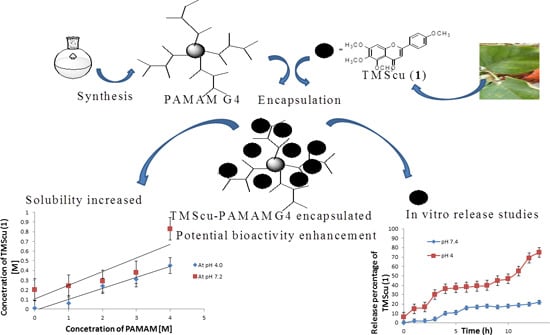
Synthesis of Polyamidoamine Dendrimer for Encapsulating Tetramethylscutellarein for Potential Bioactivity Enhancement
Abstract
:1. Introduction
2. Results and Discussion
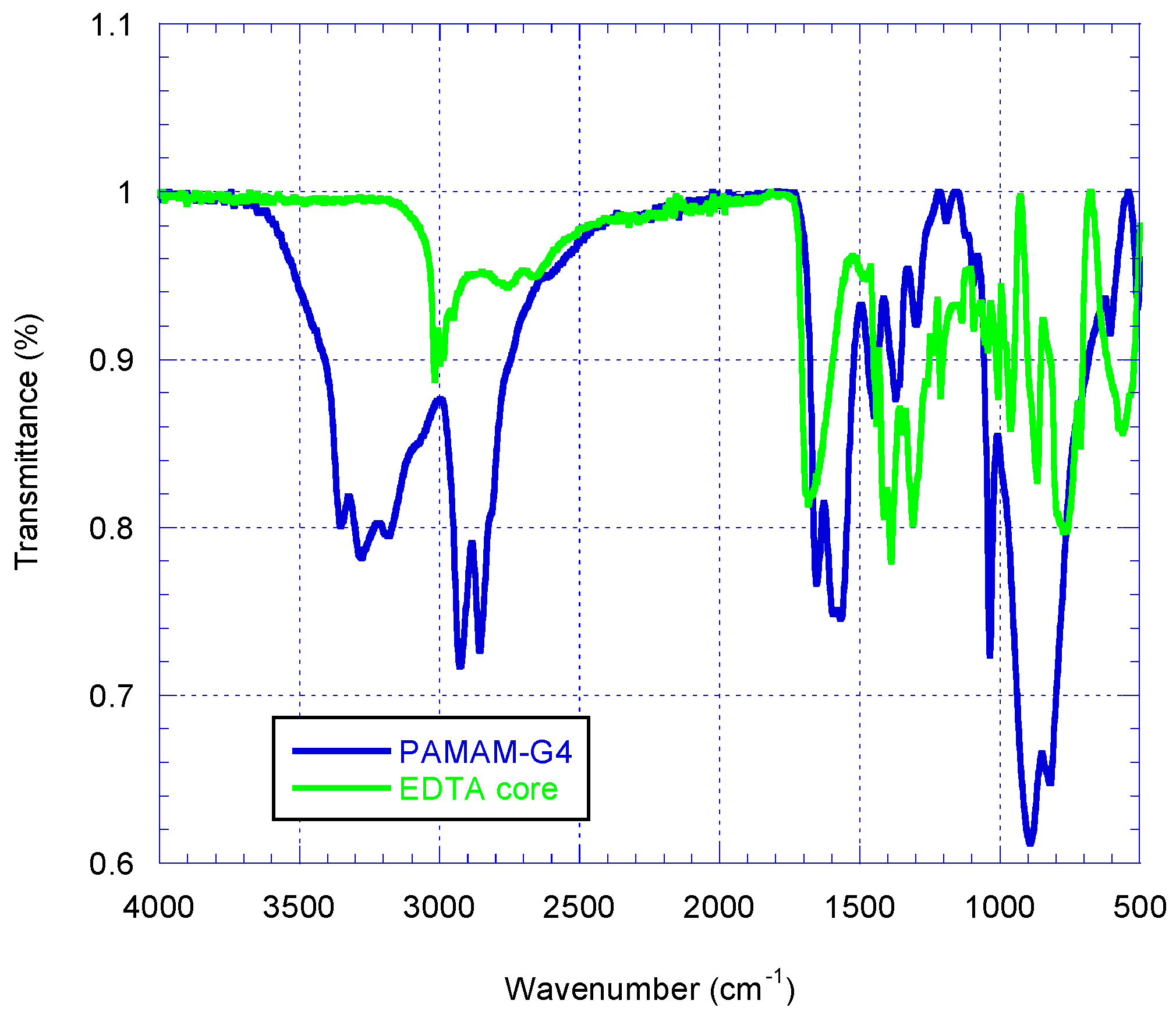
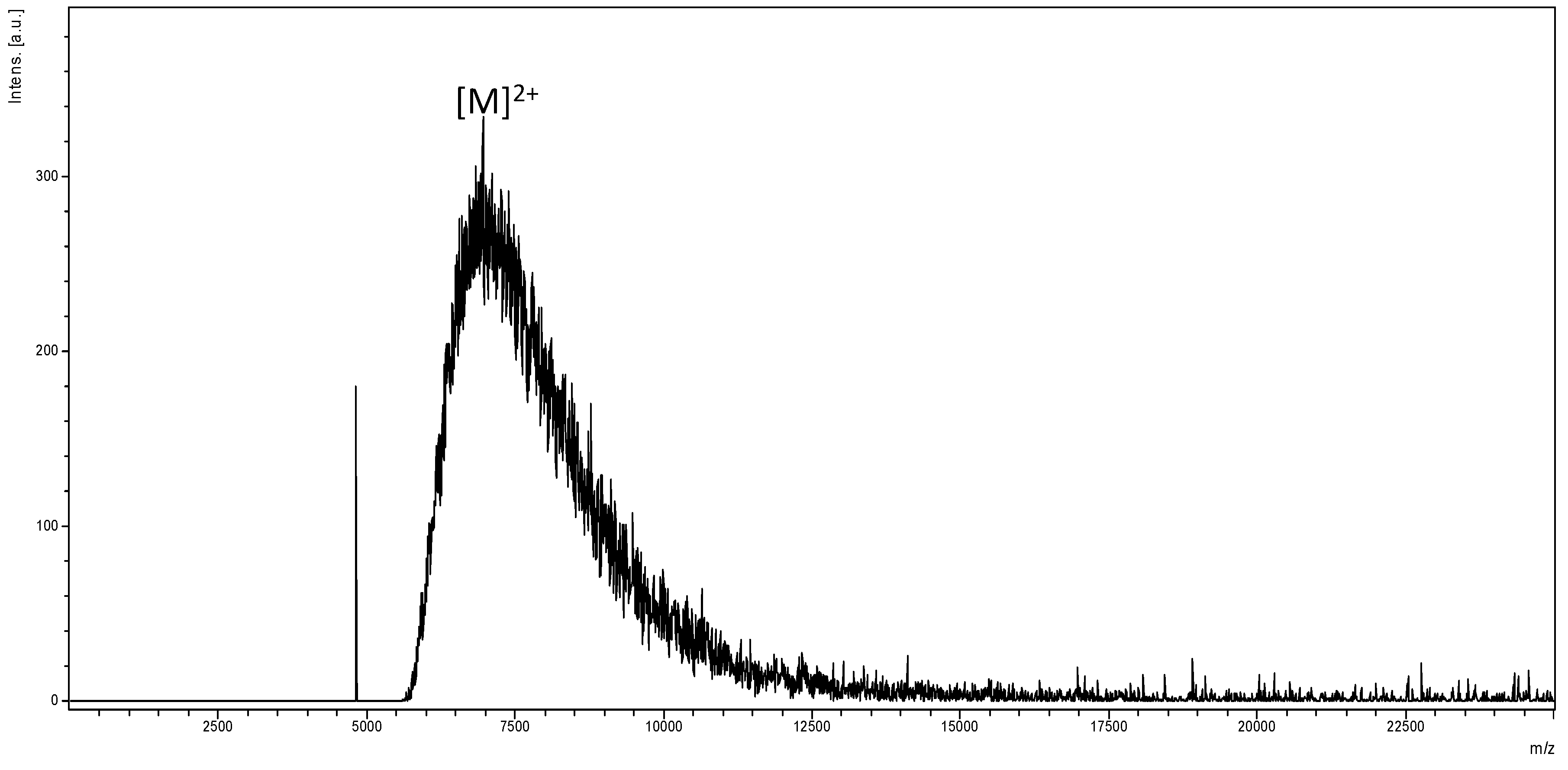
2.1. Synthesis and Characterization of Polyamidoamine (PAMAM) Generation Four (G4) Dendrimer
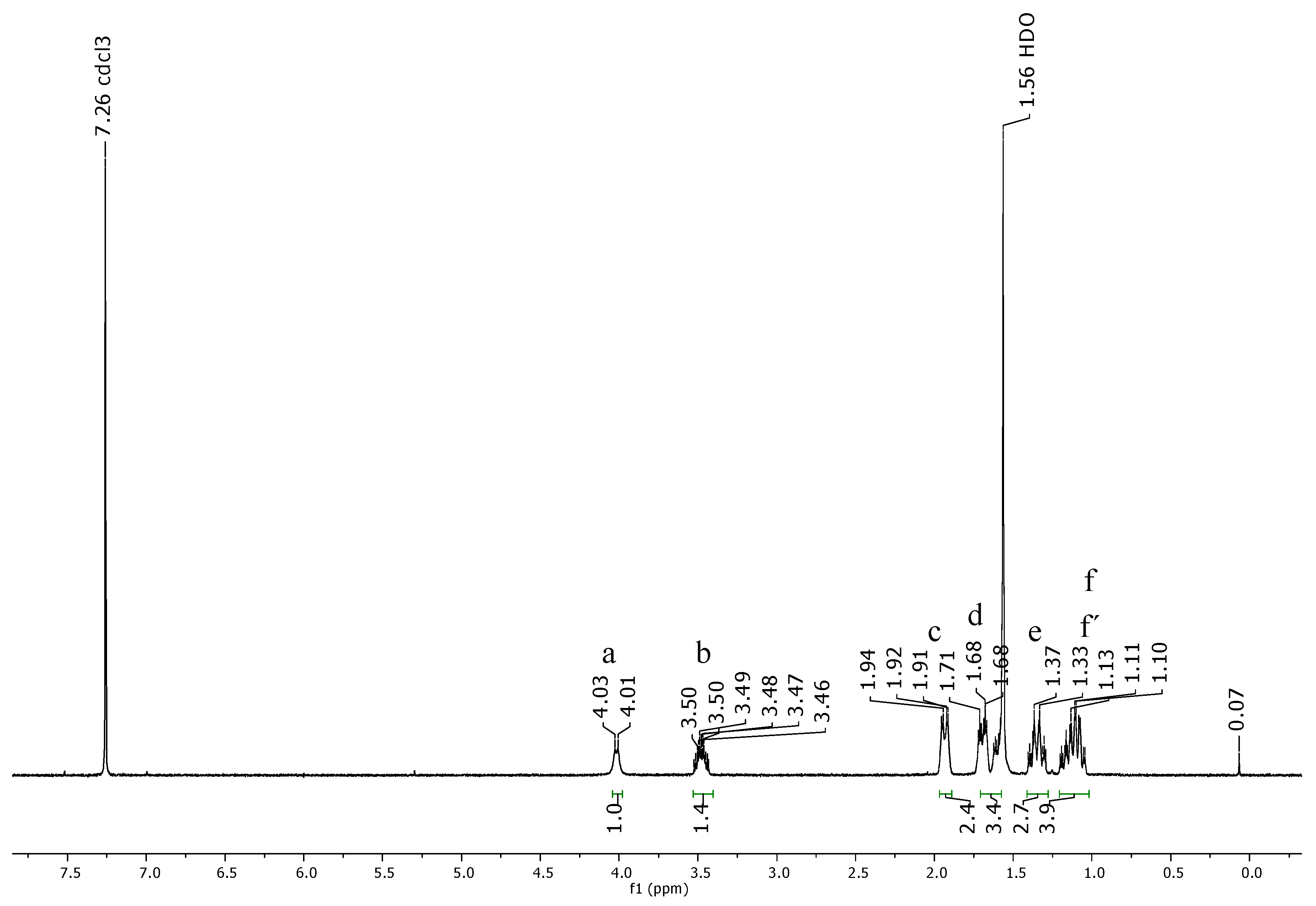
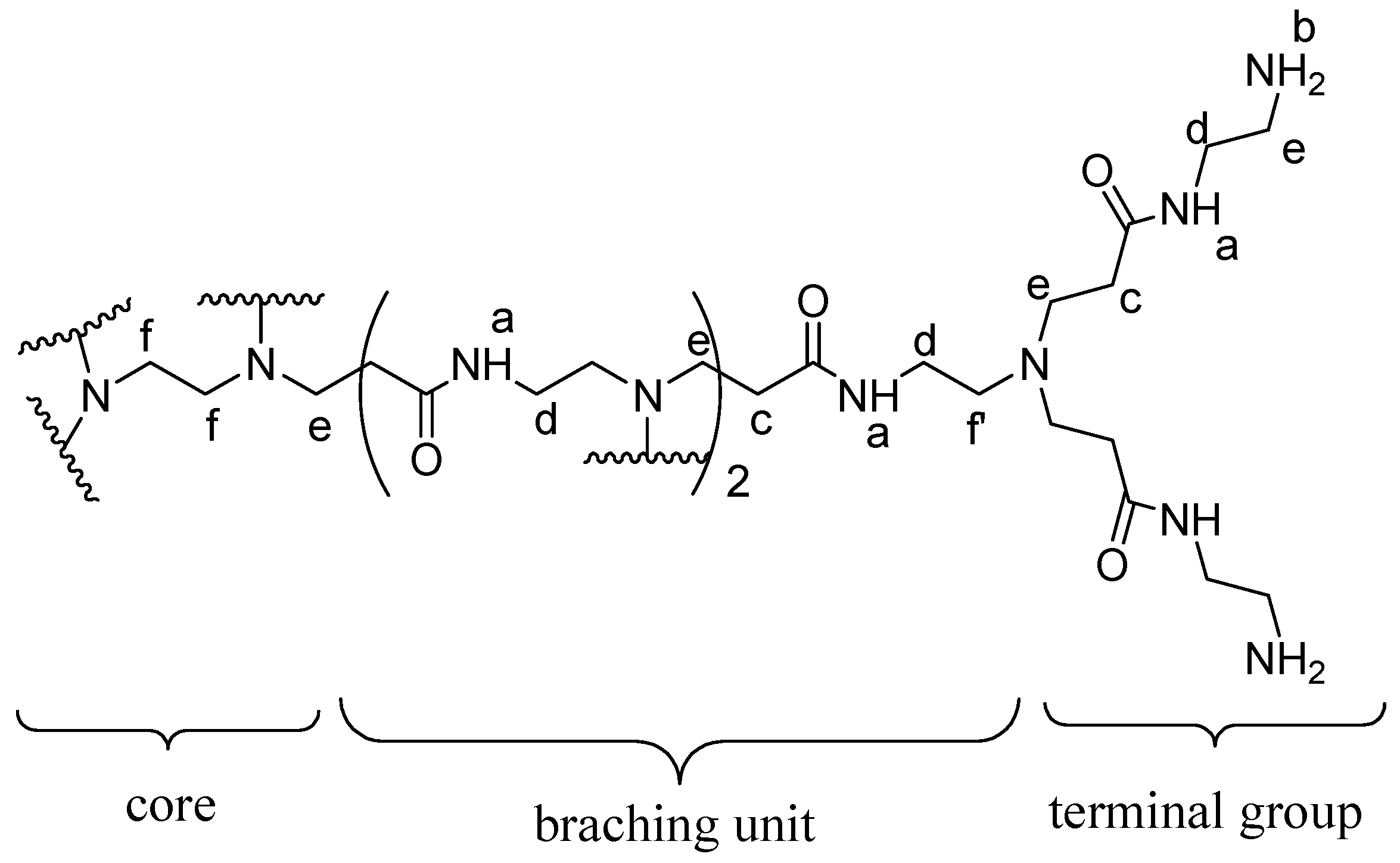
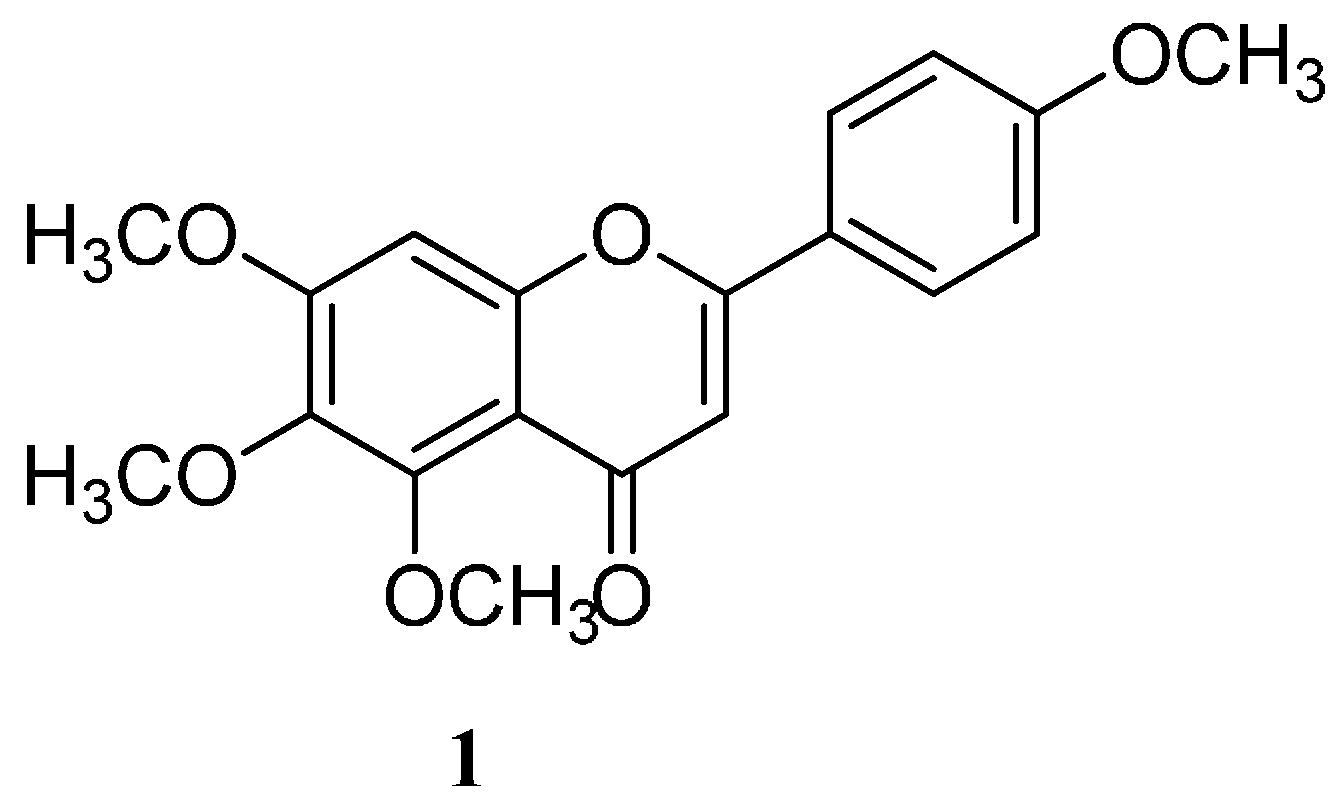
2.2. Encapsulation and Characterization of Tetramethylscutellarein (TMScu (1))-Dendrimer Complex
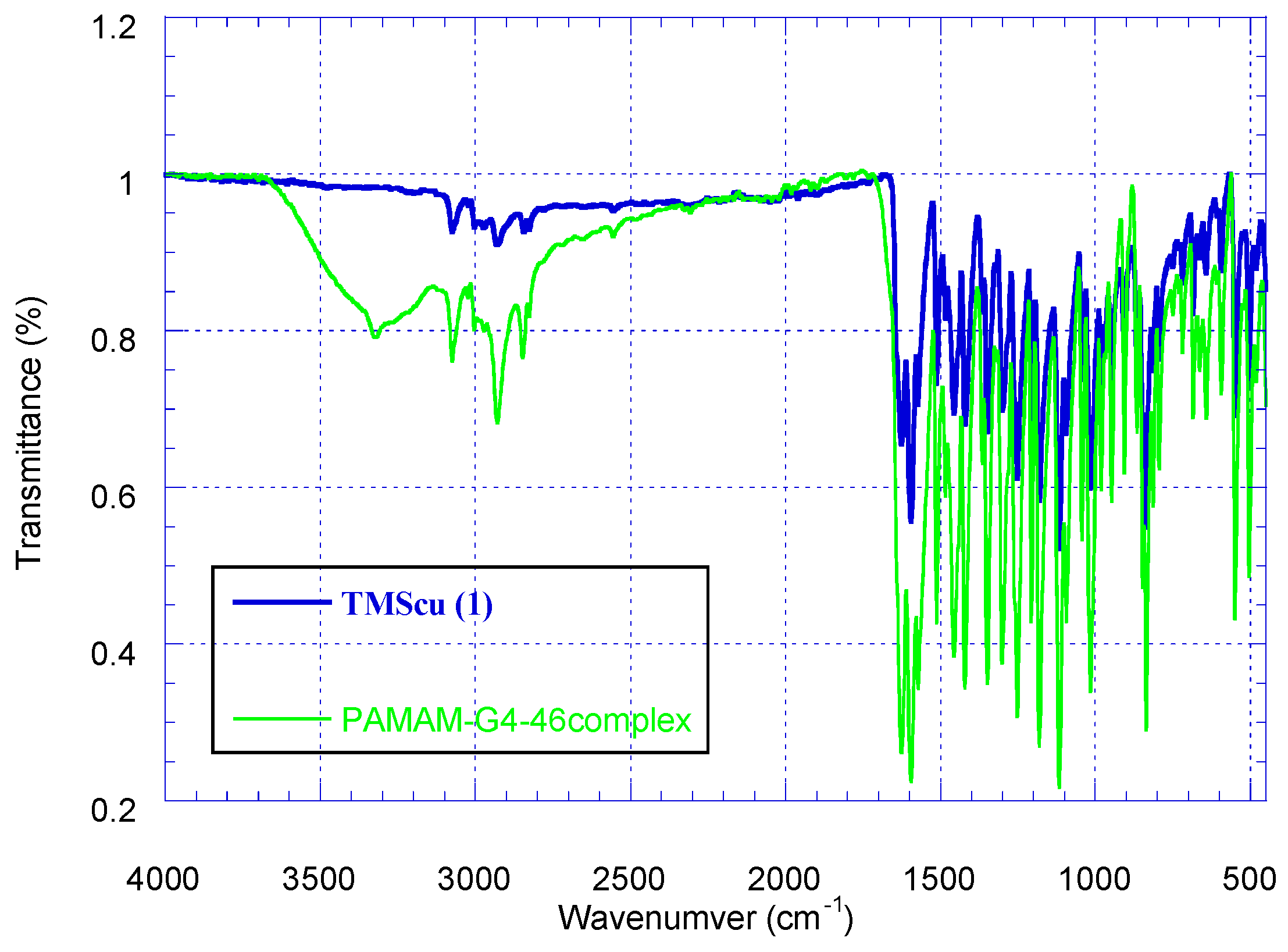
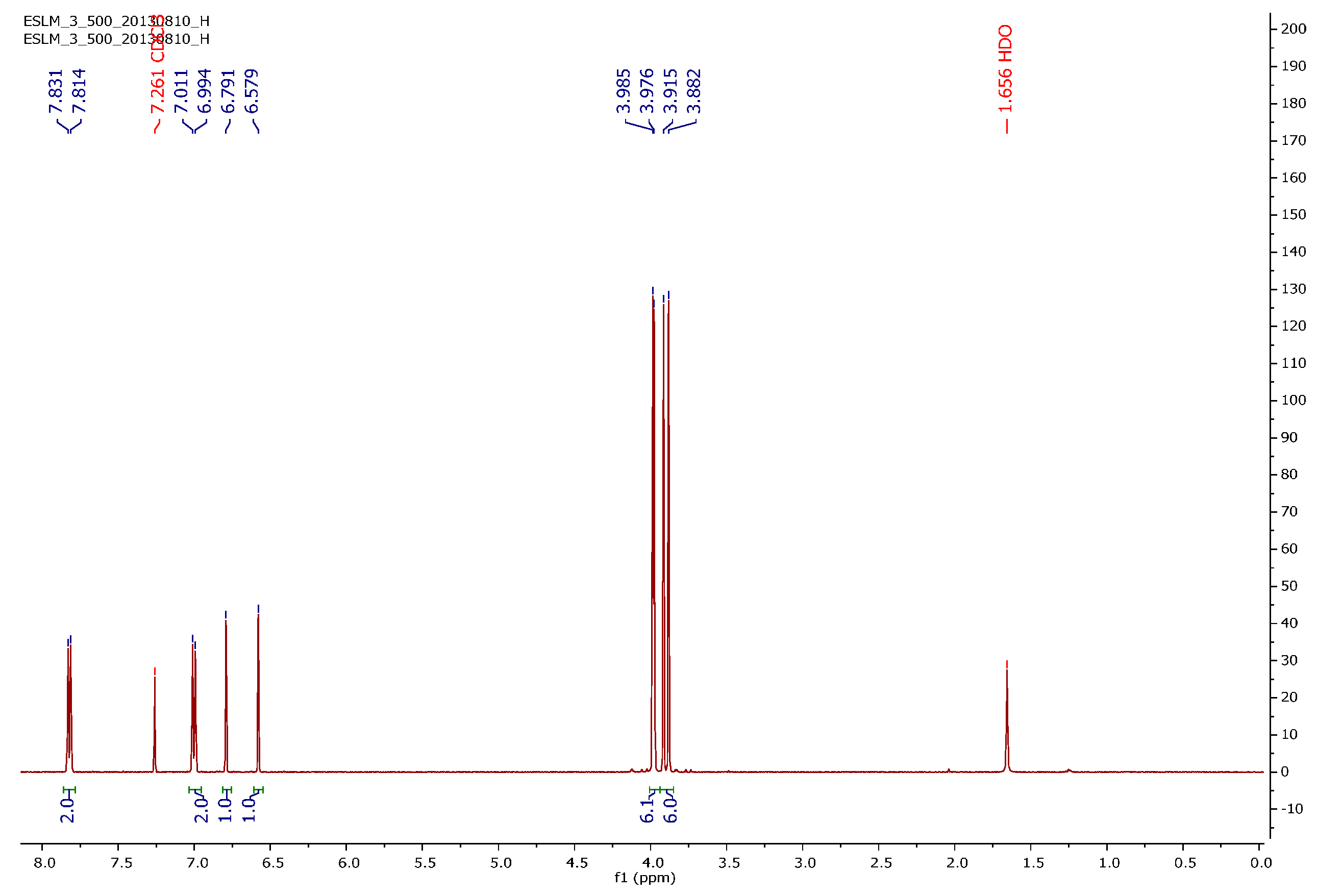
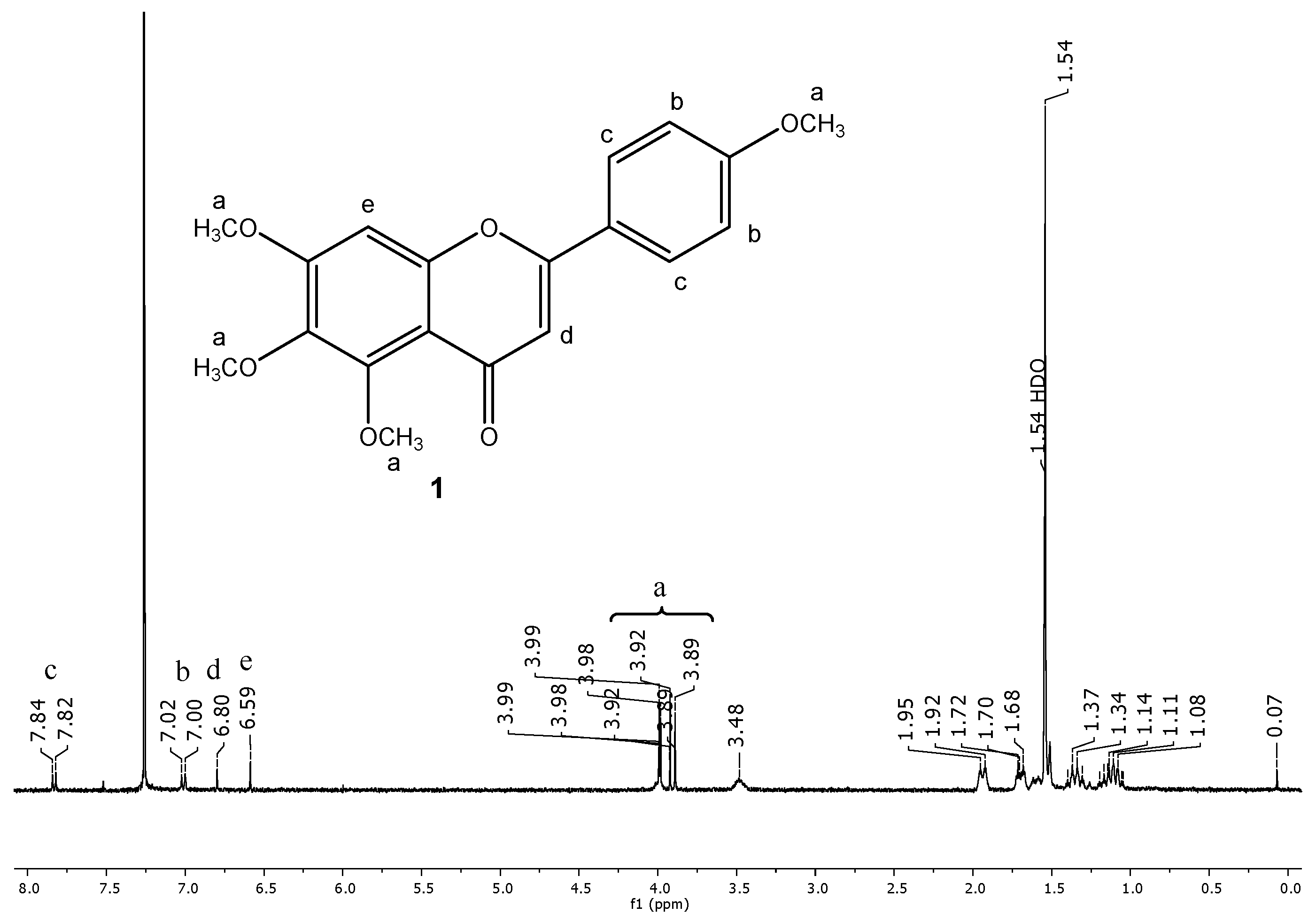
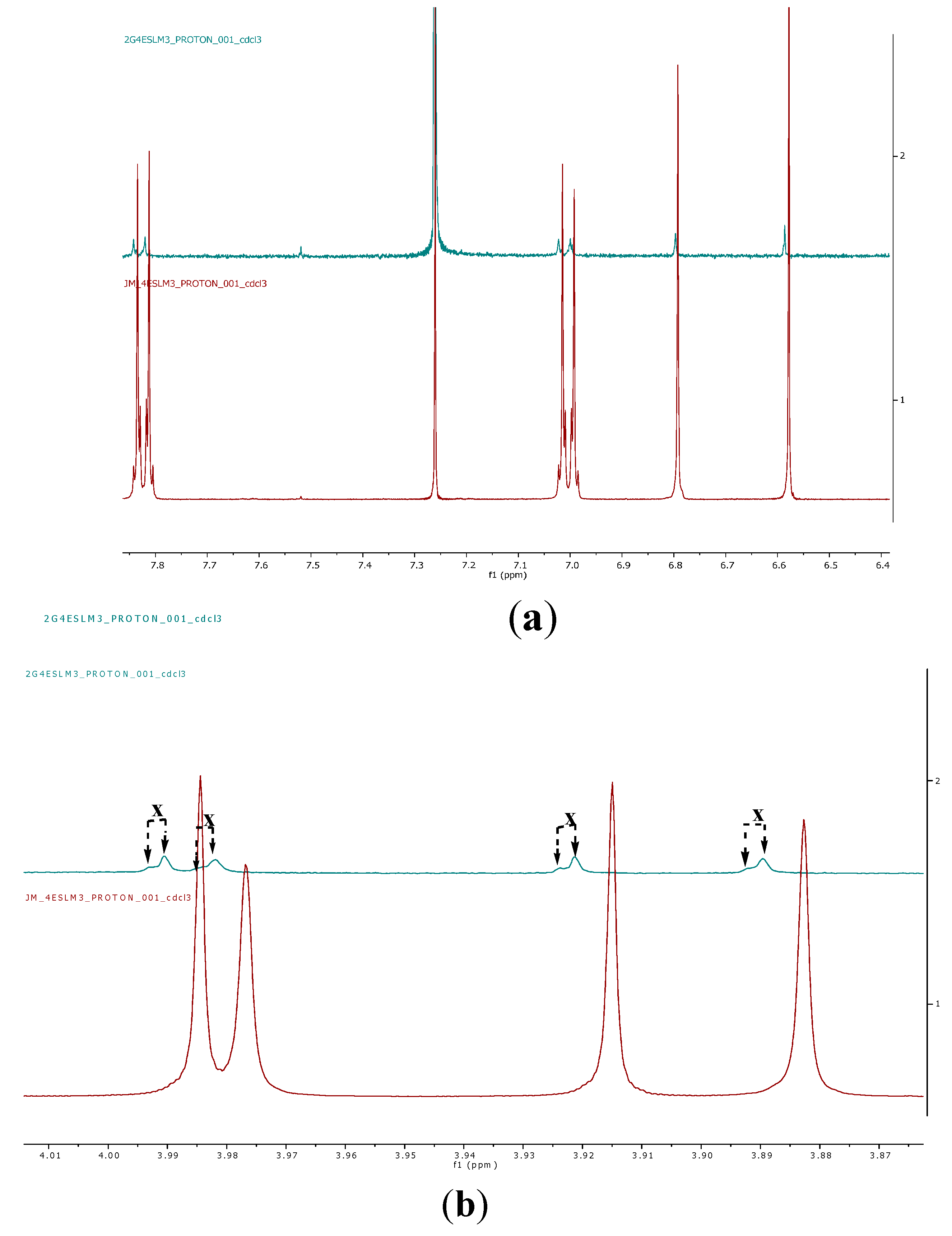
2.3. Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.4. Phase Solubility Studies of the TMScu (1)-Dendrimer G4 Formulation

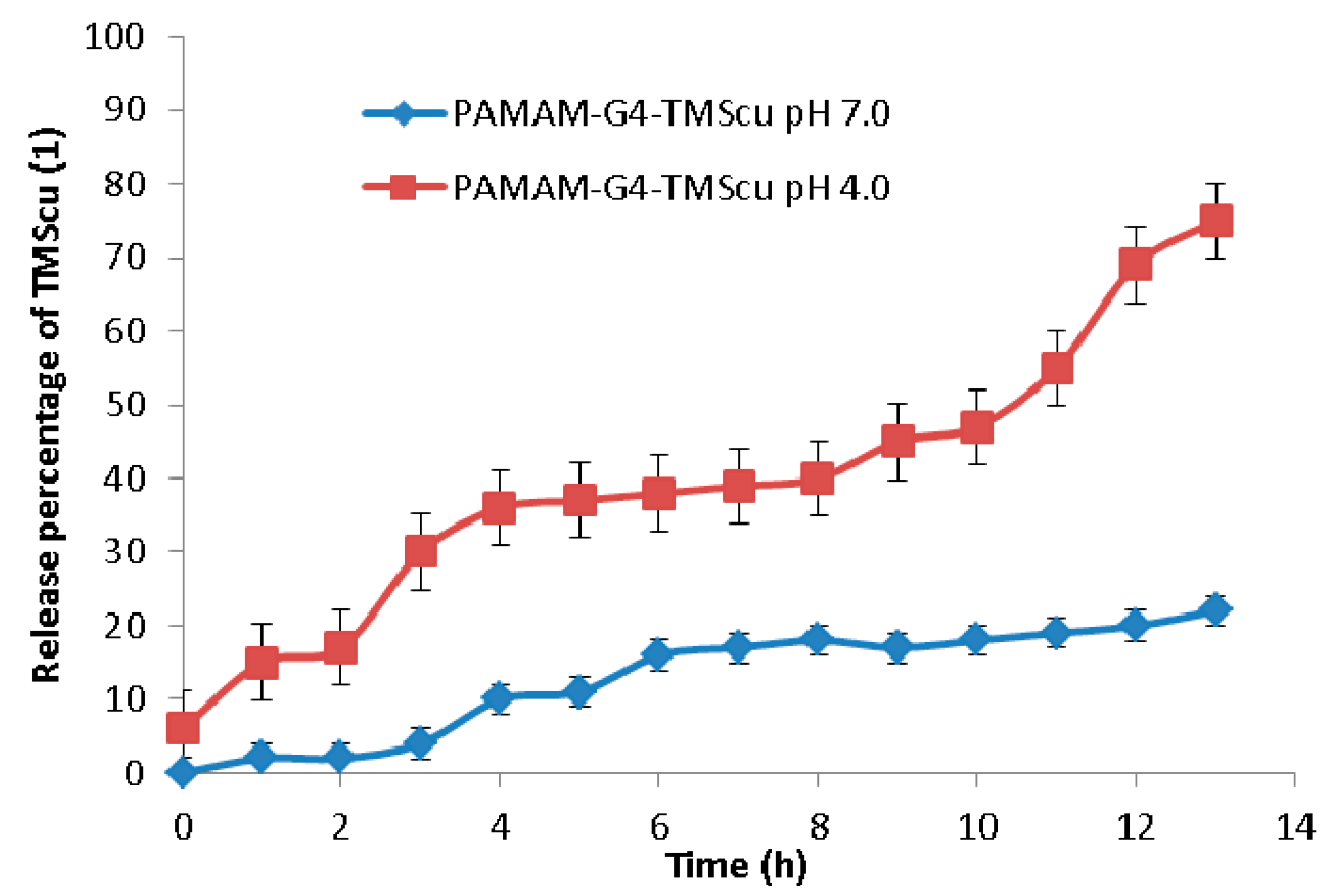
2.5. In Vitro Release of the Loaded TMScu (1)
2.6. Stability Studies of Dendrimer G4-TMScu (1) Formulation
| Formulation | Parameter | Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| Light | Dark | ||||||
| 0 | 27 | 40 | 0 | 27 | 40 | ||
| PAMAM G4-TMScu (1) | Color change | − | − | +++ | − | − | + |
| Turbidity | − | + | + | − | + | ++ | |
| Precipitation | − | + | ++ | − | − | +++ | |
3. Experimental Section
3.1. Materials and Reagents
3.2. General Experimental Procedures
3.3. Synthesis of Polyamidoamine (PAMAM) Dendrimer Generation 4
3.4. PAMAM G4
3.5. The Autoflex Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrum (MALDI-TOF-MS) Analysis


3.6. Selection of Model Compound for Encapsulation Study
3.7. Encapsulation of TMScu (1) in PAMAM Dendrimer G4
3.8. Encapsulation Efficiency (EE) and Loading Capacity (LC)
3.9. Solubilization Study of TMScu (1)
3.10. In Vitro Release Studies of Loaded Compound (TMScu, 1)
3.11. Stability Studies of TMScu (1)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Papadimitrious, S.; Bikiaris, D. Novel self-assembled core-shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release. J. Control. Release 2009, 138, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Patel, P.M. Dendrimer applications—A review. Int. J. Pharm. Biol. Sci. 2013, 4, 454–463. [Google Scholar]
- Wong, P.T.; Choi, S.K. Mechanisms and implications of dual-acting methotrexate in follate-targeting nanotherapeutic delivery. Int. J. Mol. Sci. 2015, 16, 1772–1790. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. Effect of PAMAM dendrimer on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18, 8607–8617. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.M.; Tekade, R.K.; Gupta, U.; Gajbhiye, V.; Jain, N.K. Dendrimer-mediated solubilisation, formulation development and in vitro–in vivo assessment of Piroxicam. Mol. Pharm. 2009, 6, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Vogtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Synthesis, Properties, Applications; Wiley-VCH Verlag GmbH & Co.: KGaA, Weinheim, Germany, 2009. [Google Scholar]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [PubMed]
- Lin, H.; Zhang, W.; Dong, Z.-X.; Gu, T.; Li, N.-G.; Shi, Z.-H.; Kai, J.; Qu, C.; Shang, G.-X.; Tang, Y.-P.; et al. A new and practical synthetic method for the synthesis of 6-O-methyl-scutellarein: One metabolite of scutellarine in vivo. Int. J. Mol. Sci. 2015, 16, 7587–7594. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green polyphenol epiggallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Hou, S.X.; He, W.L.; Feng, J.L.; Wang, X.C.; Fei, H.X.; Chen, Z.H. Study on the preparation of resveratrol chitosan nanoparticles with free amino groups on the surface. Zhongguo Zhong Yao Za Zhi 2006, 31, 205–208. [Google Scholar] [PubMed]
- Pandith, H.; Zhang, X.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W.; Baek, S.J. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J. Ethnopharmacol. 2013, 147, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Munissi, J.J.E.; Nyandoro, S.S.; Kombo, M.K.; Mgina, A.C.; Gruhonjic, A.; Alao, P.F.; Landberg, G.; Lu, Y.; Bin, W.; Pan, F.; et al. Antimycobacterial and cytotoxic flavonoids from Erythrina schliebenii. Manuscript. J. Nat. Prod. In Preparation to be submitted to. 2015. [Google Scholar]
- Hong, H.; Liu, G.Q. Scutellarin attenuates oxidative glutamate toxicity in PC12 cells. Planta Med. 2004, 70, 427–431. [Google Scholar] [PubMed]
- Müller, R.; Laschober, C.; Szymanski, W.W.; Allmaier, G. Determination of molecular weight, particle size, and density of high number generation PAMAM dendrimers using MALDI-TOF-MS and nES-GEMMA. Macromolecules 2007, 40, 5599–5605. [Google Scholar] [CrossRef]
- Kim, Y.; Klutz, A.M.; Jacobson, K.A. Systemic investigation of polyamidoamine dendrimers surface-modified with poly(ethylene glycol) for drug delivery applications: Synthesis, characterization, and evaluation of cytotoxicity. Bioconjug. Chem. 2008, 19, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Gautan, S.P.; Sharma, A.K.G.A.; Madhu, T.G. Synthesis and characterization of ester and amine terminated PAMAM dendrimer. Global J. Med. Res. 2013, 13, 6–15. [Google Scholar]
- Zheng, P.; Gao, L.; Sun, X.; Mei, S.H. Thermolysis behaviours of the first generation dendritic polyamidoamine. Iran. Polym. J. 2009, 18, 257–264. [Google Scholar]
- Garea, S.A.; Ghebaur, A. FT-IR spectroscopy and thermogravimetrical characterization of prodrugs based on different dendritic polymers and antitumoral drugs. Mater. Plast. 2012, 49, 1–4. [Google Scholar]
- Ritawidya, R.; Pujiyanto, A.; Mujinali, W.; Setiawan, H.; Ramli, M.; Kurniasih, D.; Yanuar, A.; Mutalib, A.; Kardono, L.B. Synthesis and characterization of poly(amidoamine) dendrimers encapsulated 198 Au nanoparticles. Atom Indones. 2012, 38, 118–126. [Google Scholar] [CrossRef]
- Kolhe, P.; Misra, E.; Kannan, R.M.; Kannan, S.; Lai, M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm. 2003, 259, 143–160. [Google Scholar] [CrossRef]
- Intra, J.; Salem, A.K. Fabrication, characterization and in vitro evaluation of poly(d,l-lactide-co-glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in non-viral gene delivery. J. Pharm. Sci. 2010, 99, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Doile, M.M.; Fortunanto, K.A.; Schmucker, I.C.; Schucko, S.K.; Silva, M.A.S.; Rodrigues, P.O. Physicochemical properties and dissolution studies of dexamethasone acetate-β-cyclodextrin inclusion complexes produced by different methods. Pharm. Sci. Technol. 2008, 9, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jun-Jun, L.; Zheng-Hong, W.; Qi-Neng, P. The effect of polyamidoamine dendrimers (PAMAM) on the solubility and pharmacokinetics of breviscapine. Acta Pharm. Sin. 2009, 44, 197–202. [Google Scholar] [CrossRef]
- Kakde, D.; Jain, D.; Shrivastava, V.; Kakde, R.; Patil, A.T. Cancer therapeutics—Opportunities, challenges and advances in drug delivery. J. Appl. Pharm. Sci. 2011, 1, 1–10. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shadrack, D.M.; Mubofu, E.B.; Nyandoro, S.S. Synthesis of Polyamidoamine Dendrimer for Encapsulating Tetramethylscutellarein for Potential Bioactivity Enhancement. Int. J. Mol. Sci. 2015, 16, 26363-26377. https://doi.org/10.3390/ijms161125956
Shadrack DM, Mubofu EB, Nyandoro SS. Synthesis of Polyamidoamine Dendrimer for Encapsulating Tetramethylscutellarein for Potential Bioactivity Enhancement. International Journal of Molecular Sciences. 2015; 16(11):26363-26377. https://doi.org/10.3390/ijms161125956
Chicago/Turabian StyleShadrack, Daniel M., Egid B. Mubofu, and Stephen S. Nyandoro. 2015. "Synthesis of Polyamidoamine Dendrimer for Encapsulating Tetramethylscutellarein for Potential Bioactivity Enhancement" International Journal of Molecular Sciences 16, no. 11: 26363-26377. https://doi.org/10.3390/ijms161125956