Chemical Modeling of Acid-Base Properties of Soluble Biopolymers Derived from Municipal Waste Treatment Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Solution Behavior of Soluble Bio-Organic Substances (SBOs)
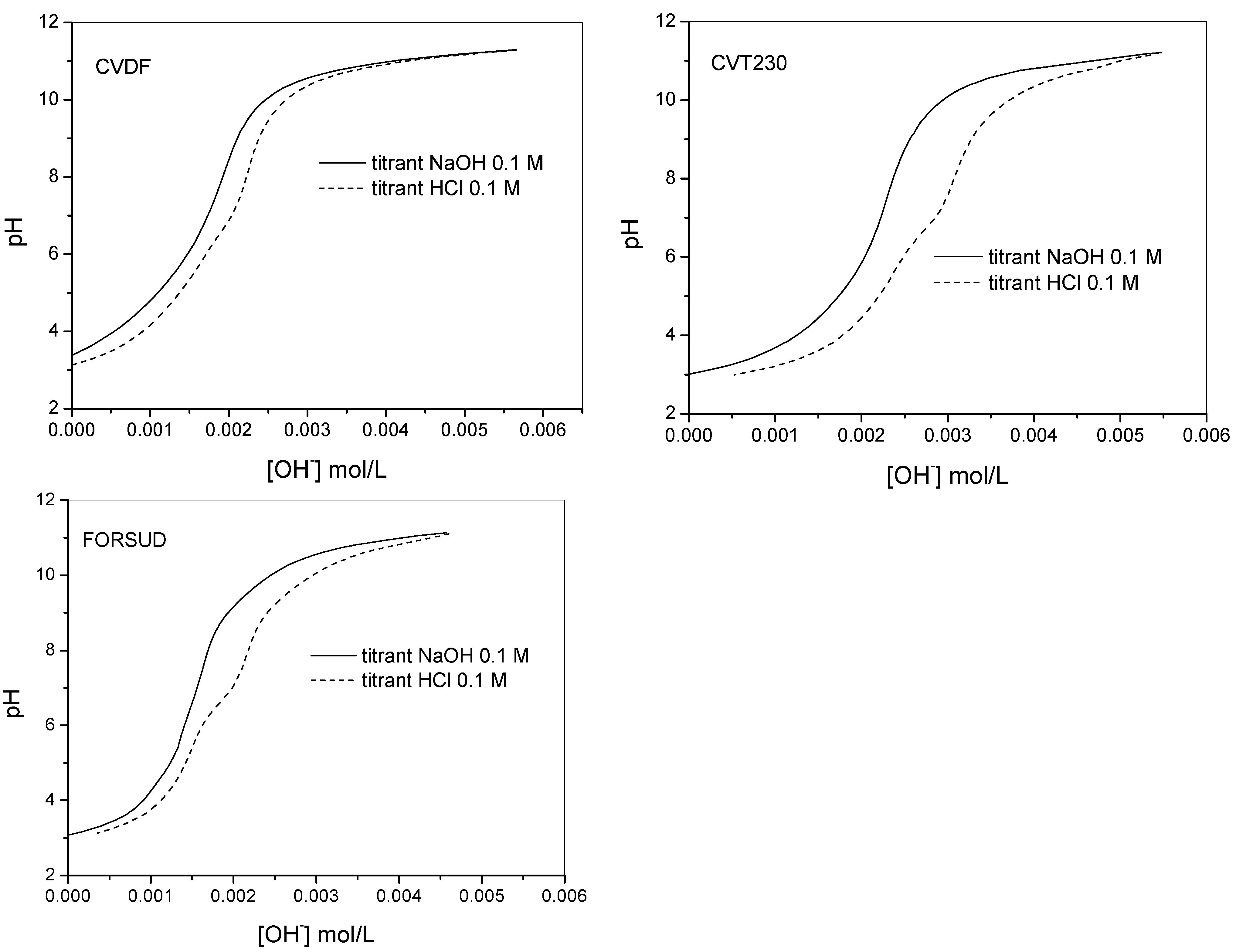
2.2. Proton Binding Capacity of SBOs
2.2.1. Protonation Constants
| SBO | Ash (w/w %) | C (w/w %) a | N (w/w %) a | Aliphatic b | NR b,c | OR b,c | Ar b | PhOY b,d | COY b,e | CMC (g·L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| CVDF | 0.56 | 48.5 | 5.2 | 0.31 | 0.06 | 0.16 | 0.23 | 0.12 | 0.11 | 2.76 |
| CVT230 | 0.81 | 52.5 | 5.2 | 0.28 | 0.07 | 0.02 | 0.22 | 0.11 | 0.11 | 4.2 |
| FORSUD | 0.01 | 57.5 | 8.8 | 0.43 | 0.09 | 0.15 | 0.15 | 0.06 | 0.13 | 1.02 |
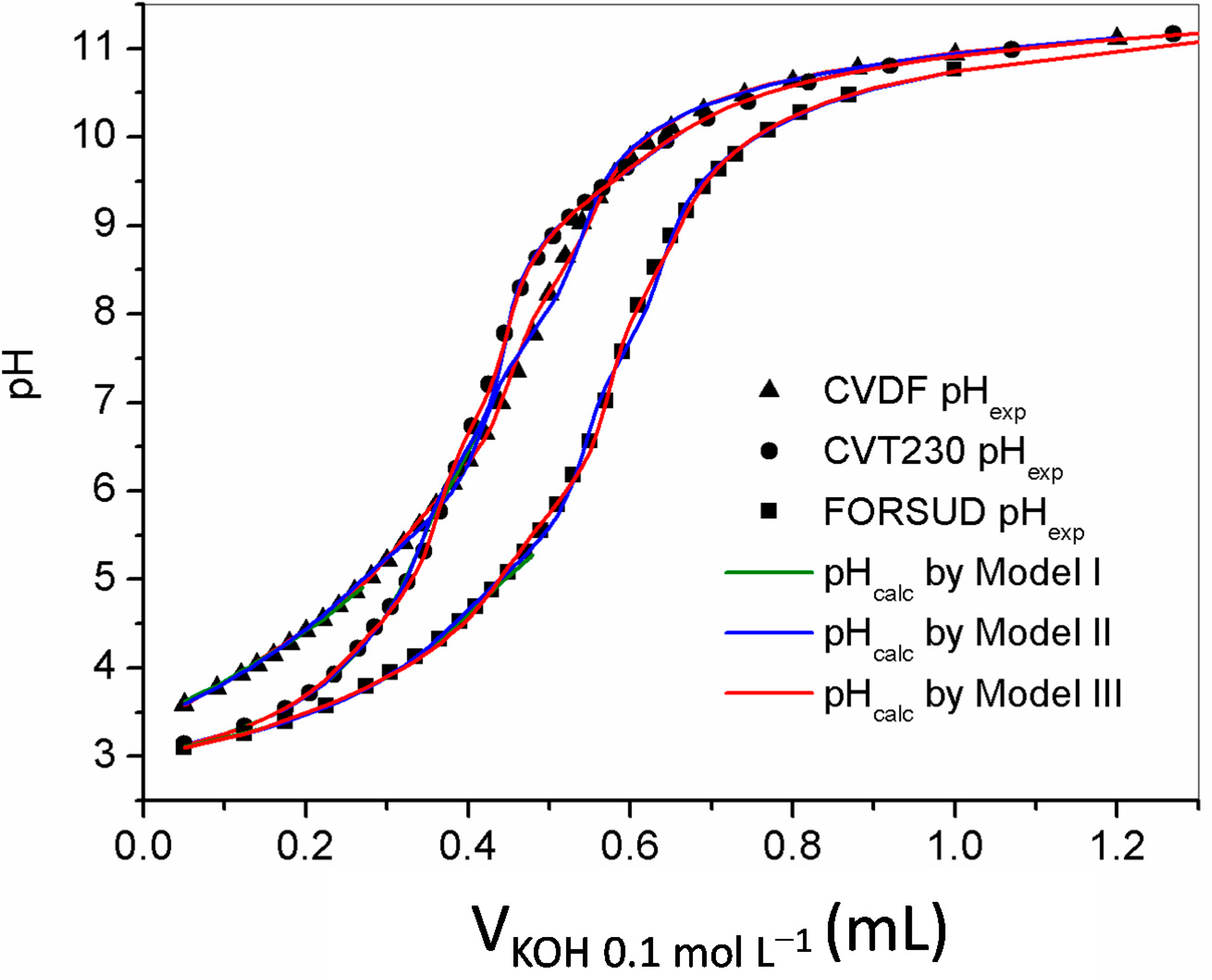
| SBO | Chemical Model | Acidic Site | logβ a | logK a | Site Concentration (mmol·g−1) |
|---|---|---|---|---|---|
| CVDF | Model I | Hcvdf1 b | 5.69 ± 0.02 c | 3.0 ± 0.4 | |
| H2cvdf1 | 9.83 ± 0.03 | 4.14 | |||
| Weighted standard deviation of the fit d | 2.14 | ||||
| Model II | Hcvdf1 | 5.42 ± 0.02 c | 2.6 ± 0.2 | ||
| H2cvdf1 | 9.41 ± 0.04 | 3.99 | |||
| Hcvdf2 | 7.78 ± 0.04 | 0.8 ± 0.2 | |||
| Hcvdf3 | 10.23 ± 0.02 | 0.9 ± 0.1 | |||
| Weighted standard deviation of the fit | 2.55 | ||||
| Model III | Hcvdf1 | 4.34 ± 0.02 | 1.70 ± 0.08 | ||
| Hcvdf2 | 6.03 ± 0.03 | 1.0 ± 0.1 | |||
| Hcvdf3 | 8.33 ± 0.04 | 0.7 ± 0.1 | |||
| Hcvdf4 | 10.40 ± 0.02 | 0.9 ± 0.2 | |||
| Weighted standard deviation of the fit | 1.73 | ||||
| CVT 230 | Model I | Hcvt1 | 5.29 ± 0.01 | 3.0 ± 0.1 | |
| H2cvt1 | 8.86 ± 0.04 | 3.57 | |||
| Weighted standard deviation of the fit | 2.43 | ||||
| Model II | Hcvt1 | 5.10 ± 0.02 | 2.70 ± 0.05 | ||
| H2cvt1 | 8.61 ± 0.06 | 3.51 | |||
| Hcvt2 | 7.58 ± 0.04 | 0.8 ± 0.1 | |||
| Hcvt3 | 9.97 ± 0.03 | 1.3 ± 0.2 | |||
| Weighted standard deviation of the fit | 3.03 | ||||
| Model III | Hcvt1 | 3.87 ± 0.01 | 1.76 ± 0.03 | ||
| Hcvt2 | 5.76 ± 0.02 | 1.07 ± 0.07 | |||
| Hcvt3 | 8.26 ± 0.04 | 0.71 ± 0.08 | |||
| Hcvt4 | 10.16 ± 0.02 | 1.2 ± 0.2 | |||
| Weighted standard deviation of the fit | 1.75 | ||||
| FORSUD | Model I | Hforsud1 | 6.12 ± 0.03 | 1.61 ± 0.02 | |
| H2forsud1 | 10.32 ± 0.07 | 4.20 | |||
| Weighted standard deviation of the fit | 1.66 | ||||
| Model II | Hforsud1 | 6.37 ± 0.05 | 1.67 ± 0.04 | ||
| H2forsud1 | 10.65 ± 0.08 | 4.28 | |||
| Hforsud2 | 9.34 ± 0.02 | 1.602 ± 0.004 | |||
| Weighted standard deviation of the fit | 1.80 | ||||
| Model III | Hforsud1 | 4.50 ± 0.04 | 1.015 ± 0.006 | ||
| Hforsud2 | 6.77 ± 0.06 | 0.67 ± 0.04 | |||
| Hforsud3 | 9.37 ± 0.02 | 1.58 ± 0.01 | |||
| Weighted standard deviation of the fit | 1.43 |
2.2.2. Protogenic Sites Concentrations
| SBO | PhOY a | COY a | CONR b | NR b | COOH c |
|---|---|---|---|---|---|
| CVDF | 4.84 | 4.55 | 1.85 | 1.85 | 2.7 |
| CVT230 | 4.76 | 4.75 | 1.92 | 1.78 | 2.83 |
| FORSUD | 2.76 | 6.16 | 4.48 | 1.82 | 1.68 |
3. Experimental Section
3.1. Chemicals
3.2. Preparation and Characterization of SBOs
3.3. Electromotive Force Measurements
3.4. Data Analysis and Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Montoneri, E.; Mainero, D.; Boffa, V.; Perrone, D.G.; Montoneri, C. Biochemenergy: A project to turn an urban wastes treatment plant into biorefinery for the production of energy, chemicals and consumer’s products with friendly environmental impact. Int. J. Glob. Environ. Issues 2011, 11, 170–196. [Google Scholar] [CrossRef]
- Vargas, A.K.N.; Savarino, P.; Montoneri, E.; Tabasso, S.; Cavalli, R.; Bianco Prevot, A.; Guardani, R.; Roux, G.A.C. Use of biowaste-derived biosurfactants in the production of emulsions for industrial use. Ind. Eng. Chem. Res. 2014, 53, 8621–8629. [Google Scholar]
- Montoneri, E.; Tomasso, L.; Colajanni, N.; Zelano, I.; Alberi, F.; Cossa, G.; Barberis, R. Urban wastes to remediate industrial sites: A case of polycyclic aromatic hydrocarbons contamination and a new process. Int. J. Environ. Sci. Technol. 2014, 11, 251–262. [Google Scholar] [CrossRef]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste cleaning waste: photodegradation of monochlorophenols in the presence of waste derived photosensitizer. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Gomis, J.; Bianco Prevot, A.; Montoneri, E.; Gonzalez, M.C.; Amat, A.M.; Martire, D.O.; Arques, A.; Carlos, L. Waste sourced bio-based substances for solar-driven wastewater remediation: Photodegradation of emerging pollutants. Chem. Eng. J. 2014, 235, 236–243. [Google Scholar] [CrossRef]
- Baxter, M.D.; Acosta, E.; Montoneri, E.; Tabasso, S. Waste biomass-extracted surfactants for heavy oil removal. Ind. Eng. Chem. Res. 2014, 53, 3612–3621. [Google Scholar]
- Boffa, V.; Perrone, D.G.; Magnacca, G.; Montoneri, E. Role of a waste-derived biosurfactant in the sol-gel synthesis of nanocrystalline titanium dioxide. Ceram. Int. 2014, 40, 12161–12169. [Google Scholar] [CrossRef]
- Deganello, F.; L.aTummino, M.; Calabrese, C.; Testa, M.L.; Avetta, P.; Fabbri, D.; Bianco Prevot, A.; Montoneri, E.; Magnacca, G. New sustainable LaFeO3 photocatalysts prepared from biowaste sourced soluble substances. New J. Chem. 2014. [Google Scholar] [CrossRef]
- Magnacca, G.; Laurenti, E.; Vigna, E.; Franzoso, F.; Tomasso, L.; Montoneri, E.; Boffa, V. Refuse derived bio-organics and immobilized soybean peroxidase for green chemical technology. Process Biochem. 2012, 47, 2025–2031. [Google Scholar] [CrossRef]
- Franzoso, F.; Tabasso, S.; Antonioli, D.; Montoneri, E.; Persico, P.; Laus, M.; Mendichi, R.; Negre, M. Films made from poly (vinyl alcohol-co-ethylene) and soluble biopolymers isolated from municipal biowaste. J. Appl. Polym. Sci. 2014, 132, 1301. [Google Scholar]
- Sortino, O.; Montoneri, E.; Patanè, C.; Rosato, R.; Tabasso, S.; Ginepro, M. Benefits for agriculture and the environment from urban waste. Sci. Total Environ. 2014, 487C, 443–451. [Google Scholar] [CrossRef]
- Montoneri, C.; Montoneri, E.; Tomasso, L.; Piva, A. Compost derived substances decrease feed protein N mineralization in swine cecal fermentation. J. Agric. Sci. 2013, 13, 31–44. [Google Scholar]
- Dinuccio, E.; Biagini, D.; Rosato, R.; Balsari, P.; Lazzaroni, C.; Montoneri, E. Use of acid soluble bio-organic substances extract as rabbits feed additive to reduce manure gases emission during storage. Adv. Anim. Biosci. 2013, 4, 515. [Google Scholar]
- Lubal, P.; Široký, D.; Fetsch, D.; Havel, J. The acidobasic and complexation properties of humic acids: Study of complexation of Czech humic acids with metal ions. Talanta 1998, 47, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Masini, J.C.; Abate, G.; Lima, E.C.; Hahn, L.C.; Nakamura, M.S.; Lichtig, J.; Nagatomy, H.R. Comparison of methodologies for determination of carboxylic and phenolic groups in humic acids. Anal. Chim. Acta 1998, 364, 223–233. [Google Scholar] [CrossRef]
- Fiol, S.; Lopez, R.; Ramos, A.; Antelo, J.M.; Arce, F. Study of the acid-base properties of three fulvic acids extracted from different horizons of a soil. Anal. Chim. Acta 1999, 385, 443–449. [Google Scholar] [CrossRef]
- Santos, E.B.H.; Esteves, V.I.; Rodrigues, J.P.C.; Duarte, A.C. Humic substances’ proton-binding equilibria: Assessment of errors and limitations of potentiometric data. Anal. Chim. Acta 1999, 392, 333–341. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Modeling the acid-base properties and metal complexation of humic substances with the stockholm humic model. J. Colloid Interface Sci. 2001, 244, 102–112. [Google Scholar] [CrossRef]
- Ritchie, J.; Perdue, M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Cooke, J.D.; Hamilton-Taylor, J.; Tipping, E. On the acid-base properties of humic acid in soil. Environ. Sci. Technol. 2007, 41, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.D.; Perdue, E.M. Analytical constrain on acidic functional groups in humic substances. Organ. Geochem. 2008, 39, 783–799. [Google Scholar] [CrossRef]
- Crea, F.; de Stefano, C.; Gianguzza, A.; Pettignano, A.; Piazzese, D.; Sammartano, S. Acid-base properties of synthetic and natural polyelectrolytes: Experimental results and models for the dependence on different aqueous media. J. Chem. Eng. Data 2009, 54, 589–605. [Google Scholar] [CrossRef]
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.G.; Montoneri, C.; Mendichi, R.; Acosta, E.J.; Kiran, S. Behavior and properties in aqueous solution of biopolymers isolated from urban refuse. Biomacromolecules 2010, 11, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.G.; Musso, G.; Mendichi, R.; Chierotti, M.R.; Gobetto, R. Biosurfactants from urban green waste. ChemSuSChem 2009, 2, 239–247. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, C.; Mineo, P.; Rigano, C.; Sammartano, S. Ionic strength dependence of formation constants. XVII. The calculation of equilibrium concentrations and formation constants. Ann. Chim. (Rome) 1993, 83, 243–277. [Google Scholar]
- De Stefano, C.; Princi, P.; Rigano, C.; Sammartano, S. Computer analysis of equilibrium data in solution. ESAB2M: an improved version of the ESAB program. Ann. Chim. (Rome) 1987, 77, 643–675. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabasso, S.; Berto, S.; Rosato, R.; Marinos, J.A.T.; Ginepro, M.; Zelano, V.; Daniele, P.G.; Montoneri, E. Chemical Modeling of Acid-Base Properties of Soluble Biopolymers Derived from Municipal Waste Treatment Materials. Int. J. Mol. Sci. 2015, 16, 3405-3418. https://doi.org/10.3390/ijms16023405
Tabasso S, Berto S, Rosato R, Marinos JAT, Ginepro M, Zelano V, Daniele PG, Montoneri E. Chemical Modeling of Acid-Base Properties of Soluble Biopolymers Derived from Municipal Waste Treatment Materials. International Journal of Molecular Sciences. 2015; 16(2):3405-3418. https://doi.org/10.3390/ijms16023405
Chicago/Turabian StyleTabasso, Silvia, Silvia Berto, Roberta Rosato, Janeth Alicia Tafur Marinos, Marco Ginepro, Vincenzo Zelano, Pier Giuseppe Daniele, and Enzo Montoneri. 2015. "Chemical Modeling of Acid-Base Properties of Soluble Biopolymers Derived from Municipal Waste Treatment Materials" International Journal of Molecular Sciences 16, no. 2: 3405-3418. https://doi.org/10.3390/ijms16023405