Genome-Wide Identification and Analysis of the VQ Motif-Containing Protein Family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis)
Abstract
:1. Introduction
2. Results
2.1. Identification and Sequence Analysis of VQ Genes in Chinese Cabbage
| Gene Name | Gene Locus | Chr. No. | Strand Direction | Location | CDS | Protein | ||
|---|---|---|---|---|---|---|---|---|
| Length (aa) | Mol.Wt. (KDa) | pI | ||||||
| BrVQ1-1 | Bra025998 | A06 | − | 6,588,703–6,588,999 | 297 | 98 | 10.81 | 4.75 |
| BrVQ1-2 | Bra016616 | A08 | + | 19,301,503–19,301,796 | 294 | 97 | 10.92 | 5.13 |
| BrVQ3-1 | Bra025892 | A06 | − | 8,759,573–8,760,265 | 693 | 230 | 25.07 | 8.67 |
| BrVQ3-2 | Bra012276 | A07 | + | 8,889,633–8,890,172 | 540 | 179 | 19.29 | 5.1 |
| BrVQ4 | Bra030082 | A07 | + | 6,712,561–6,713,295 | 735 | 244 | 26.74 | 9.66 |
| BrVQ5 | Bra035492 | A08 | − | 7,791,236–7,791,901 | 666 | 221 | 25.40 | 6.58 |
| BrVQ8 | Bra033934 | A02 | − | 108,00,121–10,800,534 | 414 | 137 | 15.36 | 10.19 |
| BrVQ9-1 | Bra035028 | A07 | − | 21,850,607–21,851,491 | 885 | 294 | 31.58 | 10.12 |
| BrVQ9-2 | Bra008356 | A02 | − | 14,998,946–14,999,815 | 870 | 289 | 31.19 | 10.39 |
| BrVQ10-1 | Bra008359 | A02 | + | 15,035,463–15,035,777 | 315 | 104 | 11.61 | 5.83 |
| BrVQ10-2 | Bra003642 | A07 | − | 14,203,152–14,203,469 | 318 | 105 | 11.69 | 5.01 |
| BrVQ10-3 | Bra035035 | A07 | + | 21,882,989–21,883,270 | 282 | 93 | 10.44 | 4.67 |
| BrVQ11-1 | Bra035182 | A07 | + | 22,479,008–22,479,511 | 504 | 167 | 18.96 | 7.96 |
| BrVQ11-2 | Bra003566 | A07 | − | 13,824,994–13,825,515 | 522 | 173 | 19.66 | 9.69 |
| BrVQ11-3 | Bra008473 | A02 | + | 15,858,716–15,859,210 | 495 | 164 | 18.79 | 8.74 |
| BrVQ12 | Bra039937 | A09 | + | 31,714,821–31,715,237 | 417 | 138 | 16.09 | 9.66 |
| BrVQ14-1 | Bra023004 | A03 | + | 8,183,495–8,184,808 | 1314 | 437 | 48.10 | 8.58 |
| BrVQ14-2 | Bra017329 | A04 | + | 15,346,657–15,347,685 | 1029 | 342 | 37.24 | 10.53 |
| BrVQ14-3 | Bra005358 | A05 | − | 5,012,814–5,014,478 | 1185 | 394 | 43.13 | 10.09 |
| BrVQ15 | Bra016956 | A04 | − | 17,404,492–17,405,181 | 690 | 229 | 24.84 | 7.87 |
| BrVQ16-1 | Bra000216 | A03 | + | 9,940,673–9,941,098 | 426 | 141 | 15.57 | 8.91 |
| BrVQ16-2 | Bra004604 | A05 | + | 1,001,763–1,002,185 | 423 | 140 | 15.29 | 4.89 |
| BrVQ18-1 | Bra004825 | A05 | + | 1,984,033–1,984,575 | 543 | 180 | 20.11 | 9.24 |
| BrVQ18-2 | Bra037658 | A04 | + | 18,293,805–18,294,347 | 543 | 180 | 19.98 | 9.33 |
| BrVQ19-1 | Bra027262 | A05 | + | 20,006,387–20,007,064 | 678 | 225 | 24.21 | 9.44 |
| BrVQ19-2 | Bra021096 | A01 | + | 23,997,911–23,998,561 | 651 | 216 | 23.49 | 9.33 |
| BrVQ20 | Bra037588 | A01 | + | 22,145,216–22,146,079 | 864 | 287 | 30.28 | 6.54 |
| BrVQ21-1 | Bra037569 | A01 | + | 22,005,523–22,006,176 | 654 | 217 | 22.73 | 6.05 |
| BrVQ21-2 | Bra022345 | A05 | + | 182,87,618–18,288,268 | 651 | 216 | 23.06 | 6.29 |
| BrVQ21-3 | Bra001716 | A03 | − | 18,018,055–18,018,699 | 645 | 214 | 22.95 | 6.22 |
| BrVQ22-1 | Bra023849 | A01 | + | 2,0411,389–20,411,979 | 591 | 196 | 20.61 | 9.69 |
| BrVQ22-2 | Bra041035 | Scaffold000402 | − | 11,806–12,390 | 585 | 194 | 20.40 | 9.89 |
| BrVQ23-1 | Bra007265 | A09 | − | 28,278,350–28,278,799 | 450 | 149 | 16.52 | 4.88 |
| BrVQ23-2 | Bra014675 | A04 | + | 2,158,610–2,159,095 | 486 | 161 | 18.10 | 5.1 |
| BrVQ23-3 | Bra014674 | A04 | + | 2,155,783–2,156,247 | 465 | 154 | 17.35 | 5.17 |
| BrVQ24-1 | Bra007279 | A09 | + | 28,339,520–28,340,206 | 687 | 228 | 23.79 | 6.59 |
| BrVQ24-2 | Bra014665 | A04 | − | 2,098,440–2,099,141 | 702 | 233 | 24.57 | 8.05 |
| BrVQ25-1 | Bra007373 | A09 | + | 28,879,454–28,879,999 | 546 | 181 | 20.12 | 6.19 |
| BrVQ25-2 | Bra014594 | A04 | − | 1,651,117–1,651,650 | 534 | 177 | 19.56 | 6.7 |
| BrVQ26-1 | Bra007505 | A09 | + | 29,533,410–29,533,874 | 465 | 154 | 17.58 | 7.02 |
| BrVQ26-2 | Bra014514 | A04 | − | 1,142,901–1,143,341 | 441 | 146 | 16.68 | 8 |
| BrVQ26-3 | Bra003400 | A07 | + | 13,026,327–13,029,056 | 846 | 281 | 32.87 | 7.16 |
| BrVQ27 | Bra039565 | A01 | − | 11,929,165–11,929,719 | 555 | 184 | 19.83 | 9.76 |
| BrVQ28 | Bra013438 | A01 | + | 5,666,359–5,666,979 | 621 | 206 | 23.15 | 5.37 |
| BrVQ29-1 | Bra010608 | A08 | + | 15,506,771–15,507,115 | 345 | 114 | 12.72 | 9.05 |
| BrVQ29-2 | Bra017849 | A03 | + | 30,916,676–30,917,020 | 345 | 114 | 12.84 | 9.05 |
| BrVQ30-1 | Bra010666 | A08 | + | 15,875,832–15,876,719 | 888 | 295 | 32.18 | 6.18 |
| BrVQ30-2 | Bra011838 | A01 | − | 282,249–283,121 | 873 | 290 | 31.74 | 7.94 |
| BrVQ31 | Bra005995 | A03 | + | 1,534,982–153,5506 | 525 | 174 | 19.11 | 9.37 |
| BrVQ32-1 | Bra022063 | A02 | + | 18,984,720–18,985,412 | 693 | 230 | 25.41 | 10.26 |
| BrVQ32-2 | Bra024996 | A06 | − | 24,596,158–24,596,844 | 687 | 228 | 25.12 | 9.99 |
| BrVQ33-1 | Bra003032 | A10 | + | 5,893,364–5,894,047 | 684 | 227 | 25.28 | 9.99 |
| BrVQ33-2 | Bra022675 | A02 | − | 8,093,063–8,093,788 | 726 | 241 | 26.93 | 9.75 |
| BrVQ34-1 | Bra024362 | A06 | + | 15,221,647–15,222,738 | 1092 | 363 | 39.11 | 5.76 |
| BrVQ34-2 | Bra037806 | A09 | + | 3,657,352–3,658,311 | 960 | 319 | 34.26 | 5.94 |
| BrVQ34-3 | Bra031876 | A02 | + | 27,356,488–27,357,465 | 978 | 325 | 35.03 | 6.65 |
| BrVQ35 | Bra006328 | A03 | − | 3,034,196–3,038,533 | 1707 | 568 | 62.99 | 8.89 |
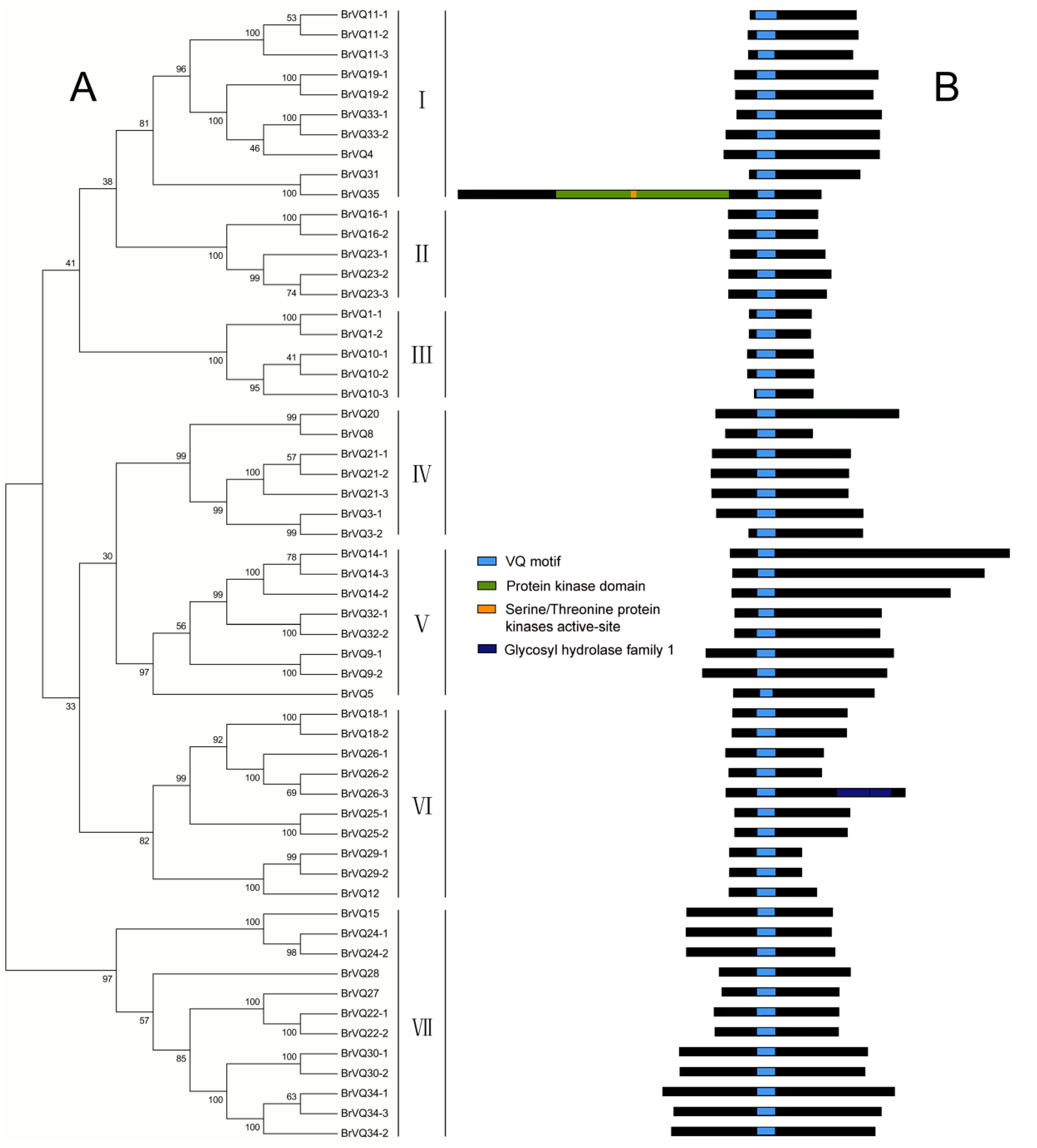
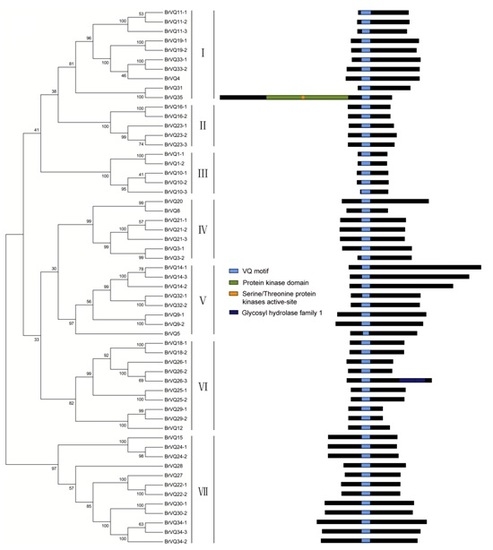
2.2. Phylogenetic Tree, Gene Structure, and Conserved Domains Analysis in Chinese Cabbage


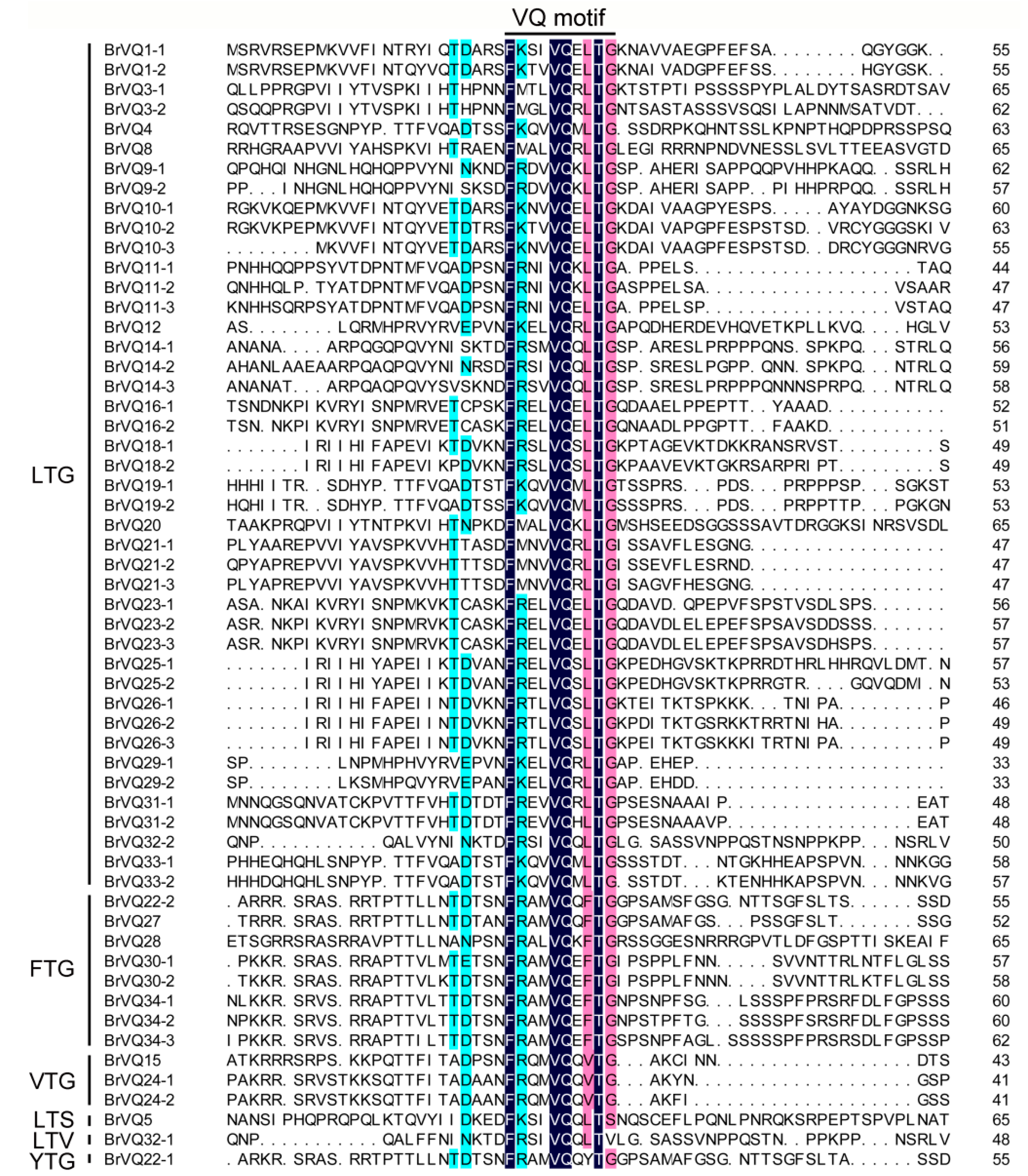
2.3. Multiple Sequence Alignment and Motif Analysis
2.4. Chromosome Mapping and Syntenic Analysis of VQ Genes in B. rapa

| tPCK Chr a | Block | Arabidopsis Gene | Chinese Cabbage Gene | ||
|---|---|---|---|---|---|
| LF b | MF1 c | MF2 c | |||
| tPCK1 | A | AtVQ1(AT1G17147) | BrVQ1-1 | BrVQ1-2 | – |
| – | – | – | BrVQ3-1 | – | – |
| – | – | – | – | – | BrVQ3-2 |
| tPCK1 | B | AtVQ4(AT1G28280) | – | – | BrVQ4 |
| tPCK1 | B | AtVQ5(AT1G32585) | – | BrVQ5 | – |
| tPCK6 | E | AtVQ8(AT1G68450) | – | BrVQ8 | – |
| tPCK6 | E | AtVQ9(AT1G78310) | BrVQ9-1 | BrVQ9-2 | – |
| tPCK6 | E | AtVQ10(AT1G78410) | BrVQ10-3 | BrVQ10-1 | BrVQ10-2 |
| tPCK6 | E | AtVQ11(AT1G80450) | BrVQ11-1 | BrVQ11-3 | BrVQ11-2 |
| tPCK3 | I | AtVQ12(AT2G22880) | BrVQ12 | – | – |
| tPCK3 | J | AtVQ14(AT2G35230) | BrVQ14-3 | BrVQ14-2 | BrVQ14-1 |
| tPCK3 | J | AtVQ15(AT2G41010) | – | BrVQ15 | – |
| tPCK3 | J | AtVQ16(AT2G41180) | BrVQ16-2 | – | BrVQ16-1 |
| tPCK3 | J | AtVQ18(AT2G44340) | BrVQ18-1 | BrVQ18-2 | – |
| tPCK2 | F | AtVQ19(AT3G15300) | BrVQ19-1 | BrVQ19-2 | – |
| tPCK2 | F | AtVQ20(AT3G18360) | – | BrVQ20 | – |
| tPCK2 | F | AtVQ21(AT3G18690) | BrVQ21-2 | BrVQ21-1 | BrVQ21-3 |
| tPCK2 | F | AtVQ22(AT3G22160) | – | BrVQ22-1 | – |
| tPCK6 | N | AtVQ23(AT3G56710) | BrVQ23-1 | BrVQ23-2/BrVQ23-3 | – |
| tPCK6 | N | AtVQ24(AT3G56880) | BrVQ24-1 | BrVQ24-2 | – |
| tPCK6 | N | AtVQ25(AT3G58000) | BrVQ25-1 | BrVQ25-2 | – |
| tPCK6 | N | AtVQ26(AT3G60090) | BrVQ26-1 | BrVQ26-2 | BrVQ26-3 |
| tPCK4 | T | AtVQ27(AT4G15120) | BrVQ27 | – | – |
| tPCK4 | U | AtVQ28(AT4G20000) | BrVQ28 | – | – |
| tPCK4 | U | AtVQ29(AT4G37710) | – | BrVQ29-2 | BrVQ29-1 |
| tPCK4 | U | AtVQ30(AT4G39720) | BrVQ30-2 | – | BrVQ30-1 |
| tPCK5 | R | AtVQ31(AT5G08480) | – | BrVQ31 | – |
| tPCK7 | V | AtVQ32(AT5G46780) | BrVQ32-2 | BrVQ32-1 | – |
| tPCK5 | Wb | AtVQ33(AT5G53830) | BrVQ33-1 | – | BrVQ33-2 |
| tPCK7 | X | AtVQ34(AT5G65170) | BrVQ34-1 | BrVQ34-3 | BrVQ34-2 |
| – | – | – | – | BrVQ35 | – |
2.5. Phylogenetic Tree of the VQ Domains in Arabidopsis, Rice and Chinese Cabbage

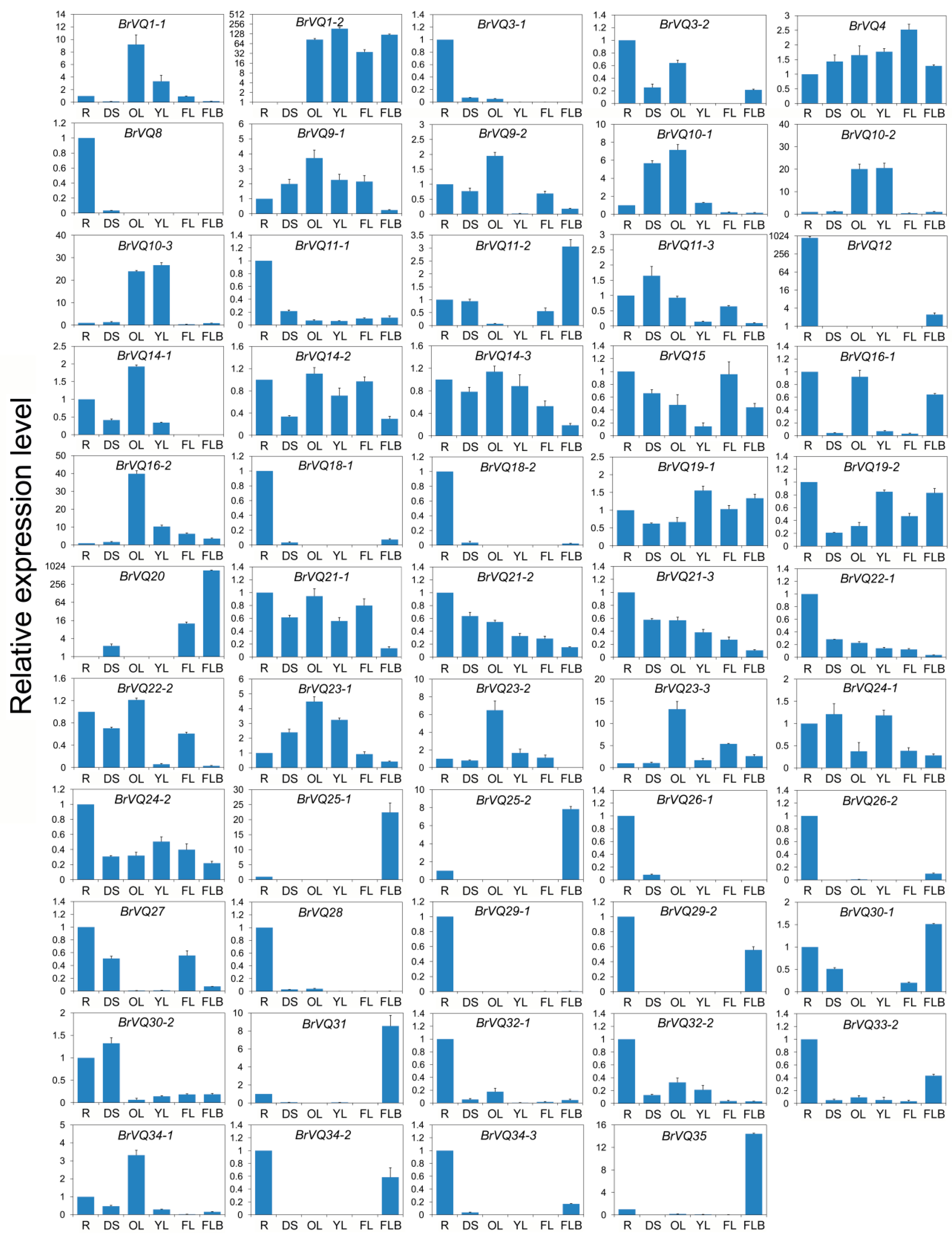
2.6. Expression Pattern of the BrVQ Genes in Different Tissues
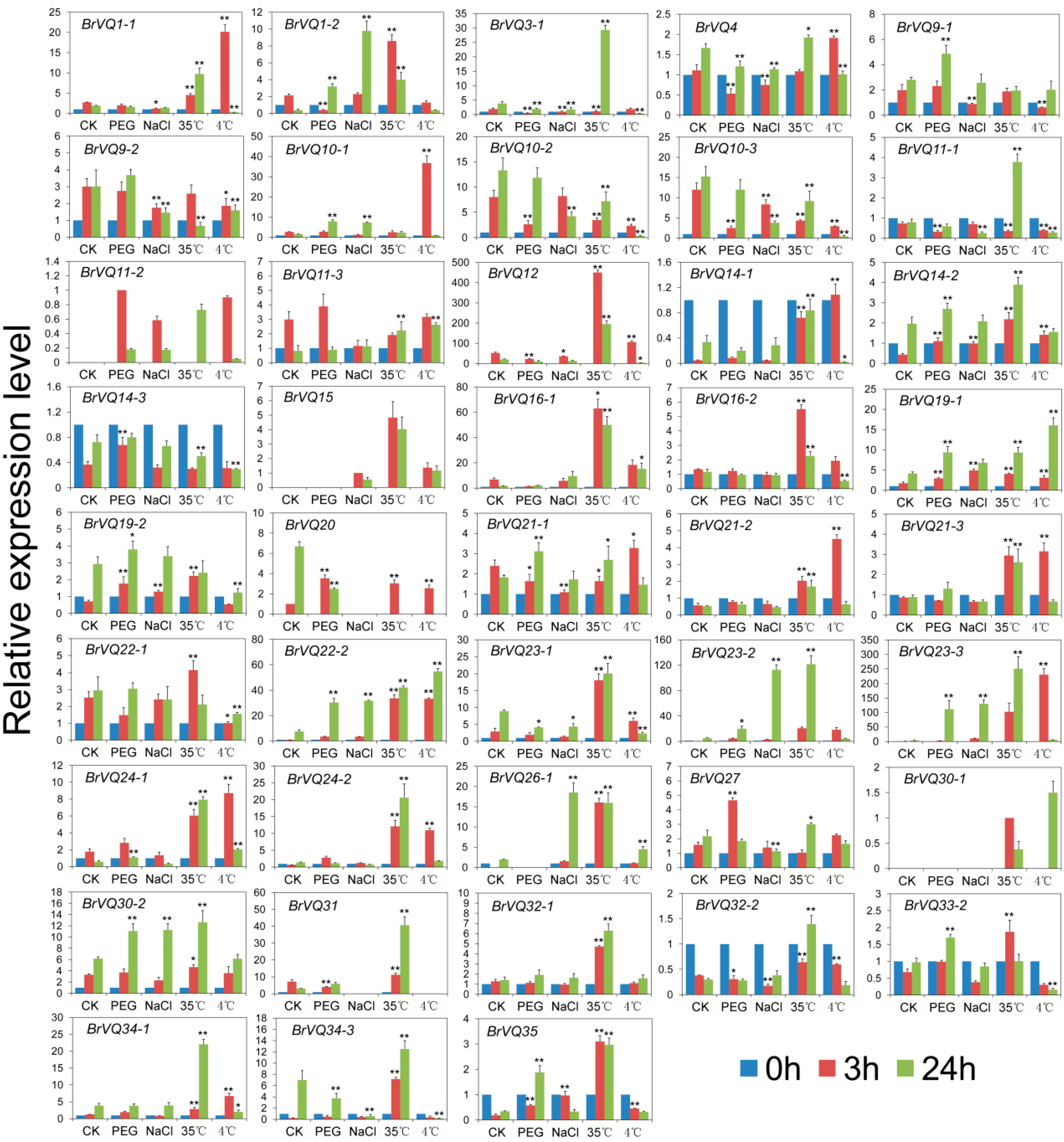
2.7. Expression Analysis of the BrVQ Genes under Abiotic Stresses
2.8. Expression Analysis of the BrVQ Genes under Phytohormone Treatment

2.9. Comparison of the Expression Patterns of BrVQ Genes and Their Orthologs in Arabidopsis
3. Discussion
3.1. VQ Gene Duplication in Chinese Cabbage
3.2. Function of the VQ Proteins in Plant Growth and Development
3.3. Function of the VQ Proteins in Abiotic and Biotic Resistance
4. Experimental Section
4.1. Plant Materials, Growth Conditions, and Stress Treatments
4.2. Sequence Retrieval
4.3. Identification and Analysis of the VQ Genes and Proteins in Chinese Cabbage
4.4. RNA Isolation and qRT-PCR
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Wray, G.A.; Hahn, M.W.; Abouheif, E.; Balhoff, J.P.; Pizer, M.; Rockman, M.V.; Romano, L.A. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 2003, 20, 1377–1419. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhou, Y.; Yang, Y.; Chi, Y.J.; Zhou, J.; Chen, J.Y.; Wang, F.; Fan, B.; Shi, K.; Zhou, Y.H.; et al. Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol. 2012, 159, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kwon, S.I.; Choi, C.; Lee, H.; Ahn, I.; Park, S.R.; Bae, S.C.; Lee, S.C.; Hwang, D.J. Expression analysis of rice VQ genes in response to biotic and abiotic stresses. Gene 2013, 529, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Sun, G.; Jin, Y.; Qiu, L. Identification of active VQ motif-containing genes and the expression patterns under low nitrogen treatment in soybean. Gene 2014, 543, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Vannozzi, A.; Wang, G.; Zhong, Y.; Corso, M.; Cavallini, E.; Cheng, Z.M. A comprehensive survey of the grapevine VQ gene family and its transcriptional correlation with WRKY proteins. Front. Plant Sci. 2015, 6, 417. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Garcia, D.; Zhang, H.; Feng, K.; Chaudhury, A.; Berger, F.; Peacock, W.J.; Dennis, E.S.; Luo, M. The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. 2010, 63, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jing, Y.; Li, J.; Xu, G.; Lin, R. Arabidopsis VQ-motif-containing-protein 29 represses seedling de-etiolation by interacting with PIF1. Plant Physiol. 2014, 164, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, L.; Wang, H.; Zhang, L.; Wang, F.; Yu, D. Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013, 74, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Perruc, E.; Charpenteau, M.; Ramirez, B.C.; Jauneau, A.; Galaud, J.P.; Ranjeva, R.; Ranty, B. A novel calmodulin-binding protein functions as a negative regulator of osmotic stress tolerance in Arabidopsis thaliana seedlings. Plant J. 2004, 38, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, E.; Jenkins, T.; Brodersen, P.; Thorgrimsen, S.; Petersen, N.H.; Zhu, S.; Qiu, J.L.; Micheelsen, P.; Rocher, A.; Petersen, M.; et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005, 24, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.L.; Fiil, B.K.; Petersen, K.; Nielsen, H.B.; Botanga, C.J.; Thorgrimsen, S.; Palma, K.; Suarez-Rodriquez, M.C.; Sandbech-Clausen, S.; Lichota, J.; et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008, 27, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.D.; Li, W.; Guo, D.; Dong, J.; Zhang, Q.; Fu, Y.; Ren, D.; Peng, M.; Xia, Y. The Arabidopsis gene SIGMA FACTOR-BINDING PROTEIN1 plays a role in the salicylate- and jasmonate-mediated defence responses. Plant Cell Environ. 2010, 33, 828–839. [Google Scholar] [PubMed]
- Lai, Z.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.; Yu, J.Q.; Chen, Z. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef] [PubMed]
- Pecher, P.; Eschen-Lippold, L.; Herklotz, S.; Kuhle, K.; Naumann, K.; Bethke, G.; Uhrig, J.; Weyhe, M.; Scheel, D.; Lee, J. The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of “VQ-motif”-containing proteins to regulate immune responses. New Phytol. 2014, 203, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Weyhe, M.; Eschen-Lipplod, L.; Pecher, P.; Scheel, D.; Lee, J. Ménage à trois: The complex relationships between mitogen-activated protein kinases, WRKY transcription factors, and VQ-motif-containing proteins. Plant Signal. Behav. 2014, 9, e29519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Liu, S.; Wu, J.; Fang, L.; Sun, S.; Liu, B.; Li, P.; Hua, W.; Wang, X. BRAD, The genetics and genomics database for Brassica plants. BMC Plant Biol. 2011, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Arora, P.K.; Mohanta, N.; Parida, P.; Bae, H. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genom. 2015, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Jaiswal, P.; Hebbard, C.; Avraham, S.; Buckler, E.S.; Casstevens, T.; Hurwitz, B.; McCouch, S.; Ni, J.; Pujar, A.; et al. Gramene: A growing plant comparative genomics resource. Nucleic Acids Res. 2008, 36, D947–D953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Fang, L.; Wang, X. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front. Plant Sci. 2012, 3, 198. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Hong, J.K.; Kim, J.A.; Jeong, M.J.; Lee, Y.H.; Lee, S.L. Genome-wide analysis of the expansin gene superfamily reveals Brassica rapa-specific evolutionary dynamics upon whole genome triplication. Mol. Genet. Genom. 2015, 290, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Calderon-Villalobos, L.I.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Hirsch-Hoffmann, M.; Hennig, L.; Gruissem, W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004, 136, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Kong, L.; Lv, Y.; Zou, M.; Lu, G.; Cao, J.; Yu, X. Genome-wide identification, phylogeny, duplication and expression analyses of two-component system genes in Chinese cabbage (Brassica rapa ssp. pekinensis). DNA Res. 2014, 21, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.S.; Baute, G.J.; Liu, S.L. Duplications and turnover in plant genomes. Plant Genome Divers. 2012, 1, 155–169. [Google Scholar]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Saha, G.; Park, J.I.; Jung, H.J.; Ahmed, N.U.; Kayum, M.A.; Chung, M.Y.; Hur, Y.; Cho, Y.G.; Watanabe, M.; Nou, I.S. Genome-wide identification and characterization of MADS-box family genes related to organ development and stress resistance in Brassica rapa. BMC Genom. 2015, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, JH.; Bancroft, I.; Cheng, F.; et al. The Brassica rapa Genome Sequencing Project Consortium: The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Mandáková, T.; Wu, J.; Xie, Q.; Lysak, M.A.; Wang, X. Deciphering the diploid ancestral genome of the Mesohexaploid Brassica rapa. Plant Cell 2013, 25, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Guo, W.; Lu, J.; Yu, H.; Qu, C.; Tang, Z.; Li, J.; Chai, Y.; Liang, Y. Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS ONE 2015, 10, e0132051. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, F.; Hou, X.L.; Wang, Z.; Huang, Z.N. Genome-wide fractionation and identification of WRKY transcription factors in Chinese cabbage (Brassica rapa ssp. pekinensis) reveals collinearity and their expression patterns under abiotic and biotic stresses. Plant Mol. Biol. Rep. 2014, 32, 781–795. [Google Scholar]
- Mun, J.H.; Kwon, S.J.; Yang, T.J.; Seol, Y.J.; Jin, M.; Kim, J.A.; Lim, M.H.; Kim, J.S.; Baek, S.; Choi, B.S.; et al. Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol. 2009, 10, R111. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, J.M.; Cui, L.; Wall, P.K.; Zhang, Q.; Zhang, X.; Leebens-Mack, J.; Ma, H.; Altman, N.; dePamphilis, C.W. Expression pattern shifts following duplication indicative of subfunctionalization and neofunctionalization in regulatory genes of Arabidopsis. Mol. Biol. Evol. 2006, 23, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Liu, D.; Guo, J.; Leseberg, C.H.; Zhang, X.; Mao, L. Functional divergence of two duplicated D-lineage MADS-box genes BdMADS2 and BdMADS4 from Brachypodium distachyon. J. Plant Physiol. 2013, 170, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Li, W.; Zou, C.; Yuan, Y. Analyses of the NAC transcription factor gene family in Gossypium raimondii UIbr.: Chromosomal location, structure, phylogeny, and expression patterns. J. Integr. Plant Biol. 2013, 55, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Galimba, K.D.; diStilio, V.S. Sub-functionalization to ovule development following duplication of a floral organ identity gene. Dev. Biol. 2015, 405, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Lin, R. VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol. 2015, 169, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Dennis, E.S.; Berger, F.; Peacock, W.J.; Chaudhury, A. MINISEED3(MINI3), A WRKY family gene, and HAIKU2(IKU2), A leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 17531–17536. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N. Abscisic Acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Lee, H.; Ishitani, M.; Zhu, J.K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 2002, 277, 8588–8596. [Google Scholar] [CrossRef] [PubMed]
- Fragnière, C.; Serrano, M.; Abou-Mansour, E.; Métraux, J.P.; L’Haridon, F. Salicylic acid and its location in response to biotic and abiotic stress. FEBS Lett. 2011, 585, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Interpro. Available online: http://www.ebi.ac.uk/interpro/ (accessed on 11 November 2015).
- SMART. Available online: http://smart.embl.de/ (accessed on 11 November 2015).
- TAIR. Available online: http://www.Arabidopsis.org/ (accessed on 11 November 2015).
- RGAP. Available online: http://rice.plantbiology.msu.edu (accessed on 11 November 2015).
- BLAST. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 11 November 2015).
- GSDS. Available online: http://gsds.cbi.pku.edu.cn/ (accessed on 11 November 2015).
- ProtParam. Available online: http://web.expasy.org/protparam/ (accessed on 11 November 2015).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Gribskov, M.; Fana, F.; Harper, J.; Hope, D.A.; Harmon, A.C.; Smith, D.W.; Tax, F.E.; Zhang, G. PlantsP: A functional genomics database for plant phosphorylation. Nucleic Acids Res. 2001, 29, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, F.; Li, J.; Ding, Q.; Zhang, Y.; Li, H.; Zhang, J.; Gao, J. Genome-Wide Identification and Analysis of the VQ Motif-Containing Protein Family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Int. J. Mol. Sci. 2015, 16, 28683-28704. https://doi.org/10.3390/ijms161226127
Zhang G, Wang F, Li J, Ding Q, Zhang Y, Li H, Zhang J, Gao J. Genome-Wide Identification and Analysis of the VQ Motif-Containing Protein Family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). International Journal of Molecular Sciences. 2015; 16(12):28683-28704. https://doi.org/10.3390/ijms161226127
Chicago/Turabian StyleZhang, Gaoyuan, Fengde Wang, Jingjuan Li, Qian Ding, Yihui Zhang, Huayin Li, Jiannong Zhang, and Jianwei Gao. 2015. "Genome-Wide Identification and Analysis of the VQ Motif-Containing Protein Family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis)" International Journal of Molecular Sciences 16, no. 12: 28683-28704. https://doi.org/10.3390/ijms161226127
APA StyleZhang, G., Wang, F., Li, J., Ding, Q., Zhang, Y., Li, H., Zhang, J., & Gao, J. (2015). Genome-Wide Identification and Analysis of the VQ Motif-Containing Protein Family in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). International Journal of Molecular Sciences, 16(12), 28683-28704. https://doi.org/10.3390/ijms161226127