Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review
Abstract
:1. Introduction
2. Radiation-Induced Changes
2.1. Apoptosis
2.2. Inflammatory Response and Oxidative Stress
2.3. Neurogenesis, Neurons and Neural Functions


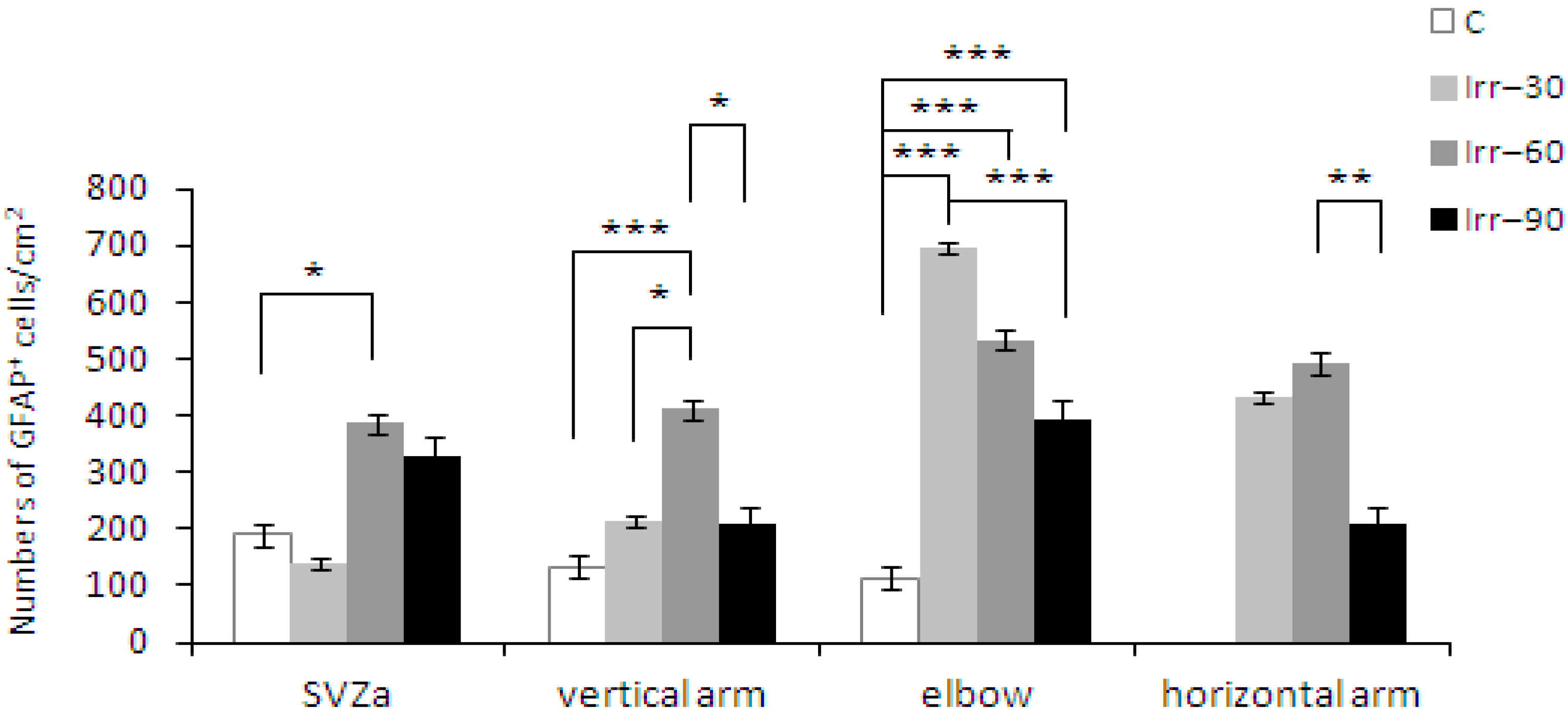
2.4. Glial Cells
2.5. Endothelial Cells
2.6. Neurocognitive Functions
3. Preclinical Approaches to Preserve/Mitigate Radiation Injury
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johannesen, T.B.; Lien, H.H.; Hole, K.H.; Lote, K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother. Oncol. 2003, 69, 169–176. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Silasi, G.; Diaz-Heijtz, R.; Besplug, J.; Rodriguez-Juarez, R.; Titov, V.; Kolb, B.; Kovalchuk, O. Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem. Biophys. Res. Commun. 2004, 325, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Giedzinski, E.; Rola, R.; Otsuka, S.; Palmer, T.D.; Fike, J.R. Radiation response of neural precursor cells: Linking cellular sensitivity to cell cyle checkpoints, apoptosis and oxidative stress. Radiat. Res. 2004, 161, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Raju, U.; Gumin, G.J.; Tofilon, P.J. Radiation-induced transcription factor activation in the rat cerebral cortex. Int. J. Radiat. Biol. 2000, 76, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lim, D.J.; Chung, Y.G.; Cho, T.H.; Lim, S.J.; Kim, W.J.; Suh, J.K. Expression of TNF-α and TGF-β 1 in the rat brain after a single high-dose irradiation. J. Korean Med. Sci. 2002, 17, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, C.; Gobbel, G.T.; Lamborn, K.R.; Tada, E.; Fike, J.R. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res. 1997, 57, 2694–2702. [Google Scholar] [PubMed]
- Peissner, W.; Kocher, M.; Treuer, H.; Gillardon, F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Mol. Brain Res. 1999, 71, 61–68. [Google Scholar] [CrossRef]
- Tada, E.; Parent, J.M.; Lowenstein, D.H.; Fike, J.R. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neurosience 2000, 99, 33–41. [Google Scholar] [CrossRef]
- Sasaki, R.; Matsumoto, A.; Itoh, K.; Kawabe, T.; Ota, Y.; Yamada, K.; Maruta, T.; Soejima, T.; Sugimura, K. Target cells of apoptosis in the adult murine dentate gyrus and O4 immunoreactivity after ionizing radiation. Neurosci. Lett. 2000, 279, 57–60. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, L.M.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar] [PubMed]
- Ferrer, I.; Macaya, A.; Blanco, R.; Olive, M.; Cinos, C.; Munell, F.; Planas, A.M. Evidence of internucleosomal DNA fragmentation and identification odf dying cells in X-ray-induced cell death in the developing brain. Int. J. Dev. Neurosci. 1995, 13, 21–28. [Google Scholar] [CrossRef]
- Raber, J.; Rola, R.; Lefevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rola, R.; Raber, J.; Rizk, A.; Otsuka, S.; VandenBerg, S.R.; Morhardt, D.R.; Fike, J.R. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004, 188, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Z.; Weinstein, P.R.; Fike, J.R.; Liu, J. Enviromental enrichment enhances neurogenesis and improves functional outcome after irradiation. Eur. J. Neurosci. 2007, 25, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. 2002, 115, 97–105. [Google Scholar] [CrossRef]
- Winocur, G.; Wojtowicz, M.J.; Sekeres, M.; Snyder, J.S.; Wang, S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 2006, 16, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.M.; Kristjansen, P.E.; Bolwig, T.G.; Wortwein, G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 2003, 119, 635–642. [Google Scholar] [CrossRef]
- Wong, C.S.; van der Kogel, A.J. Mechanisms of radiation injury to the central nervous system: Implications for neuroprotection. Mol. Interv. 2004, 4, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bellinzona, M.; Gobbel, G.T.; Shinohara, C.; Fike, J.R. Apoptosis is induced in the subependyma of young adult rats by ionizing radiation. Neurosci. Lett. 1996, 208, 163–166. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Cebrian-Silla, A.; Guerrero-Cazares, H.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front. Cell. Neurosci. 2014, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Inamura, T.; Wu, C.M.; Kura, S.; Nakamizo, A.; Inoha, S.; Miyazono, M.; Ikezaki, K. Effects of single low dose irradiation on subventricular zone cells in juvenile brain. Neurol. Res. 2002, 24, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Hong, N.; McDonald, R.J.; Wojtowicz, J.M. A role for adult hippocampal neurogenesis in spatial long-term memory. Neuroscience 2005, 130, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Barlind, A.; Karlsson, N.; Björk-Eriksson, T.; Isgaard, J.; Blomgren, K. Decreased cytogenesis in the granule cell layer of the hippocampus and impaired place learning after irradiation of the young mouse brain evaluated using the IntelliCage platform. Exp. Brain Res. 2010, 201, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Moravan, M.J.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat. Res. 2011, 176, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus 2006, 16, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M. Extended-term effects of head and neck irradiation in a rodent. Eur. J. Cancer 2001, 37, 1938–1945. [Google Scholar] [CrossRef]
- Forbes, M.E.; Paitsel, M.; Bourland, J.D.; Riddle, D.R. Systemic effects of fractionated, whole-brain irradiation in young adult and aging rats. Radiat. Res. 2013, 180, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Kyrkanides, S.; Olschowka, J.A.; Williams, J.P.; Hansen, J.T.; O’Banion, M.K. TNFα and IL-1β mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J. Neuroimmunol. 1999, 95, 95–106. [Google Scholar] [CrossRef]
- Ramanan, S.; Kooshki, M.; Zhao, W.; Hsu, F.C.; Robbins, M.E. PPARα ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-Kb and AP-1 pathways. Free Radic. Biol. Med. 2008, 45, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Kalm, M.; Fukuda, A.; Fukuda, H.; Ohrfelt, A.; Lannering, B.; Björk-Eriksson, T.; Blennow, K.; Márky, I.; Blomgren, K. Transient inflammation in neurogenic regions after irradiation of the developing brain. Radiat. Res. 2009, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Sonntag, W.E.; Mitschelen, M.; Yan, H.; Lee, Y.W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010, 86, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Vogel, H.; Masek, M.; Ligon, K.L.; Fisher, P.G.; Palmer, T.D. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. 2007, 62, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.; Zhao, W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004, 80, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Diz, D.I.; Robbins, M.E. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.R.; Farrell, C.L.; del Maestro, R.F. Effects of steroids and nonsteroid anti-inflammatory agents on vascular permeability in a rat glioma model. J. Neurosurg. 1986, 65, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Suleman, S.; Grossman, S.A.; Eller, S.; Carson, K. A cyclooxygenase-2 (COX-2) inhibitor compared with dexamethasone in a survival study of rats with intracerebral 9L gliosarcomas. Neuro-Oncology 2002, 4, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [PubMed]
- Kempermann, G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J. Neurosci. 2002, 22, 635–638. [Google Scholar] [PubMed]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.M. Becoming anew neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 5, 507–518. [Google Scholar]
- Abrous, D.N.; Koehl, M.; le Moal, M. Adult neurogenesis: From precursors to network and physiology. Physiol. Rev. 2005, 85, 523–569. [Google Scholar] [CrossRef] [PubMed]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lamproglou, I.; Chen, Q.M.; Boisserie, G.; Mazeron, J.J.; Poisson, M.; Baillet, F.; LePoncin, M.; Delattre, J.Y. Radiation-induced cognitive dysfunction: An experimental model in the old rat. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 65–70. [Google Scholar] [CrossRef]
- Schindler, M.K.; Forbes, M.E.; Robbins, M.E.; Riddle, D.R. Aging-dependent changes in the radiation response of the adult rat brain. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Balentova, S.; Hajtmanova, E.; Trylcova, R.; Adamkov, M.; Lehotsky, J. Ionizing radiation induced long-term alterations in the adult rat rostral migratory stream. Acta Histochem. 2014, 116, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Balentova, S.; Hajtmanova, E.; Adamkov, M.; Lehotsky, J. Differential expression of doublecortin and microglial markers in the rat brain following fractionated irradiation. Neurochem. Res. 2015, 40, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Greene-Schloesser, D.; Payne, V.; Diz, D.I.; Hsu, F.C.; Kooshki, M.; Mustafa, R.; Riddle, D.R.; Zhao, W.; Chan, M.D.; et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat. Res. 2012, 178, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bálentová, S.; Hajtmanová, E.; Plevková, J.; Lehotský, J.; Adamkov, M. Fractionated irradiation-induced altered spatio-temporal cell distribution in the rat forebrain. Acta Histochem. 2013, 115, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.W.; Sabek, O.M.; Fukatsu, K.; Wilcox, H.G.; Kiani, M.F.; Merchant, T.E. The differences in ICAM-1 and TNF-α expression between high single fractions and fractionated irradiation in mouse brain. Int. J. Radiat. Biol. 2003, 79, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Leyrer, C.M.; Greene-Schloesser, D.M.; Shing, E.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Ip, E.H.; Rapp, S.R.; Robbins, M.E.; et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology 2013, 80, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Noel, F.; Gumin, G.J.; Raju, U.; Tofilon, P.J. Increased expression of prohormone convertase-2 in the irradiated rat brain. FASEB J. 1998, 12, 1725–1730. [Google Scholar] [PubMed]
- Rosi, S.; Andres-Mach, M.; Fishman, K.M.; Levy, W.; Ferguson, R.A.; Fike, J.R. Cranial irradiation alters the behaviorally induced immediate-early gene Arc (activity-regulated cytoskeleton-associated protein). Cancer Res. 2008, 68, 9763–9770. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Schloesser, D.; Miller, L.; Robbins, M. Changes in hippocampal gene expression 48 h and 2 months after fractionated whole-brain irradiation of the young adult male rat. In Proceedings of the 14th International Congress of Radiation Research, Warsaw, Poland, 28 August–1 September 2011.
- Vlkolinský, R.; Krucker, T.; Nelson, G.A.; Obenaus, A. 56Fe-particle radiation reduces neuronal output and attenuates lipopolysaccharide-induced inhibition of long-term potentiation in the mouse hippocampus. Radiat. Res. 2008, 169, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Lonart, G.; Britten, R.A. Low (60cGy) doses of 56Fe HZE-particle radiation lead to apersistent reduction in the glutamatergic readily releasable pool in rat hippocampal synaptosomes. Radiat. Res. 2010, 174, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Coultrap, S.; Pinnix, C.; Davies, K.D.; Tailor, R.; Ang, K.K.; Browning, M.D.; Grosshans, D.R. Radiation induces acute alterations in neuronal function. PLoS ONE 2012, 7, e37677. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Molina, D.P.; Robbins, M.E.; Wheeler, K.T.; Brunso-Bechtold, J.K. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Guo, Y.P.; Jay, V.; Stewart, P.A.; Wong, C.S. Time course of radiation-induced apoptosis in the adult rat spinal cord. Radiother. Oncol. 1996, 39, 35–42. [Google Scholar] [CrossRef]
- Atkinson, S.; Li, Y.Q.; Wong, C.S. Changes in oligodendrocytes and myelin gene expression after radiation in the rodent spinal cord. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1093–1100. [Google Scholar] [CrossRef]
- Chari, D.M.; Crang, A.J.; Blakemore, W.F. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. Cell Death Differ. 2003, 7, 712–720. [Google Scholar]
- Chow, B.M.; Li, Y.Q.; Wong, C.S. Radiation-induced apoptosis in the central nervous system is p53-dependent. Cell Death Differ. 2000, 7, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Sato, M.; Tanaka, R. Radiation-induced apoptosis and injury of oligodendrocytes on neonatal rat brains. Clin. Neurol. Neurosurg. 1997, 99, 117. [Google Scholar] [CrossRef]
- Kurita, H.; Kawahara, N.; Asai, A.; Ueki, K.; Shin, M.; Kirino, T. Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol. Res. 2001, 23, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Linville, M.C.; Iversen, E.; Molina, D.P.; Yester, J.; Wheeler, K.T.; Robbins, M.E.; Brunso-Bechtold, J.K. Maintenance of white mater integrityinarat model of radiation-induced cognitive impairment. J. Neurol. Sci. 2009, 285, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Frisén, J.; Johansson, C.B.; Török, C.; Risling, M.; Lendahl, U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J. Cell Biol. 1995, 131, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Tada, E.; Yang, C.; Gobbel, G.T.; Lamborn, K.R.; Fike, J.R. Long-term impairment of subependymal repopulation following damage by ionizing radiation. Exp. Neurol. 1999, 160, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Cicciarello, R.; d’Avella, D.; Gagliardi, M.E.; Albiero, F.; Vega, J.; Angileri, F.F.; D’Aquino, A.; Tomasello, F. Time-related ultrastructural changes in an experimental model of whole brain irradiation. Neurosurgery 1996, 38, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Gaber, M.W.; Boyd, K.; Wilson, C.M.; Kiani, M.F.; Merchant, T.E. Effects of fractionated radiation on the brain vasculature in a murine model: Blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Liu, J.; Wang, J.; Zhou, D.; Zhao, Z.; Xiao, S.; Tao, E.; Suo, W.Z. Fractionated radiation-induced acute encephalopathy in a young rat model: Cognitive dysfunction and histologic findings. AJNR Am. J. Neuroradiol. 2011, 32, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.S.; McBride, W.H.; Withers, H.R. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother. Oncol. 1993, 29, 60–68. [Google Scholar] [CrossRef]
- Akiyama, K.; Tanaka, R.; Sato, M.; Takeda, N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol. Medico. Chir. 2001, 41, 590–598. [Google Scholar] [CrossRef]
- Wilson, C.M.; Gaber, M.W.; Sabek, O.M.; Zawaski, J.A.; Merchant, T.E. Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Bálentová, S.; Hajtmanová, E.; Kinclová, I.; Lehotský, J.; Dobrota, D.; Adamkov, M. Radiation-induced long-term alterations in hippocampus under experimental conditions. Klin. Onkol. 2012, 25, 110–116. [Google Scholar] [PubMed]
- Bálentová, S.; Hajtmanová, E.; Kinclová, I.; Lehotský, J.; Dobrota, D.; Adamkov, M. Long-term alterations of cell population in the adult rat forebrain following exposure to fractionated doses of ionizing radiation. Gen. Physiol. Biophys. 2013, 32, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Jung, J.S.; Kim, T.H.; Lim, S.J.; Oh, E.S.; Kim, J.Y.; Ji, K.A.; Joe, E.H.; Cho, K.H.; Han, I.O. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol. Dis. 2006, 21, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Hailer, N.P.; Grampp, A.; Nitsch, R. Proliferation of microglia and astrocytes in the dentate gyrus following entorhinal cortex lesion: A quantitative bromodeoxyuridine-labelling study. Eur. J. Neurosci. 1999, 11, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; Manning, J.; Rossi, F.; Krieger, C. The neuroinflammatory response in ALS: The roles of microglia and T Cells. Neurol. Res. Int. 2012, 2012, 803701. [Google Scholar] [CrossRef] [PubMed]
- Ladeby, R.; Wirenfeldt, M.; Garcia-Ovejero, D.; Fenger, C.; Dissing-Olesen, L.; Dalmau, I.; Finsen, B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Brain Res. Rev. 2005, 48, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mildenberger, M.; Beach, T.G.; McGeer, E.G.; Ludgate, C.M. An animal model of prophylactic cranial irradiation: Histologic effects at acute, early and delayed stages. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1051–1060. [Google Scholar] [CrossRef]
- Chiang, C.S.; Hong, J.H.; Stalder, A.; Sun, J.R.; Withers, H.R.; McBride, W.H. Delayed molecular responses to brain irradiation. Int. J. Radiat. Biol. 1997, 72, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.; Payne, V.; Tommasi, E.; Diz, D.I.; Hsu, F.C.; Brown, W.R.; Wheeler, K.T.; Olson, J.; Zhao, W. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Payne, V.S.; Forbes, M.E.; Robbins, M.E.; Riddle, D.R. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat. Res. 2010, 173, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Christie, L.A.; Lan, M.L.; Giedzinski, E.; Fike, J.R.; Rosi, S.; Limoli, C.L. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011, 71, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.M.; Jin, J.; Kang, B.G.; Lee, S.J.; Kim, K.H.; Yang, H.; Lee, Y.A.; Cho, Y.J.; Im, Y.S.; Lee, D.S.; et al. Trans-differentiation of neural stem cells: A therapeutic mechanism against the radiation induced brain damage. PLoS ONE 2012, 7, e25936. [Google Scholar] [CrossRef] [PubMed]
- Ljubimova, N.V.; Levitman, M.K.; Plotnikova, E.D.; Eidus, L. Endothelial cell population dynamics in rat brain after local irradiation. Br. J. Radiol. 1991, 64, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, T.E.; Stephens, L.C. Permanent radiation myelopathy. Br. J. Radiol. 1992, 65, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Pfeffer, M.R. Radiation-induced changes in the profile of spinal cord serotonin, prostaglandin synthesis, and vascular permeability. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 57–64. [Google Scholar] [CrossRef]
- Yuan, H.; Gaber, M.W.; McColgan, T.; Naimark, M.D.; Kiani, M.F.; Merchant, T.E. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: Modulation with anti-ICAM-1 antibodies. Brain Res. 2003, 969, 59–69. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, P.; Jain, V.; Reilly, R.M.; Wong, C.S. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat. Res. 2004, 161, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Thore, C.R.; Moody, D.M.; Robbins, M.E.; Wheeler, K.T. Vascular damage after fractionated whole-brain irradiation in rats. Radiat. Res. 2005, 164, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Blair, R.M.; Moody, D.M.; Thore, C.R.; Ahmed, S.; Robbins, M.E.; Wheeler, K.T. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: A potential rat model of vascular dementia. J. Neurol. Sci. 2007, 257, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P.; Csiszar, A.; Johnson, D.A.; Herman, T.S.; Ahmad, S.; Lee, Y.W.; Sonntag, W.E. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P.; Csiszar, A.; Mitschelen, M.; Lee, Y.W.; Sonntag, W.E. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS ONE 2012, 7, e30444. [Google Scholar] [CrossRef] [PubMed]
- Chuba, P.J.; Aronin, P.; Bhambhani, K.; Eichenhorn, M.; Zamarano, L.; Cianci, P.; Muhlbauer, M.; Porter, A.T.; Fontanesi, J. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer 1997, 80, 2005–2012. [Google Scholar] [CrossRef]
- Leber, K.A.; Eder, H.G.; Kovac, H.; Anegg, U.; Pendl, G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact. Funct. Neurosurg. 1998, 70, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ohguri, T.; Imada, H.; Kohshi, K.; Kakeda, S.; Ohnari, N.; Morioka, T.; Nakano, K.; Konda, N.; Korogi, Y. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K. White matter injury mechanisms. Curr. Mol. Med. 2004, 4, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.A.; Heiss, J.D.; Walbridge, S.; Muhlhauser, J.; Capogrossi, M.C.; Oldfield, E.H.; Merrill, M.J. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J. Neuropathol. Exp. Neurol. 1999, 58, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Li, Y.Q.; Lu, G.; Xu, Y.; Wong, C.S. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J. Neuropathol. Exp. Neurol. 1999, 58, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Nagy, A.; Pintilie, M.; Wong, C.S. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: A role for vascular endothelial growth factor. Clin. Cancer Res. 2004, 10, 3342–3353. [Google Scholar] [CrossRef] [PubMed]
- Coderre, J.A.; Morris, G.M. The radiation biology of boron neutroncapture therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar] [PubMed]
- Li, Y.Q.; Chen, P.; Haimovitz-Friedman, A.; Reilly, R.M.; Wong, C.S. Endothelial apoptosis initiates acut blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003, 63, 5950–5956. [Google Scholar] [PubMed]
- Santana, P.; Peña, L.A.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; Mcloughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef]
- Kölzer, M.; Arenz, C.; Ferlinz, K.; Werth, N.; Schulze, H.; Klingenstein, R.; Sandhoff, K. Phosphatidylinositol-3,5-Bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biol. Chem. 2003, 384, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Andratschke, N.; Nieder, C.; Price, R.; Rivera, B.; Tucker, S.; Ang, K. Modulation of rodent spinal cord radiation tolerance by administration of platelet-derived growth factor. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Price, R.E.; Rivera, B.; Andratschke, N.; Ang, K.K. Effects of insulin-like growth factor-1 (IGF-1) and amifostine in spinal cord reirradiation. Strahlenther. Onkol. 2005, 181, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T. Spinal cord compression: From laboratory to clinic. Eur. J. Cancer 1995, 31, 1748–1753. [Google Scholar] [CrossRef]
- D’Avella, D.; Cicciarello, R.; Angileri, F.F.; Lucerna, S.; la Torre, D.; Tomasello, F. Radiation-induced blood-brain barrier changes: Pathophysiological mechanisms and clinical implications. Acta Neurochir. 1998, 71, 282–284. [Google Scholar]
- Khuntia, D. Contemporary review of the management of brain metastasis with radiation. Adv. Neurosci. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Payne, V.; Peiffer, A.M.; Hsu, F.C.; Riddle, D.R.; Zhao, W.; Chan, M.D.; Metheny-Barlow, L.; Robbins, M.E. The peroxisomal proliferator-activated receptor (PPAR) α agonist, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat. Res. 2014, 181, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yoneoka, Y.; Satoh, M.; Akiyama, K.; Sano, K.; Fujii, Y.; Tanaka, R. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br. J. Radiol. 1999, 72, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, C.I.; Greene-Schloesser, D.; Kooshki, M.; Payne, V.S.; Hsu, F.C.; Robbins, M.E. The PPARδ agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic. Biol. Med. 2013, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gheusi, G.; Cremer, H.; McLean, H.; Chazal, G.; Vincent, J.D.; Lledo, P.M. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. USA 2000, 97, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Kim, Y.; Eun, B.; Park, O.H.; Kim, H.; Kim, K.; Park, C.H.; Vinsant, S.; Oppenheim, R.W.; Sun, W. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J. Neurosci. 2007, 27, 14392–14403. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Mandairon, N.; Jing, D.; Rajagopal, R.; Kapoor, R.; Chen, Z.Y.; Khan, T.; Proenca, C.C.; Kraemer, R.; Cleland, T.A.; et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J. Neurosci. 2008, 28, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Lazarini, F.; Mouthon, M.A.; Gheusi, G.; de Chaumont, F.; Olivo-Marin, J.C.; Lamarque, S.; Abrous, D.N.; Boussin, F.D.; Lledo, P.M. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS ONE 2009, 4, e7017. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Haehner, A.; Hummel, T.; Reichmann, H. Olfactory dysfunction as a diagnostic marker for Parkinson’s disease. Expert Rev. Neurother. 2009, 9, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Neuner, J.; Filser, S.; Michalakis, S.; Biel, M.; Herms, J. A30P α-Synuclein interferes with the stable integration of adult-born neurons into the olfactory network. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 2012, 9, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Isomura, A.; Harima, Y.; Kawaguchi, K.; Kori, H.; Miyachi, H.; Fujiwara, T.; Ishidate, F.; Kageyama, R. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 2013, 342, 1203–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knisely, J.P.; de Lotbiniere, A.C.; de Lanerolle, N.C.; Brines, M.L. Randomized trial of erythropoietin as a central nervous system radioprotectant. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 343–344. [Google Scholar] [CrossRef]
- Jenrow, K.A.; Brown, S.L.; Liu, J.; Kolozsvary, A.; Lapanowski, K.; Kim, J.H. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat. Oncol. 2010, 5, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Major, T.; Auyeung, G.; Policarpio, E.; Menon, J.; Droms, L.; Gutin, P.; Uryu, K.; Tchieu, J.; Soulet, D.; et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 2015, 16, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.S.; Bull, C.; Nilsson, M.K.; Zhu, C.; Björk-Eriksson, T.; Eriksson, P.S.; Blomgren, K.; Kuhn, H.G. From the cover: Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc. Natl. Acad. Sci. USA 2008, 105, 14632–14637. [Google Scholar] [CrossRef] [PubMed]
- Wong-Goodrich, S.J.E.; Pfau, M.L.; Flores, C.T.; Fraser, J.A.; Williams, C.L.; Jones, L.W. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res. 2010, 70, 9329–9338. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Andratschke, N.; Price, R.E.; Rivera, B.; Ang, K.K. Innovative prevention strategies for radiation necrosis of the central nervous system. Anticancer Res. 2002, 22, 1017–1023. [Google Scholar] [PubMed]
- Gonzalez, J.; Kumar, A.J.; Conrad, C.A.; Levin, V.A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Wen, T.C.; Matsuda, S.; Masuda, S.; Morishita, E.; Nagao, M.; Sasaki, R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 1998, 95, 4635–4640. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.L.; Ghezzi, P.; Keenan, S.; Agnello, D.; de Lanerolle, N.C.; Cerami, C.; Itri, L.M.; Cerami, A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. USA 2000, 97, 10526–10531. [Google Scholar] [CrossRef] [PubMed]
- Gorio, A.; Gokmen, N.; Erbayraktar, S.; Yilmaz, O.; Madaschi, L.; Cichetti, C.; di Giulio, A.M.; Vardar, E.; Cerami, A.; Brines, M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl. Acad. Sci. USA 2002, 99, 9450–9455. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Erythropoietin: New directions for the nervous system. Int. J. Mol. Sci. 2012, 13, 11102–11129. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.; Laszig, R.; Rübe, C.; Schäfer, U.; Haase, K.D.; Schilcher, B.; Mose, S.; Beer, K.T.; Burger, U.; Dougherty, C.; et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 2003, 362, 1255–1260. [Google Scholar] [CrossRef]
- Derosa, G. Efficacy and tolerability of pioglitazone in patients with type2 diabetes mellitus: Comparison with other oral antihyperglycaemic agents. Drugs 2010, 70, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Keating, G.M. Fenofibrate: A review of its use in dyslipidaemia. Drugs 2011, 71, 1917–1946. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J.; Kanakasabai, S.; Chearwae, W.; Chakraborty, S. PPAR regulation of inflammatory signaling in CNS diseases. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Zhao, W.; Riddle, D.R.; Robbins, M.E. Role of PPARs in radiation-induced brain injury. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Kooshki, M.; Zhao, W.; Hsu, F.C.; Riddle, D.R.; Robbins, M.E. The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.E.; Fish, B.L.; Cohen, E.P. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr. Pharm. Des. 2003, 9, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Molteni, A.; Moulder, J.E.; Cohen, E.F.; Ward, W.F.; Fish, B.L.; Taylor, J.M.; Wolfe, L.F.; Brizio-Molteni, L.; Veno, P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int. J. Radiat. Biol. 2000, 76, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Brown, S.L.; Kolozsvary, A.; Jenrow, K.A.; Ryu, S.; Rosenblum, M.L.; Carretero, O.A. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat. Res. 2004, 161, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kolozsvary, A.; Jenrow, K.A.; Brown, S.L.; Kim, J.H. Mitigation of radiation-induced optic neuropathy in rat by ACE inhibitor ramipril: Importance of ramipril dose and treatment time. J. Neurooncol. 2007, 82, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Christie, L.A.; Lan, M.L.; Donovan, P.J.; Cotman, C.W.; Fike, J.R.; Limoli, C.L. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19150–19155. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.O.; Waldmann, H.; Fairchild, P.J. Embryonic stem cells: Overcoming the immunological barriers to cell replacement therapy. Curr. Stem Cell Res. Ther. 2009, 4, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Knoepfler, P.S. Deconstructing stem cell tumorigenicity: A roadmap to safe regenerative medicine. Stem Cells 2009, 27, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q.; Ahrlund Richter, L.; Auerbach, J.M.; Benvenisty, N.; Charo, R.A.; Chen, G.; Deng, H.K.; Goldstein, L.S.; Hudson, K.L.; Hyun, I.; et al. Ethics. The ISSCR guidelines for human embryonic stem cell research. Science 2007, 315, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Laroche, S.; Rampon, C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci. 2005, 21, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Spires, T.L.; Grote, H.E.; Varshney, N.K.; Cordery, P.M.; van Dellen, A.; Blakemore, C.; Hannan, A.J. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J. Neurosci. 2004, 24, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, N.R.; Fields, V.; Pflibsen, L.; Salvatore, M.F.; Meshul, C.K. Social enrichment attenuates nigrostriatal lesioning and reverses motor impairment in a progressive 1-methyl-2-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol. Dis. 2012, 45, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.; Ada, L.; Bernhardt, J.; McElduff, P.; Pollack, M.; Nilsson, M.; Spratt, N.J. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: A pilot non-randomized controlled trial. Disabil. Rehabil. 2014, 36, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Chang, Y.T.; Yu, L.; Chen, H.I.; Jen, C.J.; Wu, S.Y.; Lo, C.P.; Kuo, Y.M. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J. Appl. Physiol. 2008, 105, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Kannangara, T.S.; Lucero, M.J.; Gil-Mohapel, J.; Drapala, R.J.; Simpson, J.M.; Christie, B.R.; van Praag, H. Running reduces stress and enhances cell genesis in aged mice. Neurobiol. Aging 2011, 32, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796-27815. https://doi.org/10.3390/ijms161126068
Balentova S, Adamkov M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. International Journal of Molecular Sciences. 2015; 16(11):27796-27815. https://doi.org/10.3390/ijms161126068
Chicago/Turabian StyleBalentova, Sona, and Marian Adamkov. 2015. "Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review" International Journal of Molecular Sciences 16, no. 11: 27796-27815. https://doi.org/10.3390/ijms161126068




