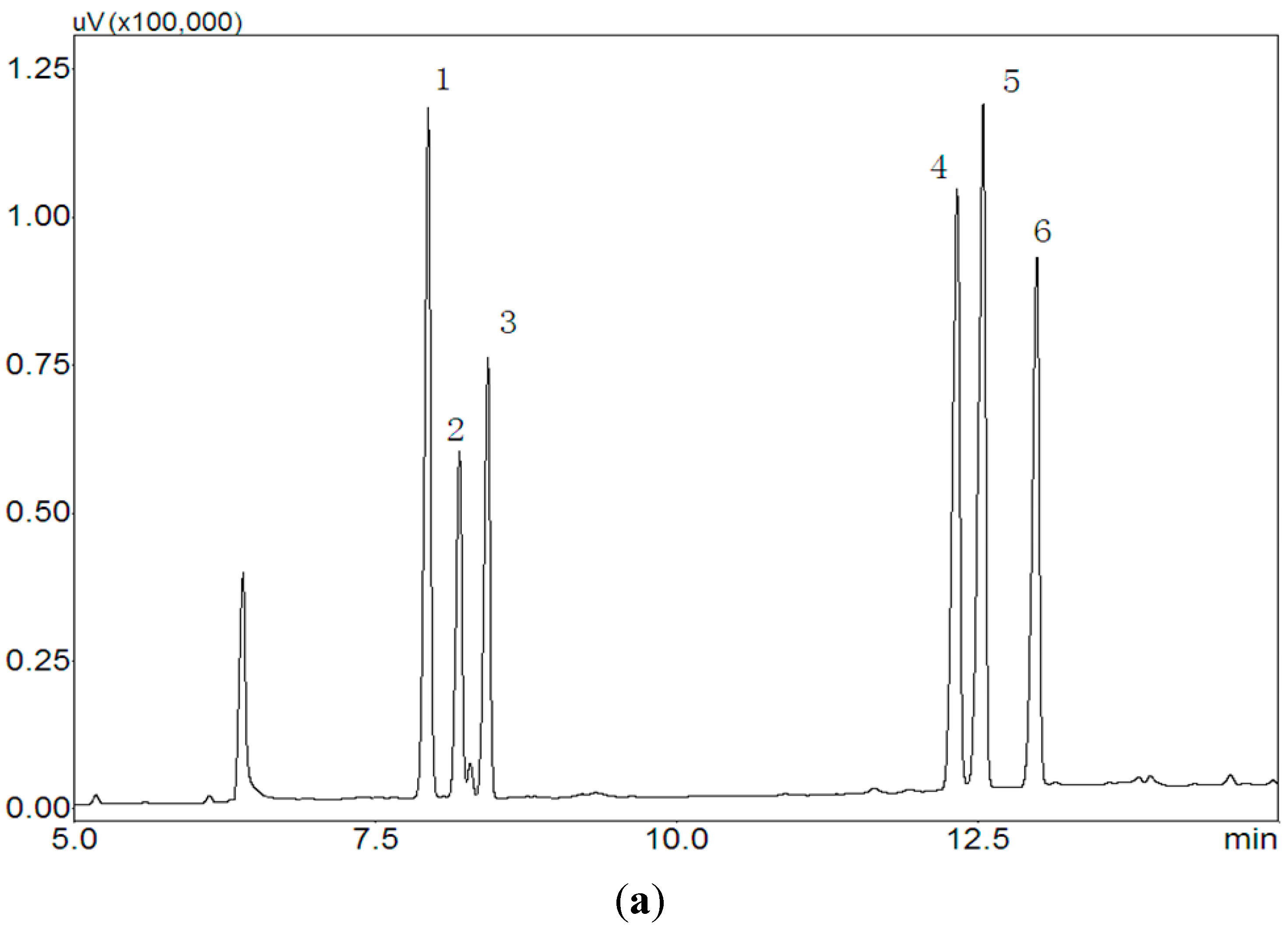
2.3. Monosaccharide Composition
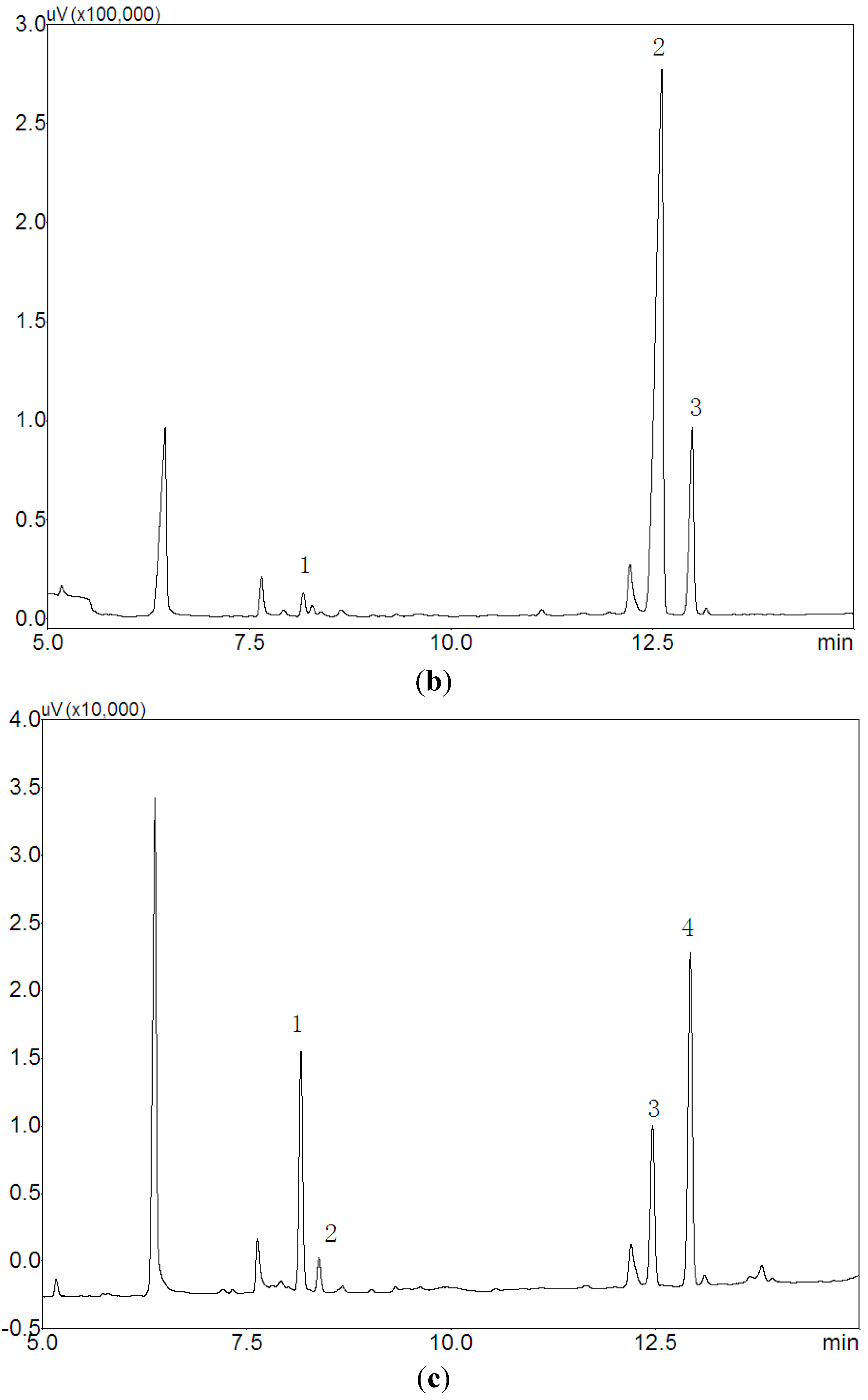

2.4. Carbohydrate-Peptide Linkage Analysis
| Amino Acids | BSP1A (mg/g) | BSP2A (mg/g) | BSP3B (mg/g) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Asp | 1.72 | 1.47 | 1.94 | 1.67 | 1.54 | 1.32 |
| Thr | 3.53 | 1.95 | 2.75 | 2.24 | 3.13 | 2.25 |
| Ser | 1.18 | 0.42 | 0.98 | 0.52 | 1.36 | 0.78 |
| Glu | 1.36 | 2.12 | 1.52 | 1.50 | 1.15 | 1.47 |
| Gly | 0.71 | 1.13 | 0.88 | 1.12 | 0.76 | 0.98 |
| Ala | 3.57 | 5.26 | 2.56 | 3.08 | 2.76 | 3.48 |
| Cys | 1.96 | 1.53 | 1.34 | 1.05 | 2.96 | 2.53 |
| Val | 1.55 | 1.26 | 1.12 | 1.35 | 1.35 | 1.48 |
| Met | – | 0.43 | – | 0.52 | – | 0.43 |
| Ile | 0.52 | 0.94 | 0.61 | 0.86 | 0.52 | 0.94 |
| Leu | 1.41 | 1.28 | 0.20 | 0.29 | 1.28 | 1.02 |
| Typ | – | 0.42 | 0.36 | – | 0.52 | 0.22 |
| Phe | 0.72 | – | 0.64 | – | 0.34 | – |
| Lys | 1.27 | 0.98 | 1.06 | 0.82 | 0.47 | 0.18 |
| His | 3.12 | 2.13 | 1.42 | 1.06 | 1.94 | 1.96 |
| Arg | 0.53 | – | 0.42 | – | 0.57 | – |
| Pro | 1.21 | 1.33 | 0.92 | 1.05 | 0.95 | 1.23 |
| Hdr | – | – | – | – | – | – |
| The total content of amino acids | 24.36 | 22.35 | 18.72 | 17.13 | 22.36 | 20.27 |
| The protein content | 23.48 | – | 17.86 | – | 21.22 | – |
| Sample | Amino Acid Residues | Before | After | Difference |
|---|---|---|---|---|
| BSP1A | Thr | 0.30 | 0.08 | 0.22 |
| Ser | 0.14 | 0.05 | 0.09 | |
| Ala | 0.52 | 0.76 | 0.24 | |
| BSP2A | Thr | 0.39 | 0.32 | 0.07 |
| Ser | 0.14 | 0.05 | 0.09 | |
| Ala | 0.61 | 0.73 | 0.12 | |
| BSP3B | Thr | 0.56 | 0.41 | 0.15 |
| Ser | 0.31 | 0.18 | 0.13 | |
| Ala | 0.78 | 0.98 | 0.20 |
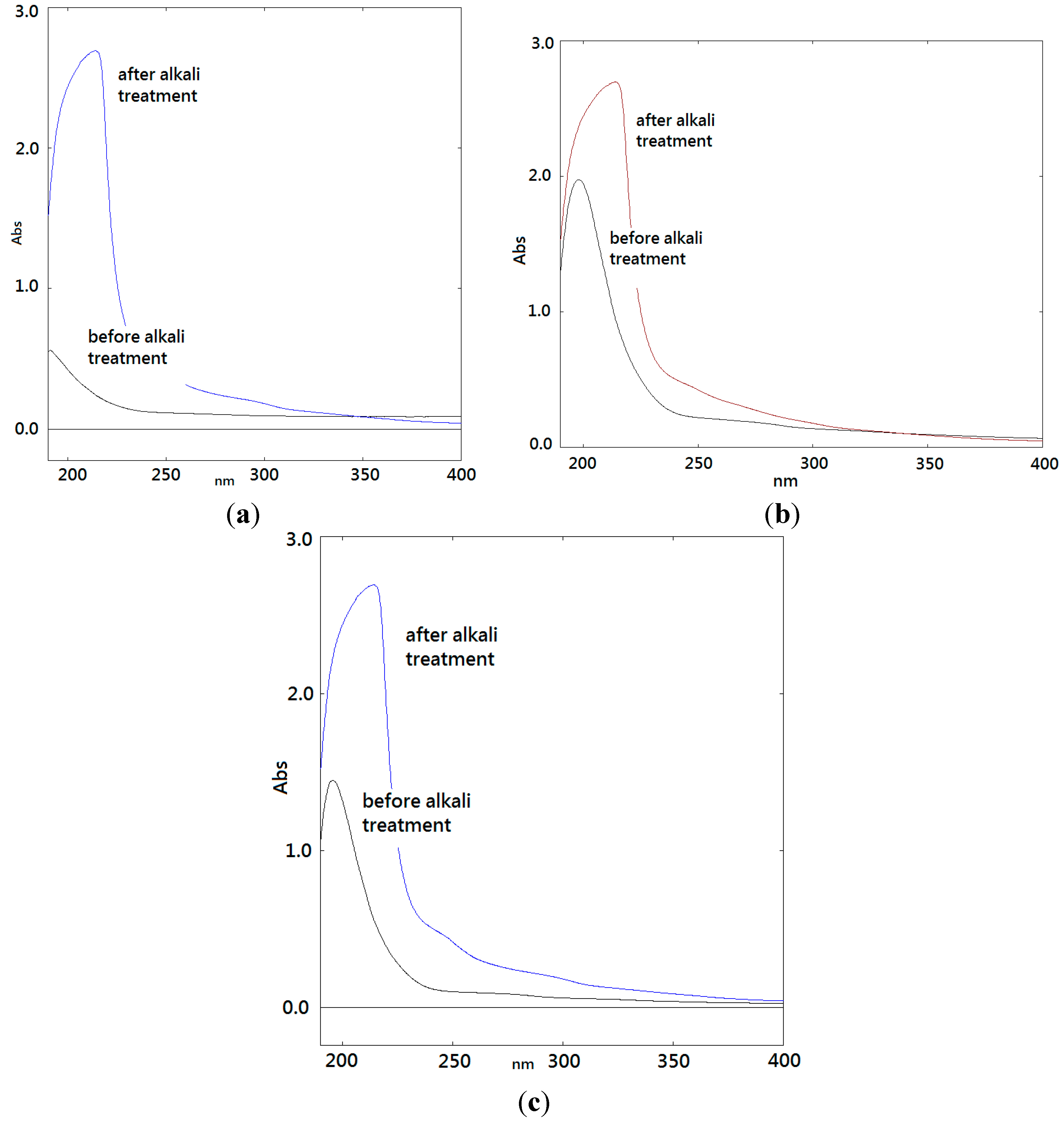
2.5. FTIR Spectra
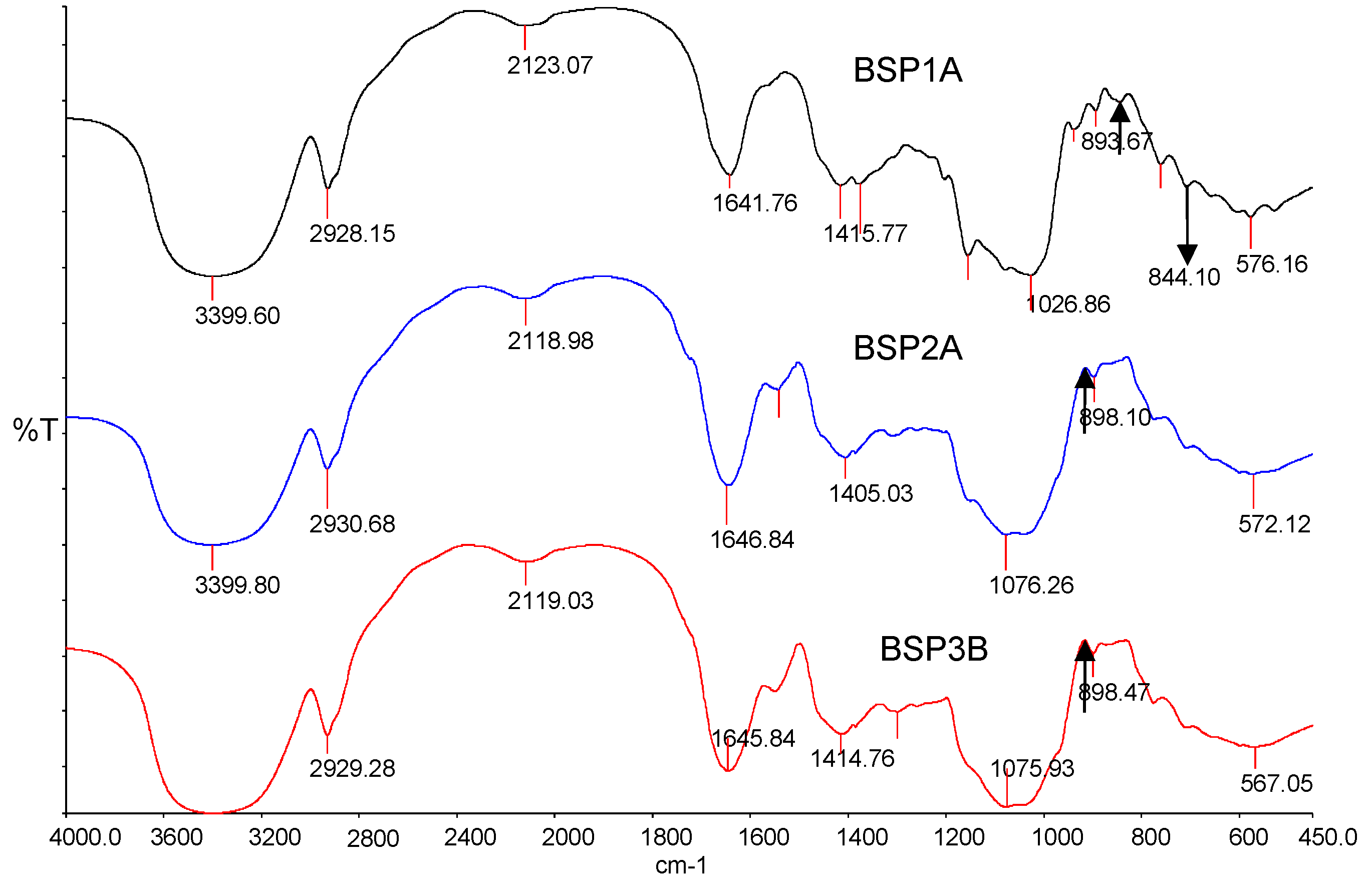
2.6. Triple Helix Structure Analysis
2.7. NMR Spectroscopy Results
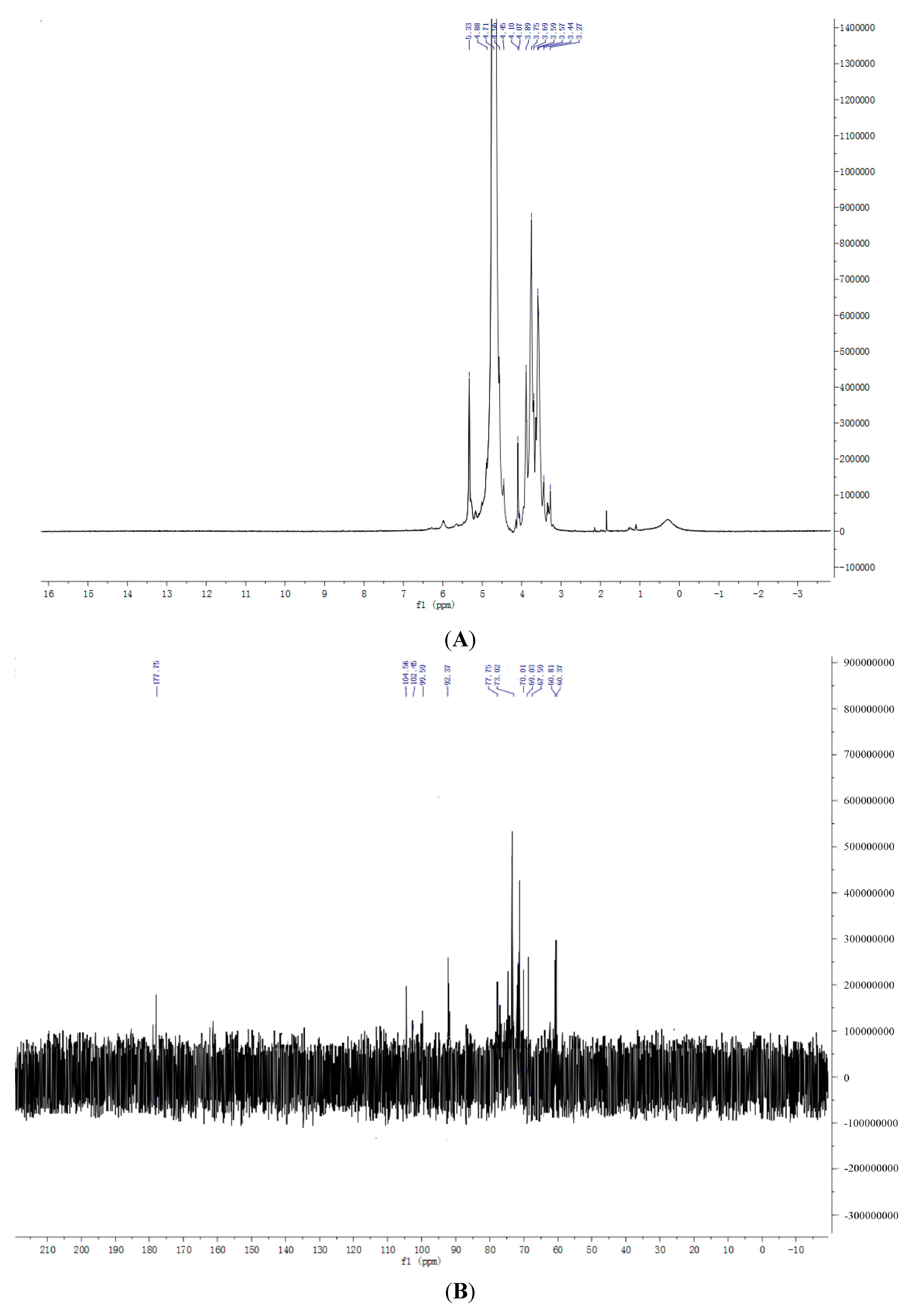
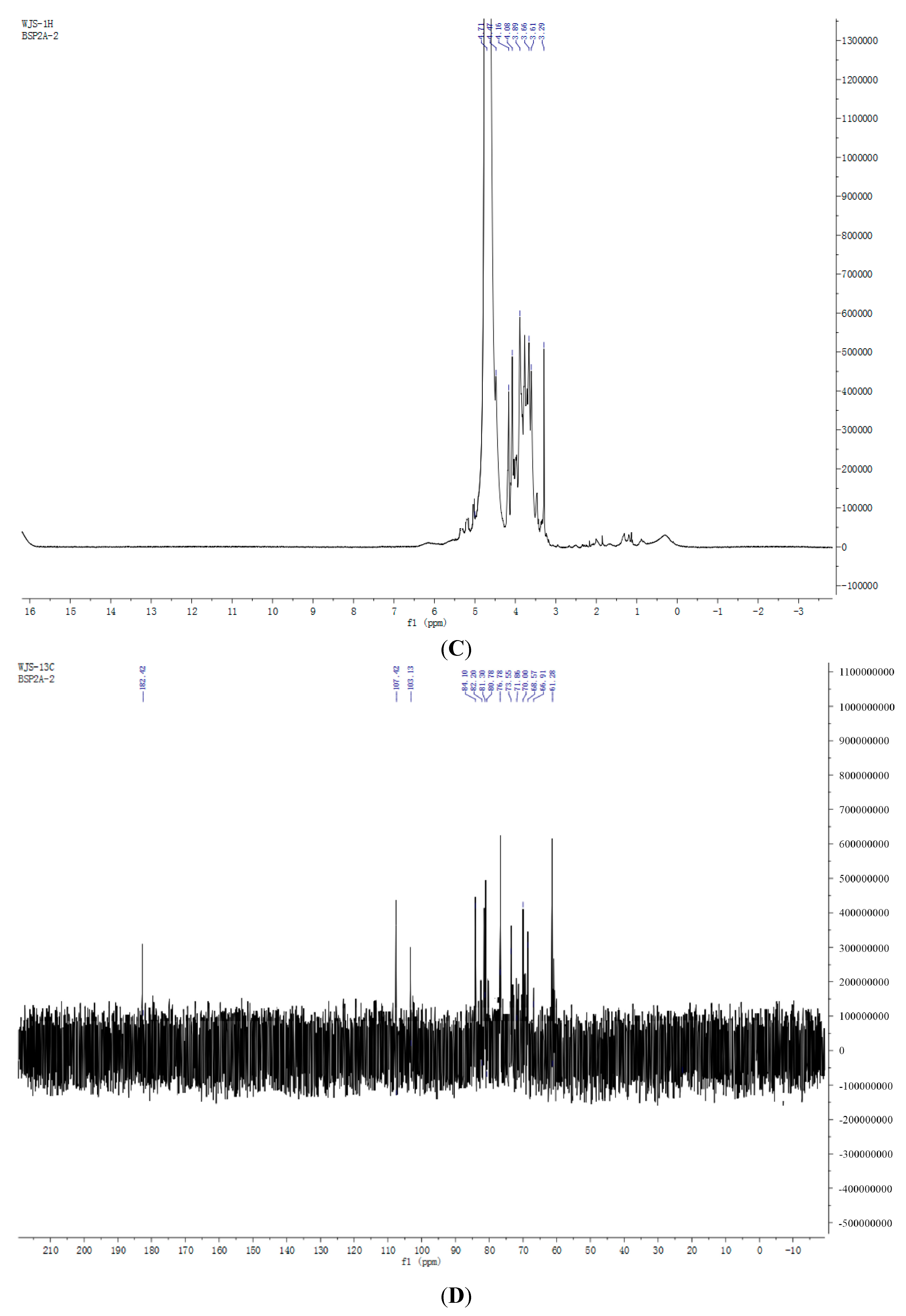
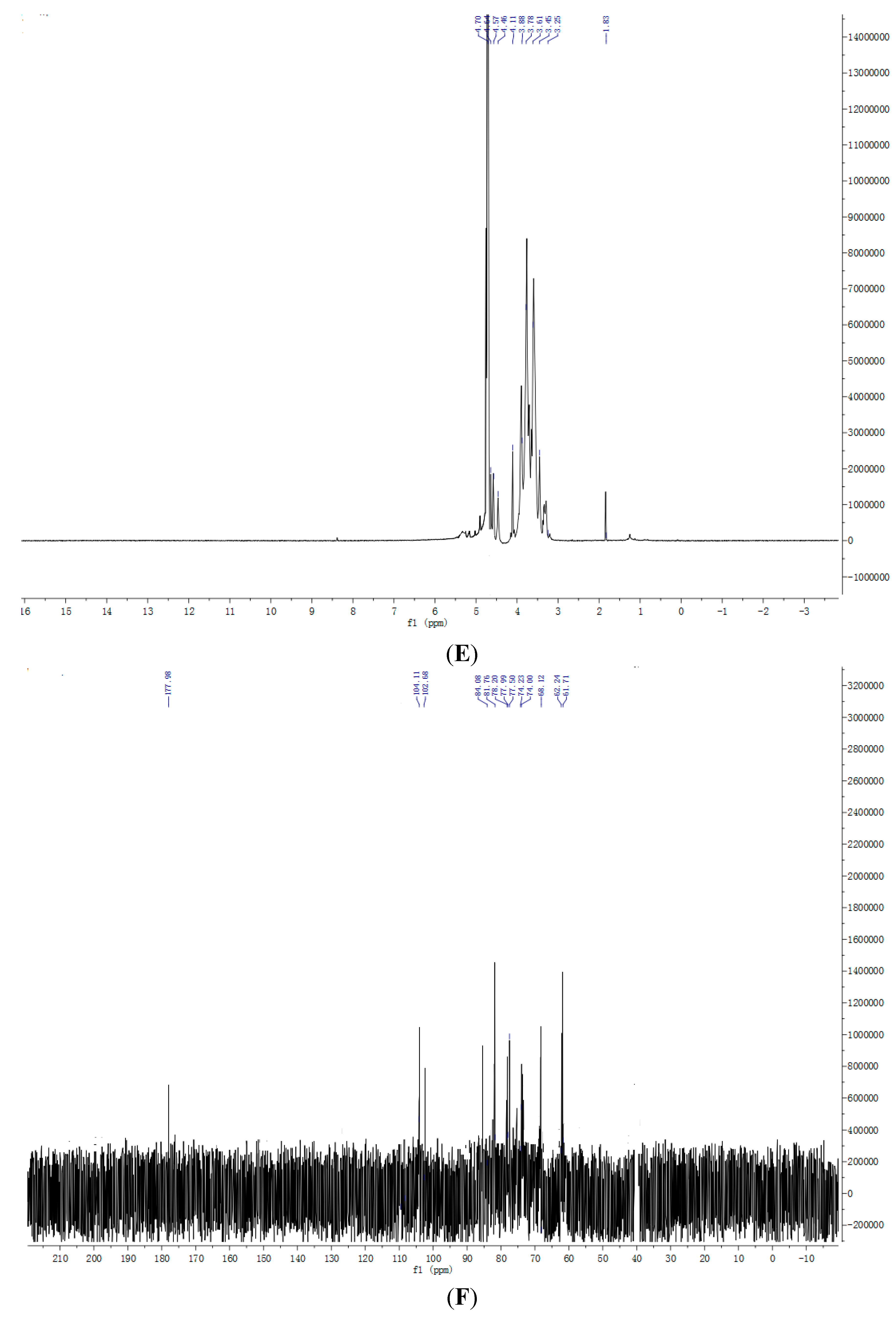
3. Experimental Section
3.1. Plant Material
3.2. Chemicals
3.3. Isolation and Purification of Bamboo Shoot Polysaccharides
3.4. Analytical Methods
3.5. Determination of Molecular Weight
3.6. Determination of Monosaccharide Composition
3.7. Carbohydrate-Peptide Linkage Analysis
3.8. Fourier Transform Infrared (FTIR)
3.9. Congo Red Test
3.10. NMR Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, A.P.; Xie, B.X.; Zhong, Q.P.; Tao, J.K. Anti-oxidative activity extracts of bamboo shoots. Food Sci. China 2008, 29, 97–100. [Google Scholar]
- Debangana, C.; Jatindra, K.S.; Sharma, G.D. Bamboo shoot: Microbiology, biochemistry and technology of fermentation—A review. Indian J. Tradit. Knowl. 2012, 11, 242–249. [Google Scholar]
- Santosh, S.; Lalit, M.B.; BalPoonam, S.; Naik, S.N. Bamboo shoot processing: Food quality and safety aspect (a review). Trends Food Sci. Technol. 2010, 21, 181–189. [Google Scholar]
- Nirmala, C.; Madho, S.B.; Sheena, H. Nutritional properties of bamboo shoots: Potential and prospects for utilization as a health food. Compr. Rev. Food Sci. F 2011, 10, 153–169. [Google Scholar]
- Santosh, S.; Poonam, S.; Lalit, M.B.; Sudhakar, P. Bamboo shoot: A potential source of food security. Mediterr. J. Nutr. Metab. 2012, 5, 1–10. [Google Scholar]
- Kato, Y.; Shiozawa, R.; Takeda, S.; Ito, S.; Matsuda, K. Structural investigation of a β-d-glucan and a xyloglucan from bamboo shoot cell-walls. Carbohydr. Res. 1982, 109, 233–248. [Google Scholar] [CrossRef]
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Isolation and characterization of anticomplementary β-2glucans from the shoots of bamboo Phyllostachys edulis. Planta Med. 2003, 69, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Aida, F.M.; Shuhaimi, M.; Dzulkifly, M.; Yazid, A.M. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules 2012, 17, 1635–1651. [Google Scholar]
- Suzuki, S.; Saito, T.; Uchiyama, M.; Akiya, S. Studies on the anti-tumor activity of polysaccharides. Isolation of hemicelluloses from Yakushima-bamboo and their growth inhibitory activities against sarcoma-180 solid tumor. Chem. Pharm. Bull. 1968, 16, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Hokputsa, S.; Harding, S.E.; Inngjerdingen, K.; Jumel, K.; Michaelsen, T.E.; Heinzne, T.; Koschella, A.; Paulsen, B.S. Bioactive polysaccharides from the stems of the Thai medicinal plant Acanthus ebracteatus: Their chemical and physical features. Carbohydr. Res. 2004, 339, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, N.; Ling, X.P.; Ye, M.L.; Zhang, C.X.; Shao, W.Y.; Yao, C.Y.; Wang, Z.Y.; Li, Q.B. The isolation and characterization of polysaccharides from longan pulp. Sep. Purif. Technol. 2008, 63, 226–230. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, S.M.; Song, K.S.; Hong, N.D.; Yoo, I.D. Characterization of carbohydrate-peptide linkage of acidic heteroglycopeptide with immunostimulating activity from mycelium of Phellinus linteus. Chem. Pharm. Bull. 1996, 44, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Quan, Y. Antioxidant and immunological activity in vitro of polysaccharides from Phellinus nigricans mycelia. Int. J. Biol. Macromol. 2014, 64, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hara, C.; Kiho, T.; Ukai, S. Anti-inflarnrnatory (1→3)β-d-glucan a sodium carbonate extract of Dictyophora indusiata fisch. Carbohydr. Res. 1983, 117, 201–213. [Google Scholar] [CrossRef]
- Cui, H.X.; Liu, Q.; Tao, Y.Z. Structure and chain conformation of a (1–6)-α-d-glucan from the root of Pueraria lobate (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydr. Polym. 2008, 74, 771–778. [Google Scholar] [CrossRef]
- Navarro, D.A.; Cerezo, A.S.; Stortz, C.A. NMR spectroscopy and chemical studies of an arabinan-rich system from the endosperm of the seed of Gleditsia triacanthos. Carbohydr. Res. 2002, 337, 255–263. [Google Scholar] [CrossRef]
- Errea, M.I.; Matulewicz, M.C. Unusual structure in the polysaccharides from the red seaweed Pterocladiella capillacea (Gelidiaceae, Gelidiales). Carbohydr. Res. 2002, 338, 943–953. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Hu, X.S.; Ni, Y.; Li, Q. Structural characterization of a polysaccharide isolated from Lady Godiva Pumpkins (Cucurbita pepo lady godiva). Macromol. Res. 2011, 19, 1172–1178. [Google Scholar] [CrossRef]
- Li, A.P.; Xie, B.X.; Tao, J.K.; Wang, J. Evaluation of the antioxidant activity of extracts from bamboo shoots. Acta Nutr. Sin. 2008, 30, 321–323. [Google Scholar]
- Meng, F.Y.; Ning, Y.L.; Qi, J.; Zhou, H.; Jie, J.; Lin, J.J.; Huang, Y.J.; Li, F.S.; Li, X.H. Structure and antitumor and immunomodulatory activities of a water-soluble polysaccharide from Dimocarpus longan Pulp. Int. J. Mol. Sci. 2014, 15, 5140–5142. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lu, R.; Yoshida, T. Structure and molecular weight of Asian lacquer polysaccharides. Carbohydr. Polym. 2003, 54, 419–424. [Google Scholar] [CrossRef]
- Yu, R.G.; Wang, L.; Zhang, H.; Zhou, C.X.; Zhao, Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia 2004, 75, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, M.; Xue, F.; Liu, H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr. Polym. 2014, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Liu, Y.; Sun, Y.; Mou, Q.; Wang, B.; Zhang, Y.; Huang, L.J. Structural characterization of LbGp1 from the fruits of Lycium barbarum L. Food Chem. 2014, 159, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.Y.; Yan, J.K. Effect of extraction media on preliminary characterizations andantioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chen, H.X.; Wang, Z.S.; Tian, J.; Wang, J. Structural characterization and antioxidant properties of polysaccharides from two Schisandra fruits. Eur. Food Res. Technol. 2013, 237, 691–701. [Google Scholar] [CrossRef]
- Satitmanwiwat, S.; Ratanakhanokchai, K.; Laohakunjit, N.; Chao, L.K.; Chen, S.T.; Pason, P.; Tachaapaikoon, C.; Kyu, K.L. Improved purity and immunostimulatory activity of β-(1→3)(1→6)-glucan from Pleurotus sajor-caju using cell wall-degrading enzymes. J. Agric. Food Chem. 2012, 60, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Rout, D.; Mondal, S.; Chakraborty, I.; Islam, S. The structure and conformation of a water-insoluble (1→3),(1→6)-β-d-glucan from the fruiting bodies of Pleurotus florida. Carbohydr. Res. 2008, 343, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Wang, X.M.; Yu, S.C.; Zhao, M. Isolation and antioxidant activities of polysaccharides extracted from the shoots of Phyllostachys edulis (Carr.). Int. J. Biol. Macromol. 2011, 49, 454–457. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zheng, J.; Xia, X.; Kan, J. Purification and Structural Identification of Polysaccharides from Bamboo Shoots (Dendrocalamus latiflorus). Int. J. Mol. Sci. 2015, 16, 15560-15577. https://doi.org/10.3390/ijms160715560
Wu J, Zheng J, Xia X, Kan J. Purification and Structural Identification of Polysaccharides from Bamboo Shoots (Dendrocalamus latiflorus). International Journal of Molecular Sciences. 2015; 16(7):15560-15577. https://doi.org/10.3390/ijms160715560
Chicago/Turabian StyleWu, Jinsong, Jiong Zheng, Xuejuan Xia, and Jianquan Kan. 2015. "Purification and Structural Identification of Polysaccharides from Bamboo Shoots (Dendrocalamus latiflorus)" International Journal of Molecular Sciences 16, no. 7: 15560-15577. https://doi.org/10.3390/ijms160715560






