Biomechanical Evaluation of Ti-Nb-Sn Alloy Implants with a Low Young’s Modulus
Abstract
:1. Introduction
2. Results
2.1. Surface Characteristics of the Specimens
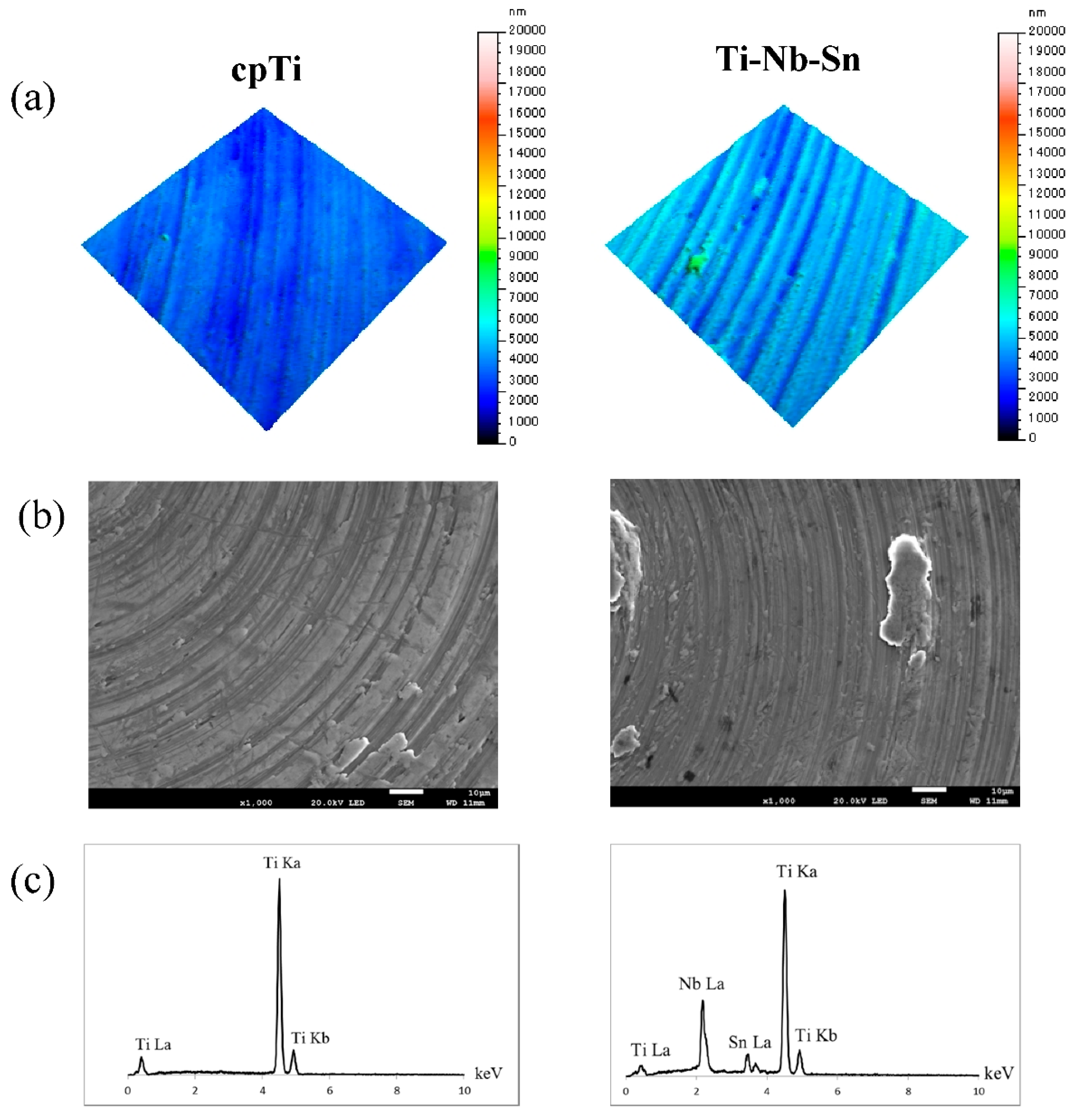
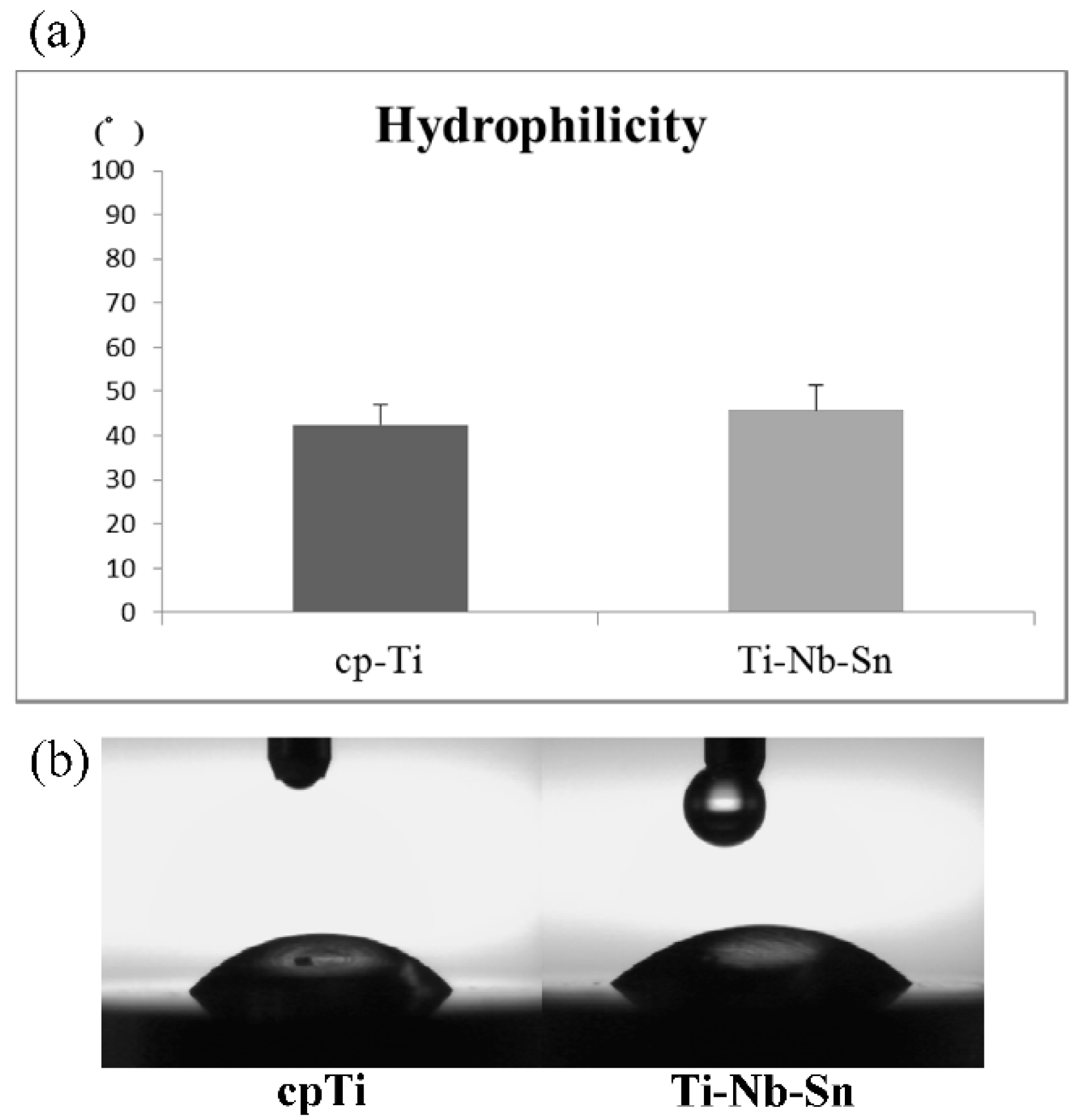
2.2. Cell Proliferation Assay

2.3. ALP Activity
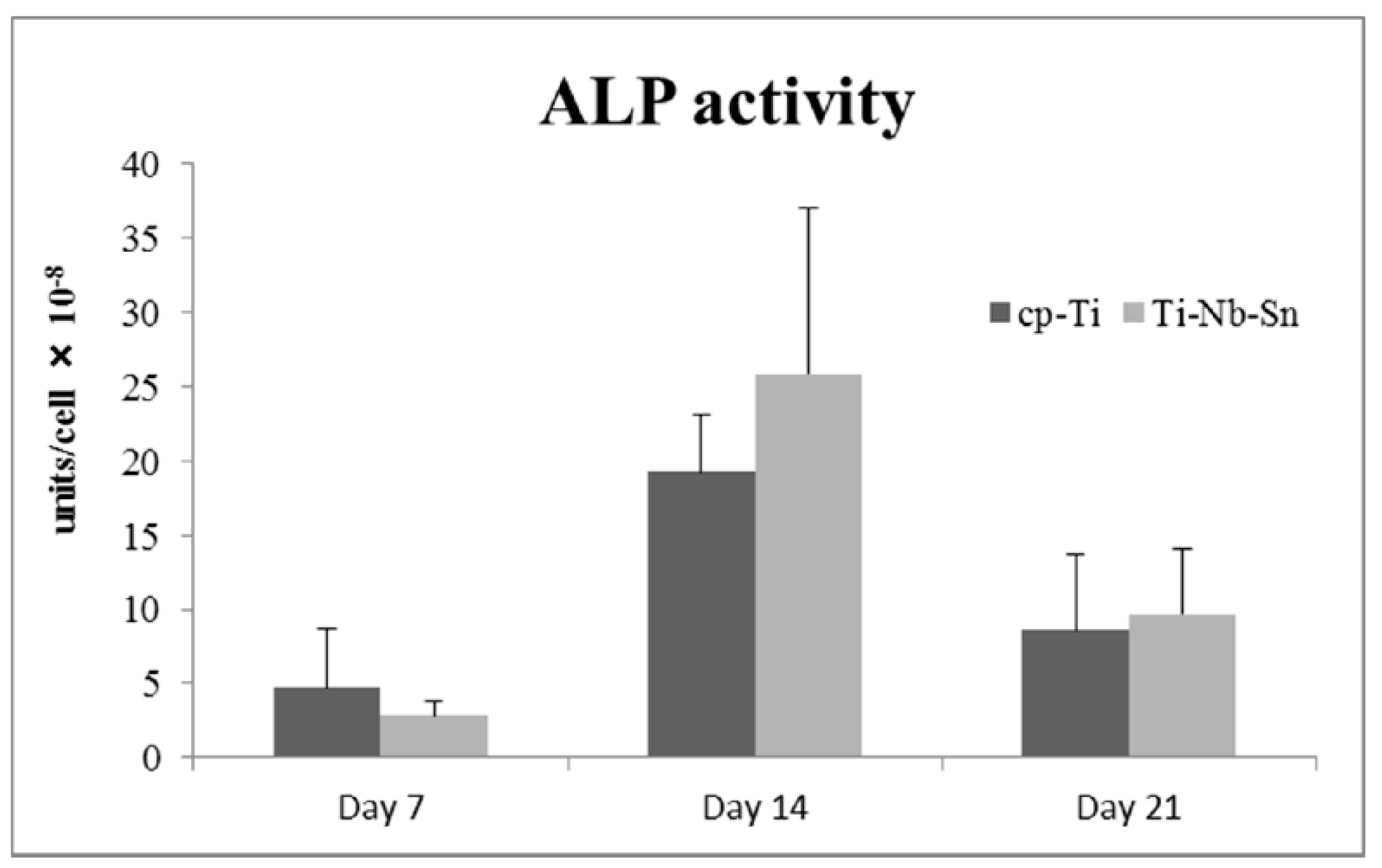
2.4. Push-in Test Values
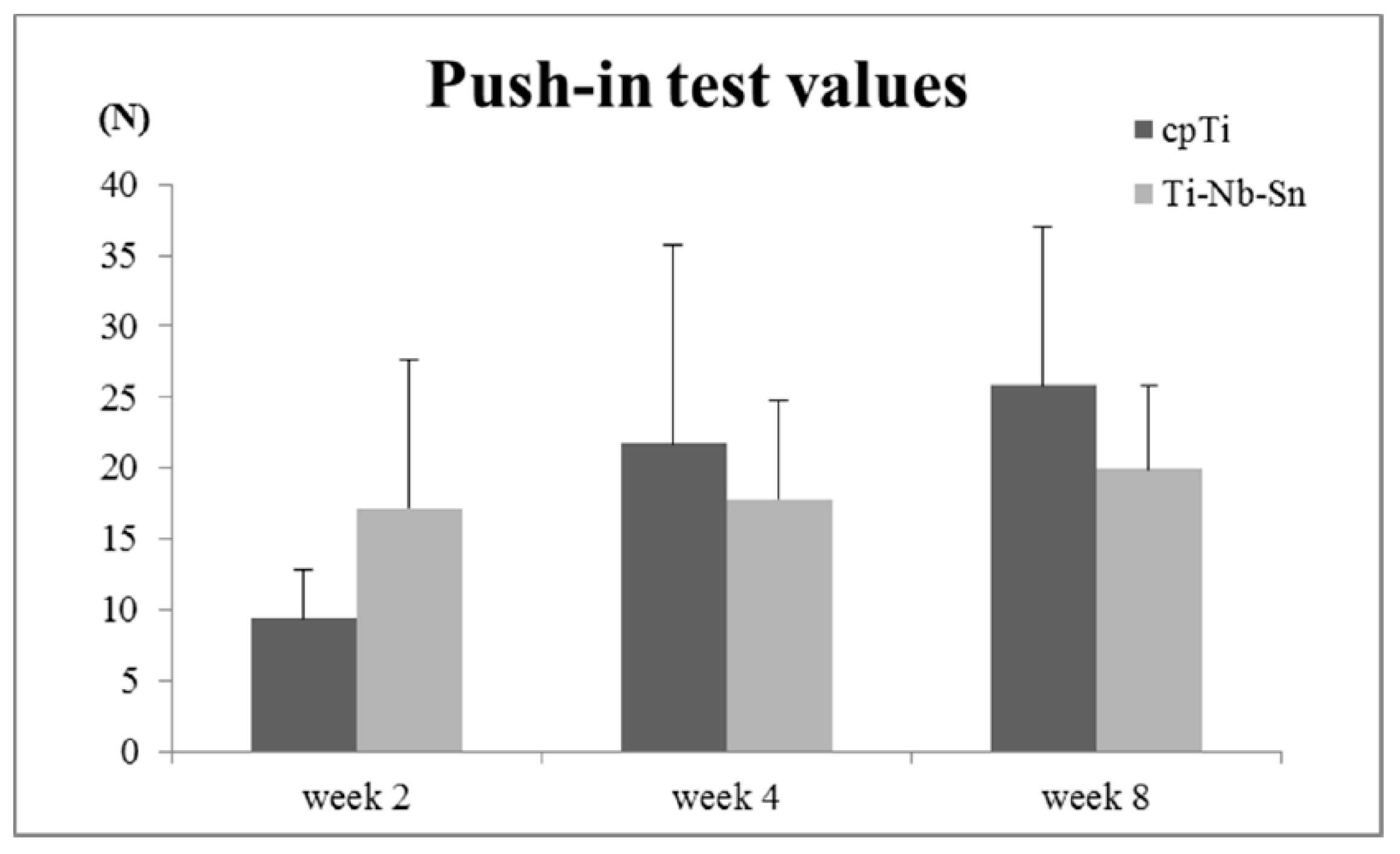
2.5. Analyses of the Implant-Tissue Interface

3. Discussion
4. Materials and Methods
4.1. Specimen Design
4.2. Surface Characterizations
4.3. Cell Culture and Proliferation
4.4. Alkaline Phosphatase (ALP) Activity
4.5. Animal Experiments
4.6. Push-in Tests
4.7. Analyses of the Implant-Tissue Interface
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salvi, G.E.; Zitzmann, N.U. The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 292–307. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Needleman, I.; Salvi, G.E.; Pjetursson, B.E. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int. J. Oral Maxillofac. Implant. 2014, 29, 346–350. [Google Scholar] [CrossRef]
- Isidor, F. Influence of forces on peri-implant bone. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 8–18. [Google Scholar] [CrossRef]
- Duyck, J.; Rønold, H.J.; van Oosterwyck, H.; Naert, I.; van der Sloten, J.; Ellingsen, J.E. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: An animal experimental study. Clin. Oral Implant. Res. 2001, 12, 207–218. [Google Scholar] [CrossRef]
- Shigemitsu, R.; Ogawa, T.; Matsumoto, T.; Yoda, N.; Gunji, Y.; Yamakawa, Y.; Ikeda, K.; Sasaki, K. Stress distribution in the peri-implant bone with splinted and non-splinted implants by in vivo loading data-based finite element analysis. Odontology 2013, 101, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Wazena, R.M; Curreyb, J.A.; Guoc, H.; Brunskid, J.B.; Helmsd, J.A.; Nancia, A. Micromotion-induced strain fields influence early stages of repair at bone-implant interfaces. Acta Biomater. 2013, 9, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Watanabe, S.; Hanada, S. Beta TiNbSn alloys with low Young’s modulus and high strength. Mater. Trans. 2005, 46, 1070–1078. [Google Scholar] [CrossRef]
- Hanada, S.; Matsumoto, H.; Watanabe, S. Mechanical compatibility of titanium implants in hard tissues. Inter. Cong. Ser. 2005, 1284, 239–247. [Google Scholar] [CrossRef]
- Zheng, T.F.; Wang, B.L.; Wang, J.G.; Lia, C.; Zhao, L.C. Corrosion behaviour of Ti-Nb-Sn shape memory alloys in different simulated body solutions. Mater. Sci. Eng. A 2006, 438–440, 891–895. [Google Scholar] [CrossRef]
- Miura, K.; Yamada, N.; Hanada, S.; Jung, T.K.; Itoi, E. The bone tissue compatibility of a new Ti-Nb-Sn alloy with a low Young’s modulus. Acta Biomater. 2011, 7, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Watanabe, S.; Masahashi, N.; Hosoda, H.; Hanawa, S. Ni-free Ti-Nb-Sn shape memory alloys. In Structural Biomaterials for the 21st Century; Niinomi, M., Okabe, T., Taleff, E.M., Lesuer, D.R., Lippard, H.F., Eds.; TMS: New Orleans, LA, USA, 2001; pp. 25–34. [Google Scholar]
- Kelly, D.J.; Jacobs, C.R. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Skerry, T.M. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008, 473, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Brett, P.M.; Harle, J.; Salih, V.; Mihoc, R.; Olsen, I.; Jones, F.H.; Tonetti, M. Roughness response genes in osteoblasts. Bone 2004, 35, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011. [Google Scholar] [CrossRef]
- Shiraishi, N.; Rong, T.; Uzuka, R.; Anada, T.; Narushima, T.; Goto, T.; Niinomi, M.; Sasaki, K.; Suzuki, O. Biomaterial evaluation of amorphous calcium phosphate coated TNTZ implants prepared using a radiofrequency magnetron sputtering systems. Mater. Trans. 2012, 53, 1343–1348. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Guo, J.; Padilla, R.J.; Ambrose, W.; de Kok, I.J.; Cooper, L.F. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Saruwatari, L.; Takeuchi, K.; Aita, H.; Ohno, N. Ti nano-nodular structuring for bone integration and regeneration. J. Dent. Res. 2008, 87, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Martra, G.; Coluccia, S.; Anpo, M. Investigations of the structure of H2O clusters adsorbed on TiO2 surfaces by near-infrared absorption spectroscopy. J. Phys. Chem. B 2005, 109, 7387–7391. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J.; Grami, S.; Jeong, S.C.; Conrad, H.J.; Hodges, J.S. Comparison of push-in versus pull-out tests on bone-implant interfaces of rabbit tibia dental implant healing model. Clin. Implant. Dent. Relat. Res. 2013, 15, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Tamai, M.; Isama, K.; Nakaoka, R.; Tsuchiya, T. Synthesis of a novel beta-tricalcium phosphate/hydroxyapatite biphasic calcium phosphate containing niobium ions and evaluation of its osteogenic properties. J. Artif. Organs 2007, 10, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ozawa, S.; Shih, J.-H.; Ryu, K.H.; Sukotjo, C.; Yang, J.-M.; Nishimura, I. Biomechanical Evaluation of Osseous Implants Having Different Surface Topographies in Rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Shiraishi, N.; Ishiko-Uzuka, R.; Anada, T.; Suzuki, O.; Masumoto, H.; Sasaki, K. Biomechanical Evaluation of Ti-Nb-Sn Alloy Implants with a Low Young’s Modulus. Int. J. Mol. Sci. 2015, 16, 5779-5788. https://doi.org/10.3390/ijms16035779
Takahashi K, Shiraishi N, Ishiko-Uzuka R, Anada T, Suzuki O, Masumoto H, Sasaki K. Biomechanical Evaluation of Ti-Nb-Sn Alloy Implants with a Low Young’s Modulus. International Journal of Molecular Sciences. 2015; 16(3):5779-5788. https://doi.org/10.3390/ijms16035779
Chicago/Turabian StyleTakahashi, Kenta, Naru Shiraishi, Risa Ishiko-Uzuka, Takahisa Anada, Osamu Suzuki, Hiroshi Masumoto, and Keiichi Sasaki. 2015. "Biomechanical Evaluation of Ti-Nb-Sn Alloy Implants with a Low Young’s Modulus" International Journal of Molecular Sciences 16, no. 3: 5779-5788. https://doi.org/10.3390/ijms16035779




