Proteomic Investigations into Hemodialysis Therapy
Abstract
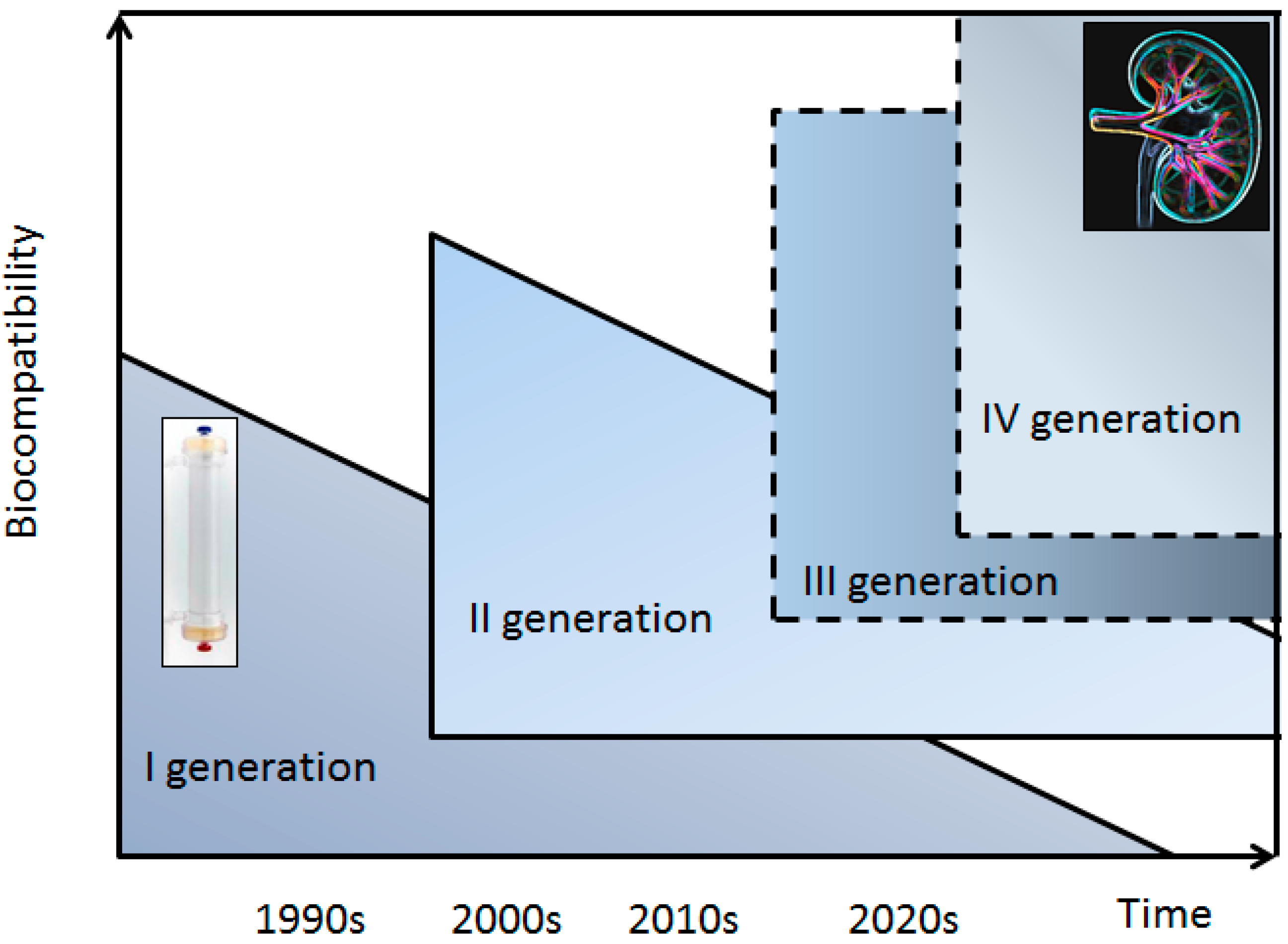
:1. Introduction
|
2. Dialytic Solute Removal
3. Proteomic and Uremic Toxicity
3.1. Characterization of Uremic Toxins
3.2. Characterization of Protein Damage Products
3.3. Other Investigations
4. Proteomic and Extracorporeal Blood Purification
4.1. Hemodialysis
4.2. Other Blood Purification Strategies
5. Proteomics and Protein Adsorption onto Dialysis Membrane
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Vanholder, R.; van Laecke, S.; Glorieux, G. The middle-molecule hypothesis 30 years after: Lost and rediscovered in the universe of uremic toxicity? J. Nephrol. 2008, 21, 140–160. [Google Scholar]
- Bonomini, V. Introducing the biology of bioincompatibility in dialysis. Nephrol. Dial. Transplant. 1991, 6 (Suppl. 2), S1–S3. [Google Scholar]
- Hakim, R.M. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993, 44, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Xiao, L.; Xu, B.; Xu, X.X.; Liu, F.Y.; Sun, L. Effects of vitamin E-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: A systematic review and meta-analysis. Ren. Fail. 2014, 36, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Sirolli, V.; Magni, F.; Urbani, A. Proteomics and nephrology. J. Nephrol. 2012, 25, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; de Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; de Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Krum, H. Cardiorenal syndrome: Role of protein-bound uremic toxins. J. Ren. Nutr. 2015, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Tetta, C. Extracorporal blood purification: More than diffusion and convection. Does this help? Curr. Opin. Crit. Care 2007, 13, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Baurmeister, U.; Vienken, J.; Ward, R.A. Should dialysis modalities be designed to remove specific uremic toxins? Semin. Dial. 2009, 22, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Dhaene, M.; Vanherweghem, J.L. Removal of β2-microglobulin by adsorption on dialysis membranes. Nephrol. Dial. Transplant. 1987, 2, 576–577. [Google Scholar] [PubMed]
- Bouman, C.S.; van Olden, R.W.; Stoutenbeek, C.P. Cytokine filtration and adsorption during pre- and postdilution hemofiltration in four different membranes. Blood Purif. 1998, 16, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Hohnholt, M.; Leypoldt, J.K.; de Spain, M. Hemodialysis membrane biocompatibility: The case of erythropoietin. Blood Purif. 1991, 9, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, K.W.; Reiger, J.; Anjorg, R.; Doht, B.; Scheler, F. Binding of antibiotics by dialysis membranes and its clinical relevance. Proc. Eur. Dial. Transplant. Assoc. 1977, 14, 607–609. [Google Scholar] [PubMed]
- Vanholder, R.; Ringoir, S. Adequacy of dialysis: A critical analysis. Kidney Int. 1992, 42, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J.A. Bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Boelaert, J.; Glorieux, G.; Eloot, S. New methods and technologies for measuring uremic toxins and quantifying dialysis adequacy. Semin. Dial. 2015, 28, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Glorieux, G.; Thornalley, P.; Schepers, E.; Meert, N.; Jankowski, J.; Jankowski, V.; Argiles, A.; Anderstam, B.; Brunet, P.; et al. Review on uremic toxins III: Recommendations for handling uraemic retention solutes in vitro-towards a standardized approach for research on uraemia. Nephrol. Dial. Transplant. 2007, 22, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Vanholder, R. The gut: The forgotten organ in uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Evenpoel, P.; Meijers, B.K.; Bammens, B.R.; Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.; Glorieux, G.; Poesen, R.; Bakker, S.J. Nonextracorporeal methods for decreasing uremic solute concentration: A future way to go? Semin. Nephrol. 2014, 34, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Westhof, T.; Vaziri, N.D.; Ingrosso, D.; Perna, A.F. Gases as uremic toxins: Is there something in the air? Semin. Nephrol. 2014, 34, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Seepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- De Loor, H.; Bammens, B.; Evenepoel, P.; de Preter, V.; Verbeke, K. Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin. Chem. 2005, 51, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Recht, N.S.; Hostetter, T.H.; Meyer, T.W. Removal of p-cresol sulfate by hemodialysis. J. Am. Soc. Nephrol. 2005, 16, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Bammens, B.; de Loor, H.; Glorieux, G.; Meijers, B.; Schepers, E.; Massy, Z.; Evenepoel, P. Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol. Dial. Transplant. 2011, 26, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, E.; Mischak, H.; Vanholder, R.C. Exploring the uremic toxins using proteomic technologies. Contrib. Nephrol. 2008, 160, 159–171. [Google Scholar] [PubMed]
- Langlois, R.G.; Trebes, J.E.; Dalmasso, E.A.; Ying, Y.; Davies, R.W.; Curzi, M.P.; Colston, B.W., Jr.; Turteltaub, K.W.; Perkins, J.; Chromy, B.A.; et al. Serum protein profile alterations in hemodialysis patients. Am. J. Nephrol. 2004, 24, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Brinkley, K.A. A proteomic analysis of proteins removed by ultrafiltration during extracorporeal renal replacement therapy. Contrib. Nephrol. 2004, 141, 280–291. [Google Scholar] [PubMed]
- Kaiser, T.; Hermann, A.; Kielstein, J.T.; Wittke, S.; Bartel, S.; Krebs, R.; Hausadel, F.; Hillmann, M.; Golovko, I.; Koester, P.; et al. Capillary electrophoresis coupled to mass spectrometry to establish polypeptide patterns in dialysis fluids. J. Chromatogr. A 2003, 1013, 157–171. [Google Scholar] [CrossRef]
- Weissinger, E.M.; Kaiser, T.; Meert, N.; de Smet, R.; Walden, M.; Mischak, H.; Vanholder, R.C. Proteomics: A novel tool to unravel the pathophysiology of uraemia. Nephrol. Dial. Transplant. 2004, 19, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Update of uremic toxin research by mass spectrometry. Mass Spectrom. Rev. 2011, 30, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Mullen, W.; Duranton, F.; Filip, S.; Gayrard, N.; Husi, H.; Schepers, E.; Neirynck, N.; Schanstra, J.P.; Jankowski, J.; et al. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol. Dial. Transplant. 2015, 30, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; van Ypersele de Strihou, C.; Kurokawa, K.; Baynes, J.W. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999, 55, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Galli, F. Protein damage and inflammation in uraemia and dialysis patients. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S5), v20–v36. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Oda, O.; Inagi, R.; Iida, Y.; Araki, N.; Yamada, N.; Horiuchi, S.; Taniguchi, N.; Maeda, K.; Kinoshita, T. β2-Microglobulin modified with advanced glycation end products is a major component of hemodialysis-associated amyloidosis. J. Clin. Investig. 1993, 92, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Pavone, B.; Bucci, S.; Sirolli, V.; Merlini, G.; del Boccio, P.; di Rienzo, M.; Felaco, P.; Amoroso, L.; Sacchetta, P.; Di Ilio, C.; et al. β2-Microglobulin causes abnormal phosphatidylserine exposure in human red blood cells. Mol. Biosyst. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; McMonagle, E. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int. 2001, 60, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Ueda, Y.; Yamada, Y.; Izuhara, Y.; Wada, T.; Jadoul, M.; Saito, A.; Kurokawa, K.; van Ypersele de Strihou, C. Carbonyl stress in uremia: Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product. J. Am. Soc. Nephrol. 1998, 9, 2349–2356. [Google Scholar] [PubMed]
- Pavone, B.; Sirolli, V.; Giardinelli, A.; Bucci, S.; Forlì, F.; di Cesare, M.; Sacchetta, P.; di Pietro, N.; Pandolfi, A.; Urbani, A.; et al. Plasma protein carbonylation in chronic uremia. J. Nephrol. 2011, 24, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Zitta, S.; Greilberger, J.; Holzer, H.; Reibnegger, G.; Hermetter, A.; Oettl, K. Effect of hemodialysis on the antioxidative properties of serum. Biochim. Biophys. Acta 2003, 1638, 267–272. [Google Scholar] [CrossRef]
- Ward, R.A.; Ouseph, R.; McLeish, K.R. Effects of high-flux hemodialysis on oxidant stress. Kidney Int. 2003, 63, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Pavone, B.; Sirolli, V.; Bucci, S.; Libardi, F.; Felaco, P.; Amoroso, L.; Sacchetta, P.; Urbani, A.; Bonomini, M. Adsorption and carbonylation of plasma proteins by dialyser membrane material: in vitro and in vivo proteomics investigations. Blood Transfus. 2010, 8 (Suppl. S3), s113–s119. [Google Scholar] [PubMed]
- Miyata, T.; Saito, A.; Kurokawa, K.; van Ypersele de Strihou, C. Advanced glycation and lipoxidation end products: Reactive carbonyl compounds-related uraemic toxicity. Nephrol. Dial. Transplant. 2001, 16 (Suppl. S4), 8–11. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. AGEs and carbonyl stress: Potential pathogenetic factors of long-term uraemic complications. Nephrol. Dial. Transplant. 2000, 15, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Michelis, R.; Gery, R.; Sela, S.; Shurtz-Swirski, R.; Grinberg, N.; Snitkovski, T.; Shasha, S.M.; Kristal, B. Carbonyl stress induced by intravenous iron during hemodialysis. Nephrol. Dial. Transplant. 2003, 18, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, K.S.; Choi, S.Y.; Know, H.Y.; Won, M.H. Oxidative modification of human ceruloplasmin by peroxyl radicals. Biochim. Biophys. Acta 2001, 1568, 30–36. [Google Scholar] [CrossRef]
- Miller, Y.I.; Altamentova, S.M.; Shaklai, N. Oxidation of low-density lipoprotein by hemoglobin stem from a heme initiated globin radical: Antioxidant role of haptoglobin. Biochemistry 1997, 36, 12189–12198. [Google Scholar] [CrossRef] [PubMed]
- Rottoli, P.; Magri, B.; Cianti, R.; Bargagli, E.; Vagaggini, C.; Nikiforakis, N.; Pallini, V.; Bini, L. Carbonylated proteins in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics 2005, 5, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.; Cheng, Y.M.; Yang, S.M. Impairments of the biological properties of serum albumin in patients on hemodialysis. Nephrology 2007, 12, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Borderie, D.; Nguyen-Khoa, T.; Drüeke, T.B.; Ekindjian, O.G.; Lacour, B. Increased plasma S-nitrosothiol levels in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Weissinger, E.M.; Nguyen-Khoa, T.; Fumeron, C.; Saltiel, C.; Walden, M.; Kaiser, T.; Mischak, H.; Drüeke, T.B.; Lacour, B.; Massy, Z.A. Effects of oral vitamin C supplementation in hemodialysis patients: A proteomic assessment. Proteomics 2006, 6, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Badiou, S.; Cristol, J.P.; Jaussent, I.; Terrier, N.; Morena, M.; Maurice, F.; Leray-Moragues, H.; Rivory, J.P.; Chalabi, L.; Delcourt, C.; et al. Fine-tuning of the prediction of mortality in hemodialysis patients by use of cytokine proteomic determination. Clin. J. Am. Soc. Nephrol. 2008, 3, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mangé, A.; Goux, A.; Badiou, S.; Patrier, L.; Canaud, B.; Maudelonde, T.; Cristol, J.P.; Solassol, J. HDL proteome in hemodialysis patients: A quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS ONE 2012, 7, e34107. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Kopecky, C.; Kubicek, M.; Haidinger, M.; Döller, D.; Katholnig, K.; Suarna, C.; Eller, P.; Tölle, M.; Gerner, C.; et al. Serum amyloid A in uremic HDL promotes inflammation. J. Am. Soc. Nephrol. 2012, 23, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; de Boer, I.; Tang, C.; Mayer, P.S.; Zelnick, L.; Afkarian, M.; Heineke, J.W.; Himmelfarb, J. A cluster of proteins implicated in kidney disease is increased in high-density lipoprotein isolated from hemodialysis subjects. J. Proteome Res. 2015, 14, 2792–2806. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, C.; Haidinger, M.; Birner-Grünberger, R.; Darnhofer, B.; Kaltenecker, C.C.; Marsche, G.; Holzer, M.; Weichhart, T.; Antlanger, M.; Kovarik, J.J.; et al. Restoration of renal function does not correct impairment of uremic HDL properties. J. Am. Soc. Nephrol. 2015, 26, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Chikazawa, Y.; Sato, K.; Nakagawa, M.; Imamura, H.; Hayama, S.; Yamaya, H.; Asaka, M.; Tomosugi, N.; Yokoyama, H.; et al. Proteomic analysis of serum, outflow dialysate and adsorbed protein onto dialysis membranes (polysulfone and PMMA) during hemodialysis treatment using SELDI-TOF-MS. Am. J. Nephrol. 2006, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Dihazi, H.; Muller, C.A.; Mattes, H.; Muller, G.A. Proteomic analysis to improve adequacy of hemo- and peritoneal dialysis: Removal of small and highmolecular weight proteins with high- and low-flux filters or a peritoneal membrane. Proteomics Clin. Appl. 2008, 2, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Ficheux, A.; Gayrard, N.; Szware, I.; Andress, D.; Soullier, S.; Duny, Y.; Goubert, G.; Thomas, M.; Bismuth-Mondolfo, J.; Daurès, J.P.; et al. The use of SDS-PAGE scanning of spent dialysate to assess uraemic toxin removal by dialysis. Nephrol. Dial. Transplant. 2011, 26, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Sirolli, V.; Lupisella, S.; Levi-Mortera, S.; Pavone, B.; Pieroni, L.; Amoroso, L.; di Vito, R.; Bucci, S.; Bernardini, S.; et al. Proteomic investigations on the effect of different membrane materials on blood protein adsorption during hemodialysis. Blood Transfus. 2012, 10 (Suppl. S2), s101–s112. [Google Scholar] [PubMed]
- Pedrini, L.A.; Krisp, C.; Gmerek, A.; Wolters, D.A. Patterns of proteins removed with high-flux membranes on high-volume hemodiafiltration detected with a multidimensional LC-MS/MS strategy. Blood Purif. 2014, 20, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Monari, E.; Cuoghi, A.; Bellei, E.; Bergamini, S.; Caiazzo, M.; Aucella, F.; Loschiavo, C.; Corazza, L.; Palladino, G.; Sereni, L.; et al. Proteomic analysis of protein extraction during hemofiltration with on-line endogenous reinfusion (HFR) using different polysulphone membranes. J. Mater. Sci. 2014, 25, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, A.; Caiazzo, M.; Monari, E.; Bellei, E.; Bergamini, S.; Sereni, L.; Aucella, F.; Loschiavo, C.; Atti, M.; Tomasi, A. New horizon in dialysis depuration: Characterization of a polysulfone membrane able to break the “albumin wall”. J. Biomater. Appl. 2015, 29, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, T.; Eloot, S.; Vanholder, R.; Glorieux, G.; van der Sande, F.M.; Scheijen, J.L.; Leunissen, K.M.; Kooman, J.P.; Schalkwijk, C.G. Protein-bound uraemic toxins, dicarbonyl stress and advanced glycation end products in conventional and extended haemodialysis and haemodiafiltration. Nephrol. Dial. Transplant. 2015, 30, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Chanard, J.; Lavaud, S.; Randoux, C.; Rieu, P. New insights in dialysis membrane biocompatibility: Relevance of adsorption properties and heparin binding. Nephrol. Dial. Transplant. 2003, 18, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Tolkoff-Rubin, N.; Schifferli, J.A. Is adsorption an important characteristic of dialysis membranes? Kidney Int. 1996, 49, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Jacobasch, H. Surface characterization of polymers for medical devices. Int. J. Artif. Organs 1999, 22, 160–176. [Google Scholar] [PubMed]
- Lonnemann, G.; Koch, K.M.; Shaldon, S.; Dinarello, C.A. Studies on the ability of hemodialysis membranes to induce, bind, and clear human interleukin-1. J. Lab. Clin. Med. 1988, 112, 76–86. [Google Scholar] [PubMed]
- Anderson, J.M.; Bonfield, T.L.; Ziats, N.P. Protein adsorption and cellular adhesion and activation on biomedical polymers. Int. J. Artif. Organs 1990, 13, 375–382. [Google Scholar] [PubMed]
- Johnson, R.J. Complement activation during extracorporeal therapy: Biochemistry, cell biology and clinical relevance. Nephrol. Dial. Transplant. 1994, 9 (Suppl. S2), 36–45. [Google Scholar] [PubMed]
- Franck, R.D.; Weber, J.; Dresbach, H.; Thelen, H.; Weiss, C.; Floege, J. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int. 2001, 60, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Parker, C.; Wilcox, L.; Janatova, J. Activation of complement by hemodialysis membranes: Polyacrylonitrile binds more C3a than cuprophan. Kidney Int. 1990, 37, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Schifferli, J. Adsorption of complement factor D by polyacrylonitrile dialysis membranes. Kidney Int. 1993, 43, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Valette, P.; Thomas, M.; Dejardin, P. Adsorption of low molecular weight proteins to hemodialysis membranes: Experimental results and simulations. Biomaterials 1999, 20, 1621–1634. [Google Scholar] [CrossRef]
- Vienken, J. Polymers in nephrology. Characteristics and needs. Int. J. Artif. Organs 2002, 25, 470–479. [Google Scholar] [PubMed]
- Bonomini, M.; Pavone, B.; Sirolli, V.; del Buono, F.; di Cesare, M.; del Boccio, P.; Amoroso, L.; di Ilio, C.; Sacchetta, P.; Federici, G.; et al. Proteomics characterization of protein adsorption onto hemodialysis membranes. J. Proteom. Res. 2006, 5, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Lupisella, S.; Sirolli, V.; Bucci, S.; Amoroso, L.; Pavone, B.; Pieroni, L.; Sacchetta, P.; Bonomini, M. Proteomic analysis of protein adsorption capacity of different hemodialysis membranes. Mol. Biosyst. 2012, 8, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, L.; Levi Mortera, S.; Greco, V.; Sirolli, V.; Ronci, M.; Felaco, P.; Fucci, G.; De Fulviis, S.; Massoud, R.; Condò, S.; et al. Biocompatibility of hemodialysis membrane materials by proteomic investigations. Mol. Biosyst. 2015, 11, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Aoike, I. Clinical significance of protein adsorbable membranes-long-term clinical effects and analysis using proteomic technique. Nephrol. Dial. Transplant. 2007, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Takita, T.; Furuhashi, M.; Takahashi, T.; Watanabe, T.; Maruyama, Y.; Hishida, A. Polymethylmethacrylate efficacy in reduction of renal itching in hemodialysis patients: Crossover study and role of tumor necrosis factor-α. Artif. Organs 2001, 25, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Mares, J.; Thongbookerd, V.; Tumaz, Z.; Moravec, J.; Matejovic, M. Specific adsorption of some complement activation proteins to polysulfone dialysis membranes during hemodialysis. Kidney Int. 2009, 76, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Schafer, R.M.; Falkenhagen, D.; Joshua, M.S.; Heidland, A.; Klinkmann, H.; Gurland, H.J. Biocompatibility of a new high-permeability modified cellulose membrane for haemodialysis. Nephrol. Dial. Transplant. 1993, 82, 47–53. [Google Scholar]
- Pesic, I.; Muller, G.A.; Baumann, C.; Dihazi, G.H.; Koziolek, M.J.; Eltoweissy, M.; Bramlage, C.; Asif, A.R.; Dihazi, H. Cellulose membranes are more effective in holding back vital proteins and exhibit less interaction with plasma proteins during hemodialysis. Biochim. Biophys. Acta 2013, 1834, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Thongboonkerd, V. Proteomics in extracorporeal blood purification and peritoneal dialysis. J. Proteomics 2010, 73, 521–526. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomini, M.; Sirolli, V.; Pieroni, L.; Felaco, P.; Amoroso, L.; Urbani, A. Proteomic Investigations into Hemodialysis Therapy. Int. J. Mol. Sci. 2015, 16, 29508-29521. https://doi.org/10.3390/ijms161226189
Bonomini M, Sirolli V, Pieroni L, Felaco P, Amoroso L, Urbani A. Proteomic Investigations into Hemodialysis Therapy. International Journal of Molecular Sciences. 2015; 16(12):29508-29521. https://doi.org/10.3390/ijms161226189
Chicago/Turabian StyleBonomini, Mario, Vittorio Sirolli, Luisa Pieroni, Paolo Felaco, Luigi Amoroso, and Andrea Urbani. 2015. "Proteomic Investigations into Hemodialysis Therapy" International Journal of Molecular Sciences 16, no. 12: 29508-29521. https://doi.org/10.3390/ijms161226189