Quantitative Analysis of PMLA Nanoconjugate Components after Backbone Cleavage
Abstract
:1. Introduction
2. Results and Discussion
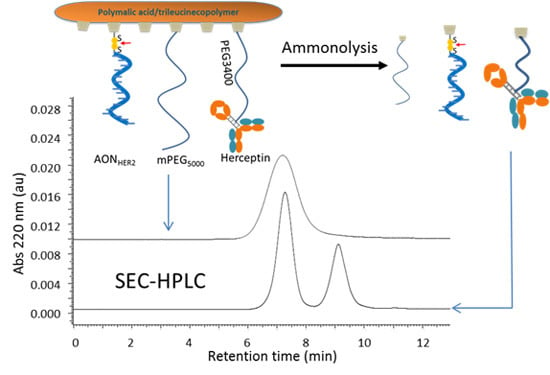
2.1. Selective Cleavage of PMLA
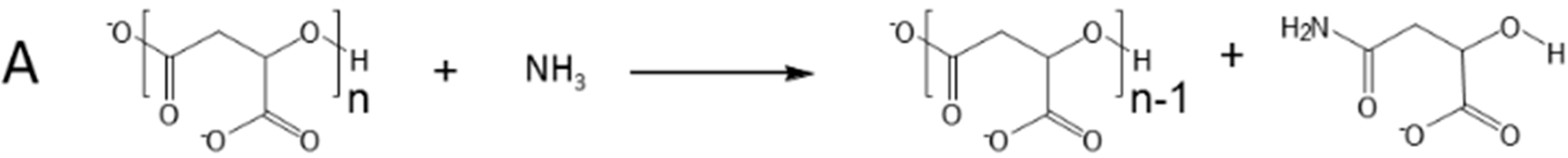

2.2. Cleavage Kinetics for Free PMLA
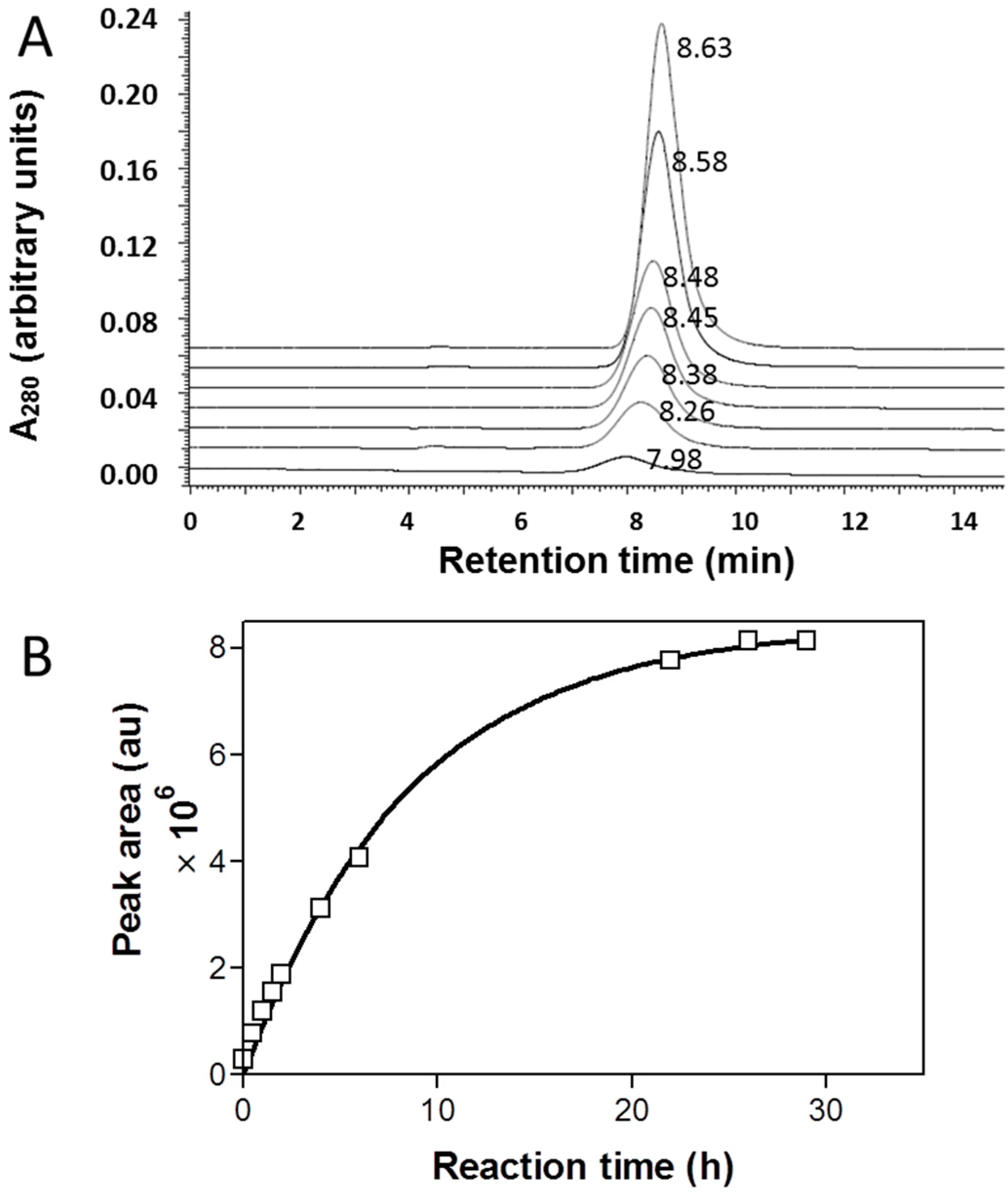
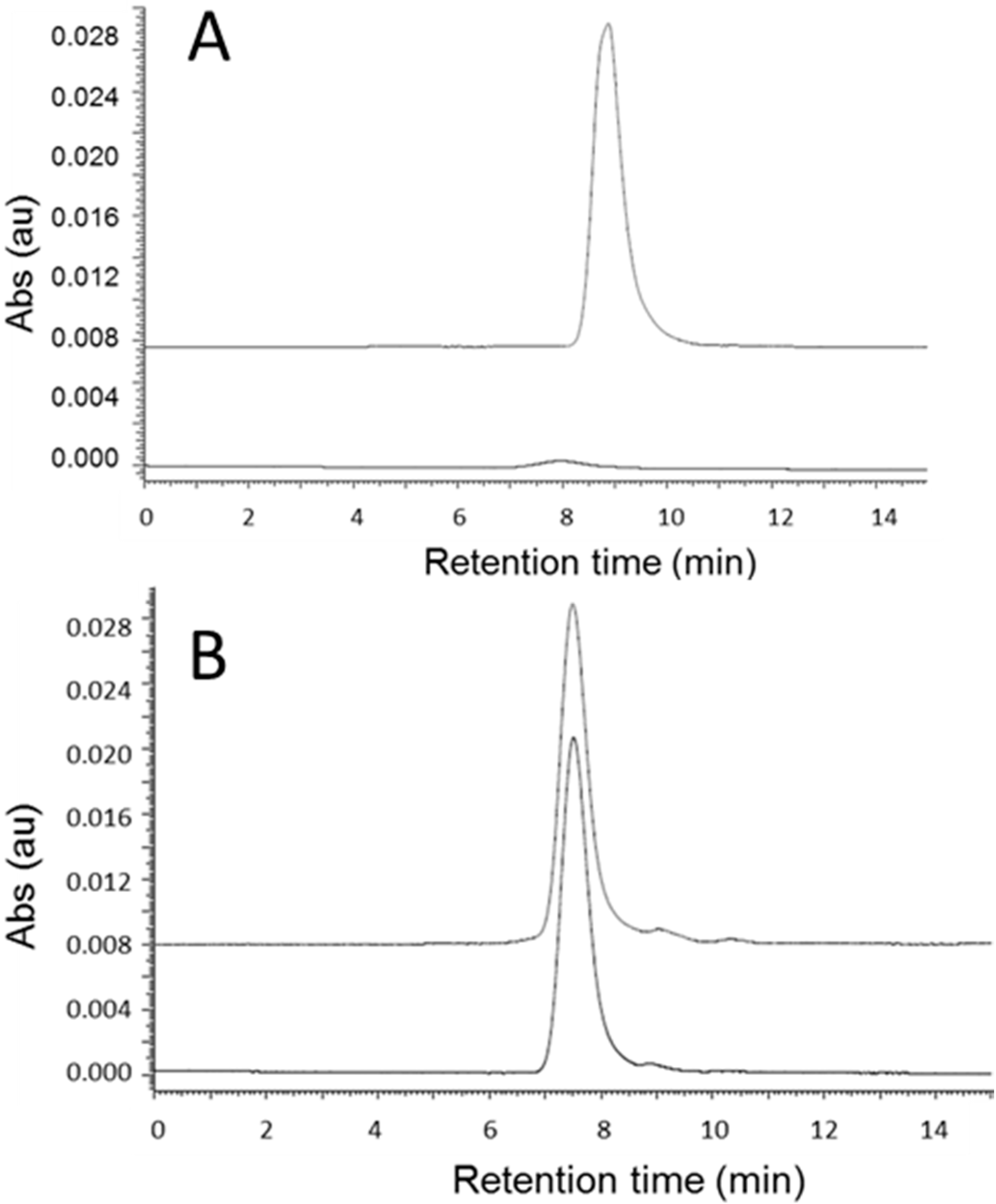
2.3. Cleavage of P/mPEG(5%)/LLL(40%)/Herceptin(0.2%)/AONHER2(2%)

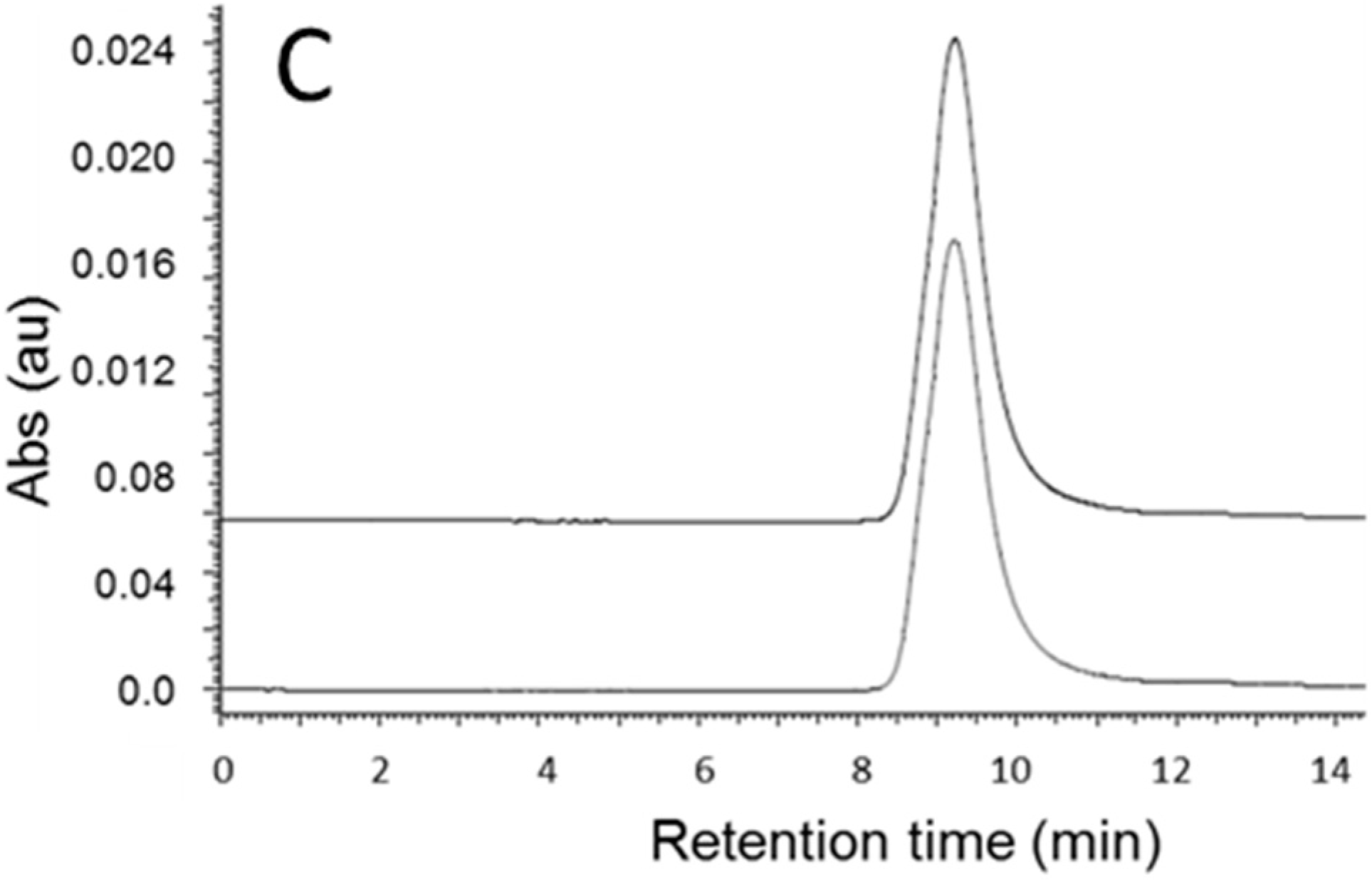
2.4. Quantitation of Antibody (Herceptin) and AONHER2
| Herceptin | AON | |
|---|---|---|
| Sample, conc (mg/mL) | 1.01 ± 0.03 a | 0.29 ± 0.01 a 0.34 ± 0.01 d |
| Synthesis, feed (mg) | 5.00 g | 10.00 g |
| Content, measured (mg) | 5.05 ± 0.15 b | 1.45 ± 0.05 b 1.7 ± 0.05 e |
| Recovery (%) | 101 c | 14.5 c 17.0 f |
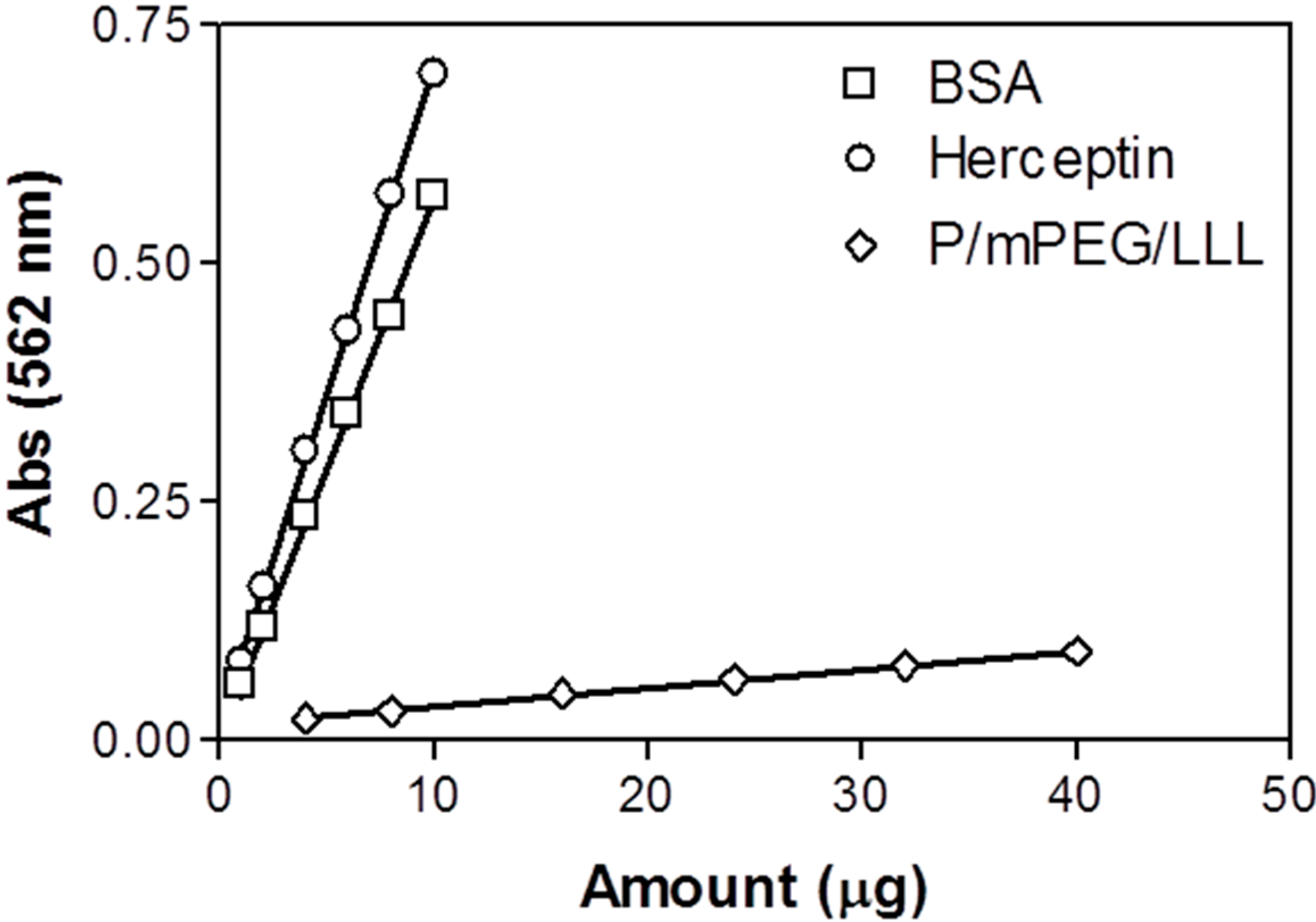
2.5. Quantitation of Herceptin in the Nanoconjugate Using the BCA Method
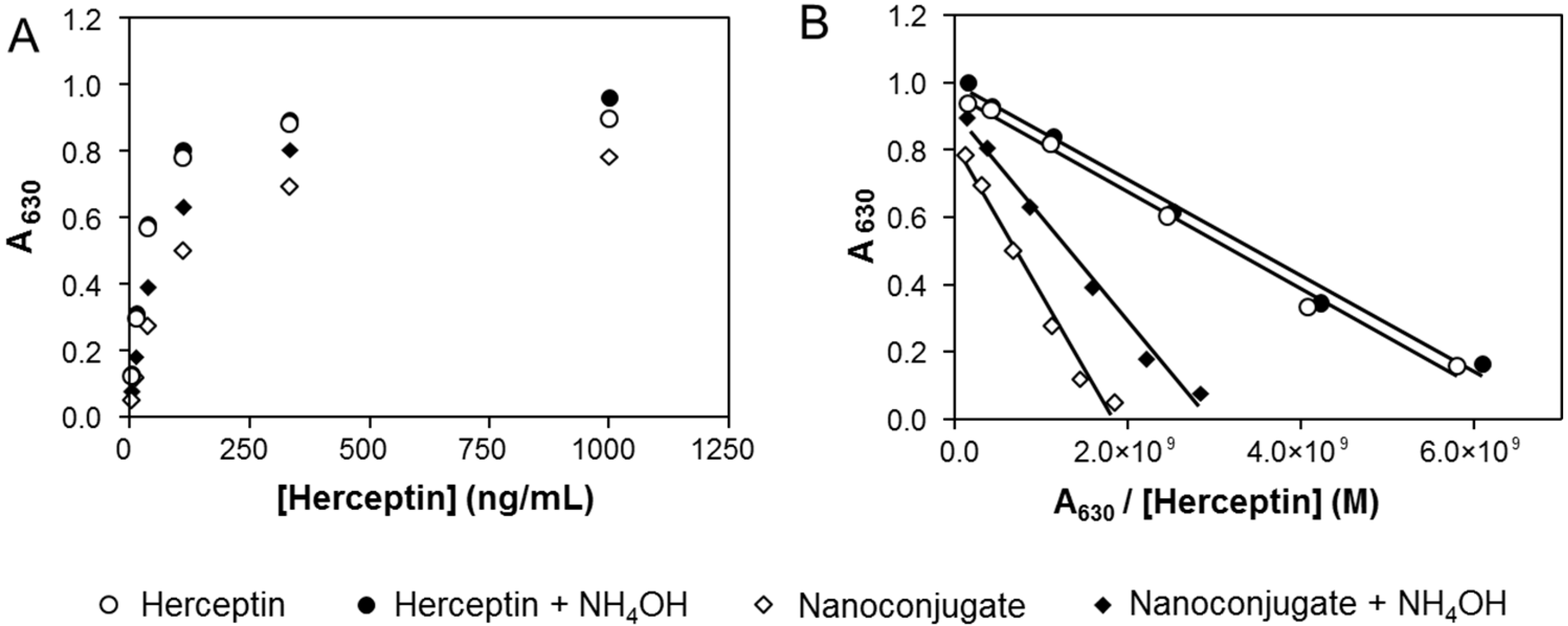
2.6. Characterization by ELISA of Herceptin after Nanoconjugate Ammonolysis
3. Experimental Section
3.1. Materials
3.2. Synthesis of P/mPEG(5%)/LLL(40%/Herceptin(0.2%)/AONHER2(2%)
3.3. Selective Cleavage of the Nanoconjugate Backbone by Ammonium Hydroxide
3.4. Quantitation of Antibody by SEC-HPLC in the Reaction Mixture after Cleavage
3.5. Herceptin Quantitation Using the BCA Protein Assay Prior to Cleavage
3.6. ELISA for Free and Conjugated Herceptin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kopecek, J. Polymer-drug conjugates: Origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Noh, Y.W.; Heo, M.B.; Cho, M.Y.; Lim, Y.T. Multifunctional hybrid nanoconjugates for efficient in vivo delivery of immunomodulating oligonucleotides and enhanced antitumor immunity. Angew. Chem. 2012, 51, 9670–9673. [Google Scholar] [CrossRef]
- Khandare, J.; Calderon, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Portilla-Arias, J.; Patil, R.; Black, K.L.; Ljubimova, J.Y.; Holler, E. Distinct mechanisms of membrane permeation induced by two polymalic acid copolymers. Biomaterials 2013, 34, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, P.C. Light scattering by polymer solutions. In Polymer Chemistry: The Basic Concepts; Marcel Decker Inc.: New York, NY, USA, 1984; p. 659. [Google Scholar]
- Li, Y.P.; Xiao, K.; Luo, J.T.; Lee, J.; Pan, S.R.; Lam, K.S. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release 2010, 144, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.T.; Xiao, K.; Li, Y.P.; Lee, J.S.; Shi, L.F.; Tan, Y.H.; Xing, L.; Cheng, R.H.; Liu, G.Y.; Lam, K.S. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconj. Chem. 2010, 21, 1216–1224. [Google Scholar] [CrossRef]
- Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Rekechenetskiy, A.; Inoue, S.; Black, K.L.; Holler, E.; Ljubimova, J.Y. Cellular delivery of doxorubicin via pH-controlled hydrazone linkage using multifunctional nano vehicle based on poly(β-l-malic acid). Int. J. Mol. Sci. 2012, 13, 11681–11693. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Nayak, T.R.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Theranostic unimolecular micelles based on brush-shaped amphiphilic block copolymers for tumor-targeted drug delivery and positron emission tomography imaging. ACS Appl. Mater. Interfaces 2014, 6, 21769–21779. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Holler, E. β-Poly(l-malate) production by non-growing microplasmodia of physarum polycephalum. Effects of metabolic intermediates and inhibitors. FEMS Microbiol. Lett. 2000, 193, 69–74. [Google Scholar]
- Lee, B.S.; Vert, M.; Holler, E. Water-Soluble Aliphatic Polyesters: Poly(malic acid)s; Polyester, I., Ed.; Wiley-VCH Verlag Gmbh: Weinheim, Germany, 2002; Volume 3a, pp. 75–103. [Google Scholar]
- Ljubimova, J.Y.; Portilla-Arias, J.; Patil, R.; Ding, H.; Inoue, S.; Markman, J.L.; Rekechenetskiy, A.; Konda, B.; Gangalum, P.R.; Chesnokova, A.; et al. Toxicity and efficacy evaluation of multiple targeted polymalic acid conjugates for triple-negative breast cancer treatment. J. Drug Target. 2013, 21, 956–967. [Google Scholar]
- Ding, H.; Inoue, S.; Ljubimov, A.V.; Patil, R.; Portilla-Arias, J.; Hu, J.; Konda, B.; Wawrowsky, K.A.; Fujita, M.; Karabalin, N.; et al. Inhibition of brain tumor growth by intravenous poly(β-l-malic acid) nanobioconjugate with pH-dependent drug release. Proc. Natl. Acad. Sci. USA 2010, 107, 18143–18148. [Google Scholar]
- Inoue, S.; Ding, H.; Portilla-Arias, J.; Hu, J.; Konda, B.; Fujita, M.; Espinoza, A.; Suhane, S.; Riley, M.; Gates, M.; et al. Polymalic acid-based nanobiopolymer provides efficient systemic breast cancer treatment by inhibiting both HER2/neu receptor synthesis and activity. Cancer Res. 2011, 71, 1454–1464. [Google Scholar]
- Inoue, S.; Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Espinoza, A.; Mongayt, D.; Markman, J.L.; Elramsisy, A.; Phillips, H.W.; et al. Nanobiopolymer for direct targeting and inhibition of egfr expression in triple negative breast cancer. PLoS ONE 2012, 7, e31070. [Google Scholar]
- Ljubimova, J.Y.; Ding, H.; Portilla-Arias, J.; Patil, R.; Gangalum, P.R.; Chesnokova, A.; Inoue, S.; Rekechenetskiy, A.; Nassoura, T.; Black, K.L.; et al. Polymalic acid-based nano biopolymers for targeting of multiple tumor markers: An opportunity for personalized medicine. J. Vis. Exp. 2014, 88, 50668. [Google Scholar]
- Patil, R.; Portilla-Arias, J.; Ding, H.; Inoue, S.; Konda, B.; Hu, J.; Wawrowsky, K.A.; Shin, P.K.; Black, K.L.; Holler, E.; et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-l-malic acid). Pharm. Res. 2010, 27, 2317–2329. [Google Scholar]
- Ding, H.; Portilla-Arias, J.; Patil, R.; Black, K.L.; Ljubimova, J.Y.; Holler, E. The optimization of polymalic acid peptide copolymers for endosomolytic drug delivery. Biomaterials 2011, 32, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Helguera, G.; Rodriguez, J.A.; Markman, J.; Luria-Perez, R.; Gangalum, P.; Portilla-Arias, J.; Inoue, S.; Daniels-Wells, T.R.; Black, K.; et al. Polymalic acid nanobioconjugate for simultaneous immunostimulation and inhibition of tumor growth in HER2/neu-positive breast cancer. J. Control. Release 2013, 171, 322–339. [Google Scholar]
- Holler, E. Poly(malic acid) from natural sources. In Handbook of Engineering Polymeric Materials; Cheremisinoff, N.P., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 93–103. [Google Scholar]
- Holler, E.; Lee, B.S. Analysis of poly(β-l-malic acid) in tissue and solution. Recent Res. Dev. Anal. Chem. 2002, 2, 177–192. [Google Scholar]
- Lee, B.S.; Fujita, M.; Khazenzon, N.M.; Wawrowsky, K.A.; Wachsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; Ljubimova, J.Y.; Holler, E. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(β-l-malic acid acid) for drug delivery. Bioconj. Chem. 2006, 17, 317–326. [Google Scholar] [CrossRef]
- Bulmus, V.; Woodward, M.; Lin, L.; Murthy, N.; Stayton, P.; Hoffman, A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 2003, 93, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, B.H. Non-inverted versus inverted plots in enzyme kinetics. Nature 1959, 184, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the bicinchoninic acid protein assay—Identification of the groups responsible for color formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Patil, R.; Portilla-Arias, J.; Black, K.L.; Ljubimova, J.Y.; Holler, E. Quantitative Analysis of PMLA Nanoconjugate Components after Backbone Cleavage. Int. J. Mol. Sci. 2015, 16, 8607-8620. https://doi.org/10.3390/ijms16048607
Ding H, Patil R, Portilla-Arias J, Black KL, Ljubimova JY, Holler E. Quantitative Analysis of PMLA Nanoconjugate Components after Backbone Cleavage. International Journal of Molecular Sciences. 2015; 16(4):8607-8620. https://doi.org/10.3390/ijms16048607
Chicago/Turabian StyleDing, Hui, Rameshwar Patil, Jose Portilla-Arias, Keith L. Black, Julia Y. Ljubimova, and Eggehard Holler. 2015. "Quantitative Analysis of PMLA Nanoconjugate Components after Backbone Cleavage" International Journal of Molecular Sciences 16, no. 4: 8607-8620. https://doi.org/10.3390/ijms16048607
APA StyleDing, H., Patil, R., Portilla-Arias, J., Black, K. L., Ljubimova, J. Y., & Holler, E. (2015). Quantitative Analysis of PMLA Nanoconjugate Components after Backbone Cleavage. International Journal of Molecular Sciences, 16(4), 8607-8620. https://doi.org/10.3390/ijms16048607