Spatiotemporal Expression of p63 in Mouse Epidermal Commitment
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Spatiotemporal Expression of Markers Related to Epidermal Commitment
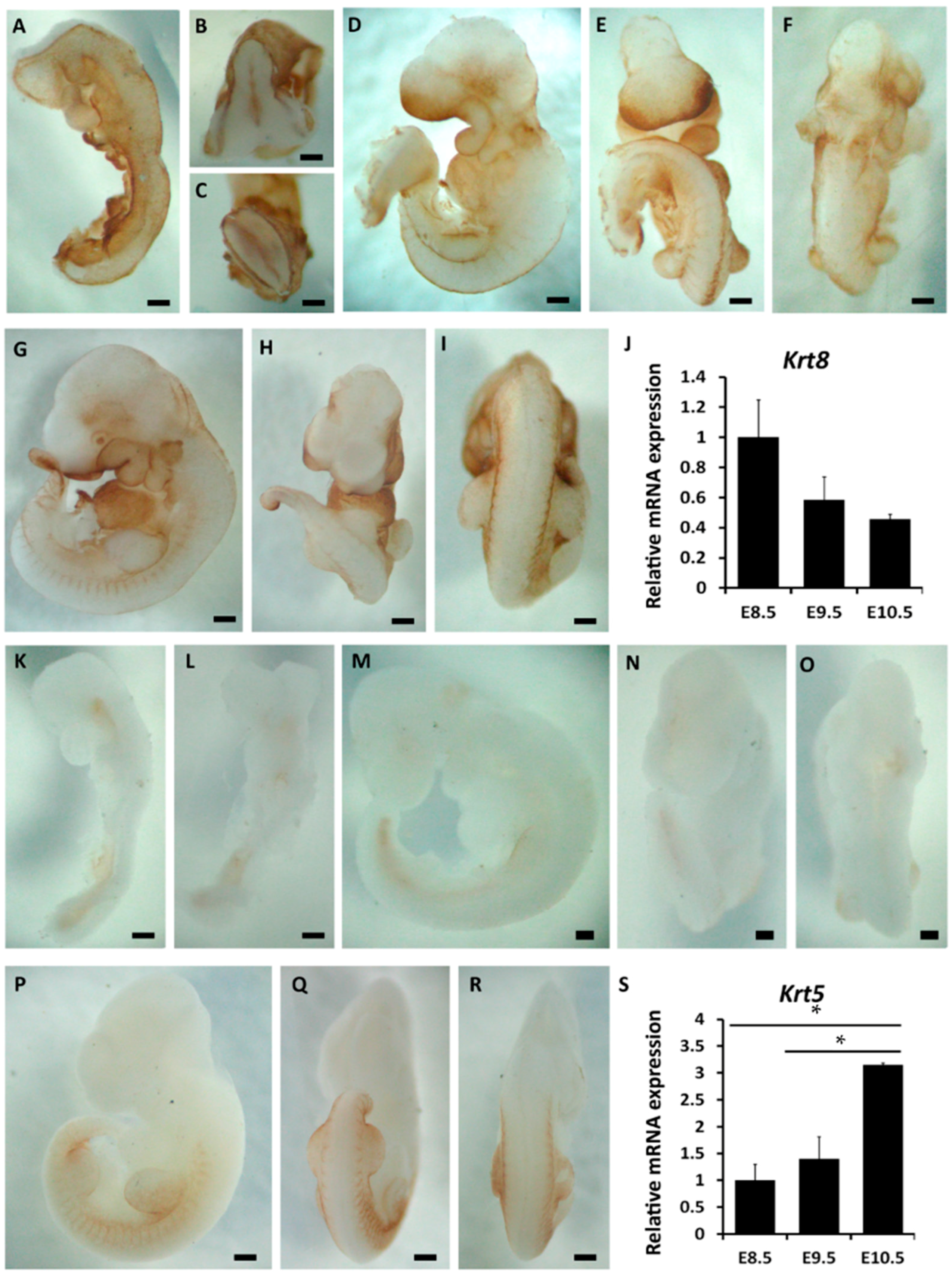
2.2. The Spatiotemporal Expression of p63 in Mouse Embryos from E8.5 to E10.5
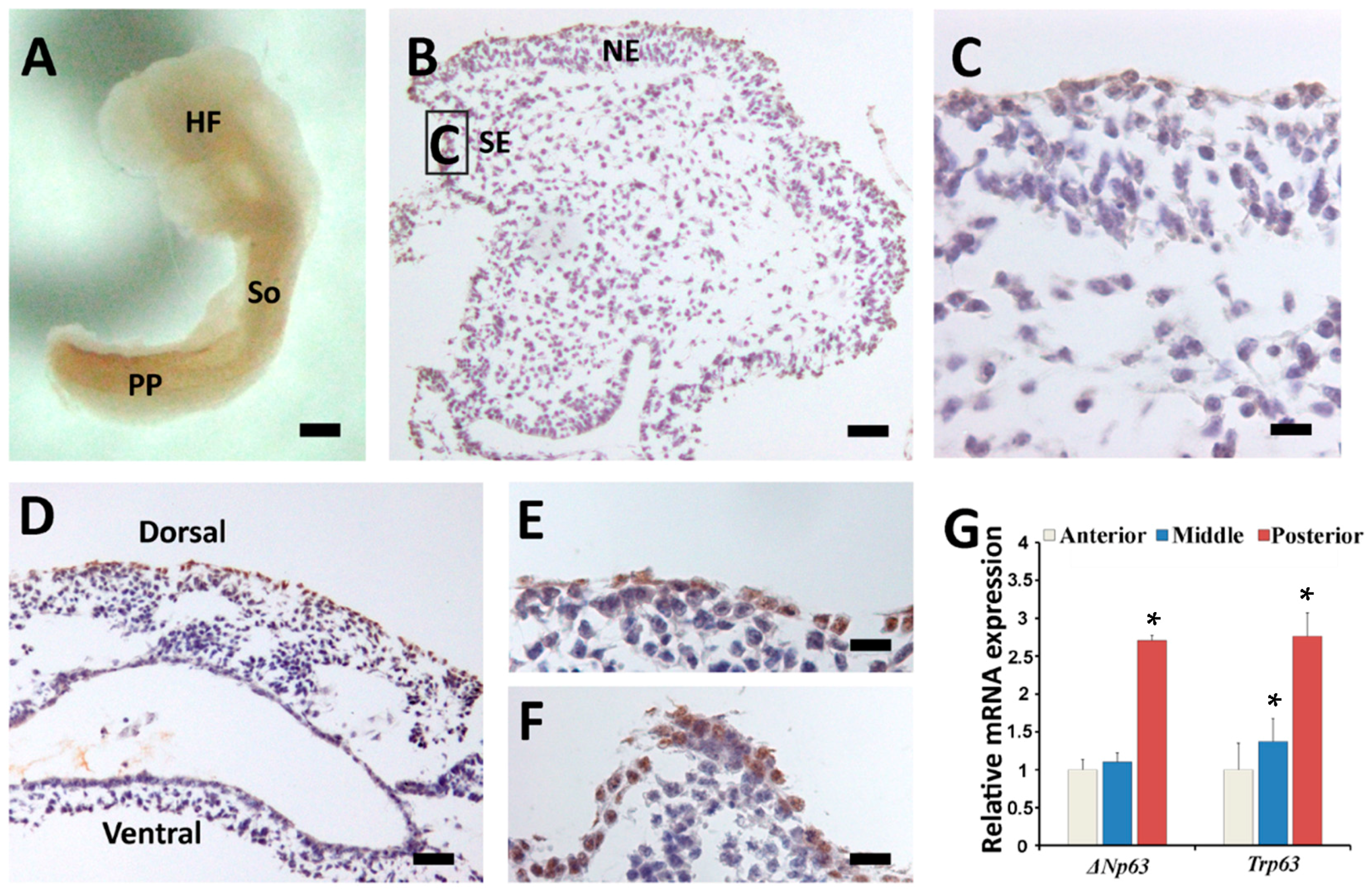

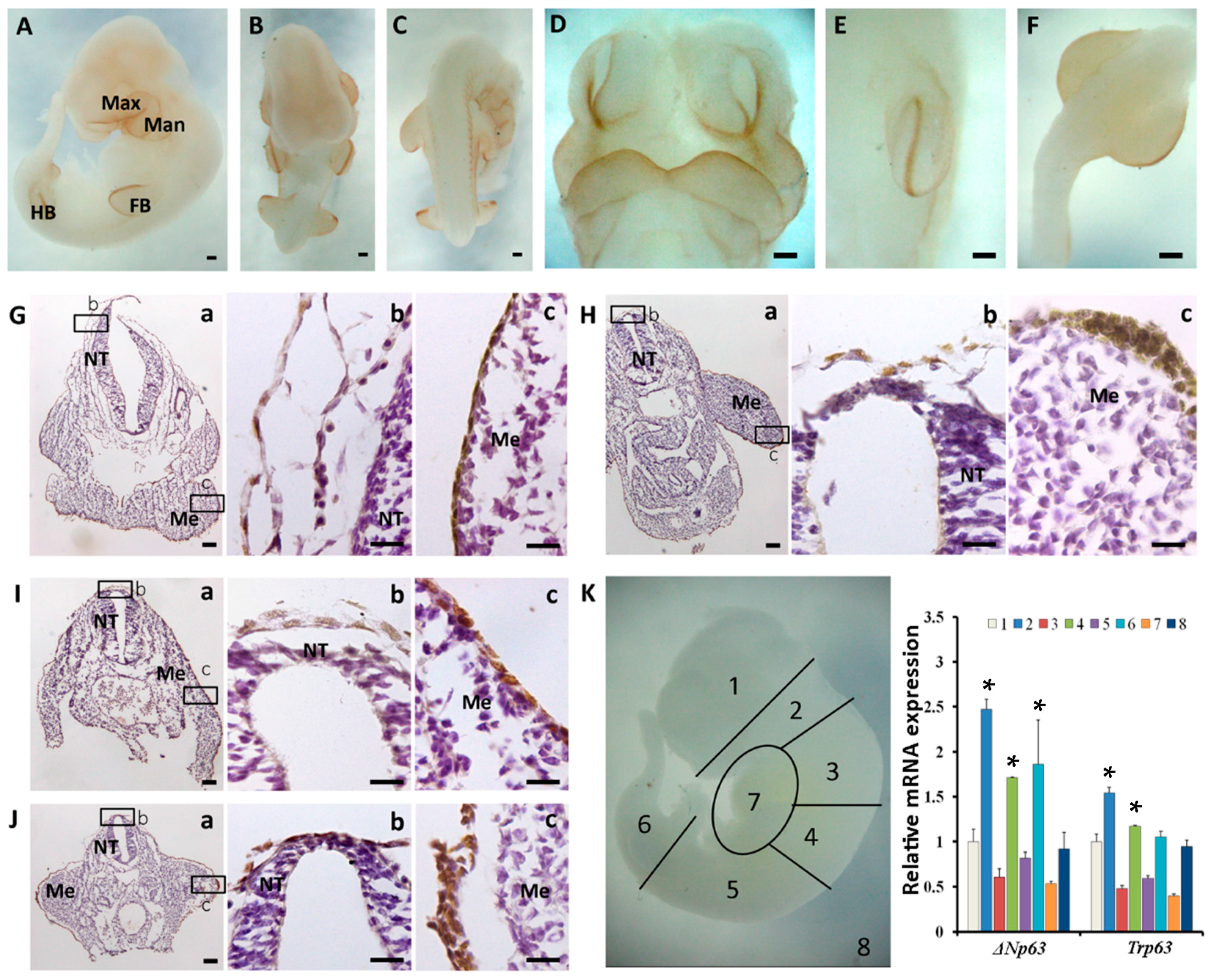
2.3. Sequential Expression of K8, p63 and K14 during Mouse Epidermal Commitment

3. Discussion
4. Experimental Section
4.1. Animal and Embryo Preparation
4.2. Whole-Mount Immunohistochemistry of Mouse Embryos
4.3. Reverse-Transcription and Polymerase Chain Reaction (qRT-PCR and RT-PCR)
4.4. Immunofluorescence Double Staining and DAB Immunohistochemistry
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Keymeulen, A.; Blanpain, C. Tracing epithelial stem cells during development, homeostasis, and repair. J. Cell Biol. 2012, 197, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.I.; Roop, D.R. The role of p63 in development and differentiation of the epidermis: Tanioku kihei memorial lecture. J. Dermatol. Sci. 2004, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- M’Boneko, V.; Merker, H.-J. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation). Cells Tissues Organs 1988, 133, 325–336. [Google Scholar]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Duan, E. Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. Int. J. Mol. Sci. 2013, 14, 10869–10895. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Tainsky, M.; Fuchs, E. Programming gene expression in developing epidermis. Development 1994, 120, 2369–2383. [Google Scholar] [PubMed]
- Fuchs, E.; Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef]
- Mehrel, T.; Hohl, D.; Rothnagel, J.A.; Longley, M.A.; Bundman, D.; Cheng, C.; Lichti, U.; Bisher, M.E.; Steven, A.C.; Steinert, P.M.; et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 1990, 61, 1103–1112. [Google Scholar] [CrossRef]
- Steven, A.C.; Steinert, P.M. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 1994, 107, 693–700. [Google Scholar] [PubMed]
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 2007, 129, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Schweitzer, R.; Sun, D.Q.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. P63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–718. [Google Scholar] [PubMed]
- Koster, M.I.; Kim, S.; Roop, D.R. P63 deficiency: A failure of lineage commitment or stem cell maintenance? J. Investig. Dermatol. 2005, 10, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Tadeu, A.M.; Horsley, V. Notch signaling represses p63 expression in the developing surface ectoderm. Development 2013, 140, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, E.N.; Oti, M.; Niehues, H.; van Heeringen, S.J.; Schalkwijk, J.; Stunnenberg, H.G.; van Bokhoven, H.; Zhou, H. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015, 16, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.I.; Kim, S.; Mills, A.A.; DeMayo, F.J.; Roop, D.R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004, 18, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006, 20, 3185–3197. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.K.; Carroll, J.S.; Leong, C.O.; Cheng, F.; Brown, M.; Mills, A.A.; Brugge, J.S.; Ellisen, L.W. P63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 2006, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ihrie, R.A.; Marques, M.R.; Nguyen, B.T.; Horner, J.S.; Papazoglu, C.; Bronson, R.T.; Mills, A.A.; Attardi, L.D. Perp is a p63-regulated gene essential for epithelial integrity. Cell 2005, 120, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Vanbokhoven, H.; Melino, G.; Candi, E.; Declercq, W. P63, a story of mice and men. J. Investig. Dermatol. 2011, 131, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.A.; Zheng, B.H.; Wang, X.J.; Vogel, H.; Roop, D.R.; Bradley, A. P63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999, 398, 708–713. [Google Scholar] [PubMed]
- Koster, M.I.; Kim, S.; Huang, J.; Williams, T.; Roop, D.R. TAp63α induces AP-2γ as an early event in epidermal morphogenesis. Dev. Biol. 2006, 289, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.A.; Birkaya, B.; Sinha, S. A functional enhancer of keratin14 is a direct transcriptional target of ΔNp63. J. Investig. Dermatol. 2007, 127, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Rufini, A.; Terrinoni, A.; Dinsdale, D.; Ranalli, M.; Paradisi, A.; de Laurenzi, V.; Spagnoli, L.G.; Catani, M.V.; Ramadan, S.; et al. Differential roles of p63 isoforms in epidermal development: Selective genetic complementation in p63 null mice. Cell Death Differ. 2006, 13, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. p63: Revving up epithelial stem-cell potential. Nat. Cell Biol. 2007, 9, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Laurikkala, J.; Mikkola, M.L.; James, M.; Tummers, M.; Mills, A.A.; Thesleff, I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development 2006, 133, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Medawar, A.; Virolle, T.; Rostagno, P.; de la Forest-Divonne, S.; Gambaro, K.; Rouleau, M.; Aberdam, D. ΔNp63 is essential for epidermal commitment of embryonic stem cells. PLoS ONE 2008, 3, e3441. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Yingling, J.M. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr. Opin. Genet. Dev. 1998, 8, 481–486. [Google Scholar] [CrossRef]
- Pispa, J.; Thesleff, I. Mechanisms of ectodermal organogenesis. Dev. Biol. 2003, 262, 195–205. [Google Scholar] [CrossRef]
- Olivera-Martinez, I.; Thelu, J.; Teillet, M.A.; Dhouailly, D. Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt-1. Mech. Dev. 2001, 100, 233–244. [Google Scholar] [CrossRef]
- Thesleff, I.; Sharpe, P. Signalling networks regulating dental development. Mech. Dev. 1997, 67, 111–123. [Google Scholar] [CrossRef]
- Sinha, S.; Degenstein, L.; Copenhaver, C.; Fuchs, E. Defining the regulatory factors required for epidermal gene expression. Mol. Cell. Biol. 2000, 20, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.-A.; Ortt, K.; Birkaya, B.; Smalley, K.; Sinha, S. An active role of the ΔN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS ONE 2009, 4, e5623. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.H.; Chao, C.F.; Lu, M.H.; Lin, H.C.; Chen, J.Y. A half-site of the p53-binding site on the keratin 14 promoter is specifically activated by p63. J. Biochem. 2012, 152, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Rollin, J.; Masse, I.; Lamartine, J.; Gidrol, X. P63 regulates human keratinocyte proliferation via -MYC-regulated gene network and differentiation commitment through cell adhesion-related gene network. J. Biol. Chem. 2012, 287, 5627–5638. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.S.; Al Khamis, A.; Ferguson, C.A.; Bach, I.; Rosenfeld, M.G.; Sharpe, P.T. Conserved regulation of mesenchymal gene expression by FGF-8 in face and limb development. Development 1999, 126, 221–228. [Google Scholar] [PubMed]
- Joyner, A.; Wall, N. Immunohistochemistry of whole-mount mouse embryos. Cold Spring Harb. Protoc. 2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.Y.; Zhao, Q.; Liu, S.; Zhang, H.S.; Shi, J.C.; Li, N.; Lei, X.H.; Zhao, H.S.; Deng, Z.L.; et al. Senescence of human skin-derived precursors regulated by Akt-FOXO3-p27(KIP1)/p15(INK4b) signaling. Cell. Mol. Life Sci. 2015, 72, 2949–2960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Zhao, H.; Qiao, J.; Liu, S.; Deng, Z.; Lei, X.; Ning, L.; Cao, Y.; Zhao, Y.; et al. Ovine hair follicle stem cells derived from single vibrissae reconstitute haired skin. Int. J. Mol. Sci. 2015, 16, 17779–17797. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.S.; Qiao, J.Q.; Zhang, S.B.; Zhang, H.S.; Lei, X.H.; Wang, X.Y.; Deng, Z.L.; Ning, L.N.; Cao, Y.J.; Guo, Y.; et al. GPR39 marks specific cells within the sebaceous gland and contributes to skin wound healing. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Rinne, T.; Brunner, H.G.; van Bokhoven, H. p63-associated disorders. Cell Cycle 2007, 6, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Liu, S.; Zhang, H.; Li, N.; Wang, X.; Cao, Y.; Ning, L.; Duan, E.; Xia, G. Spatiotemporal Expression of p63 in Mouse Epidermal Commitment. Int. J. Mol. Sci. 2015, 16, 29542-29553. https://doi.org/10.3390/ijms161226185
Zhao Q, Liu S, Zhang H, Li N, Wang X, Cao Y, Ning L, Duan E, Xia G. Spatiotemporal Expression of p63 in Mouse Epidermal Commitment. International Journal of Molecular Sciences. 2015; 16(12):29542-29553. https://doi.org/10.3390/ijms161226185
Chicago/Turabian StyleZhao, Qian, Shuang Liu, Huishan Zhang, Na Li, Xinyue Wang, Yujing Cao, Lina Ning, Enkui Duan, and Guoliang Xia. 2015. "Spatiotemporal Expression of p63 in Mouse Epidermal Commitment" International Journal of Molecular Sciences 16, no. 12: 29542-29553. https://doi.org/10.3390/ijms161226185




