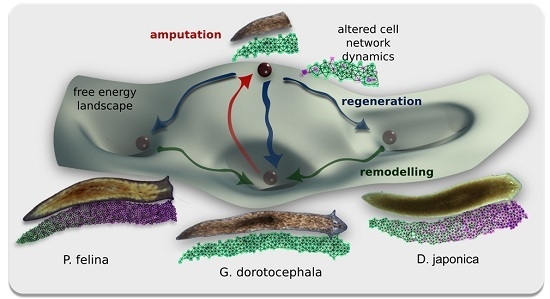
Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms
Abstract
:1. Introduction
2. Results
2.1. Octanol Treatment Induces Changes in Head Shape
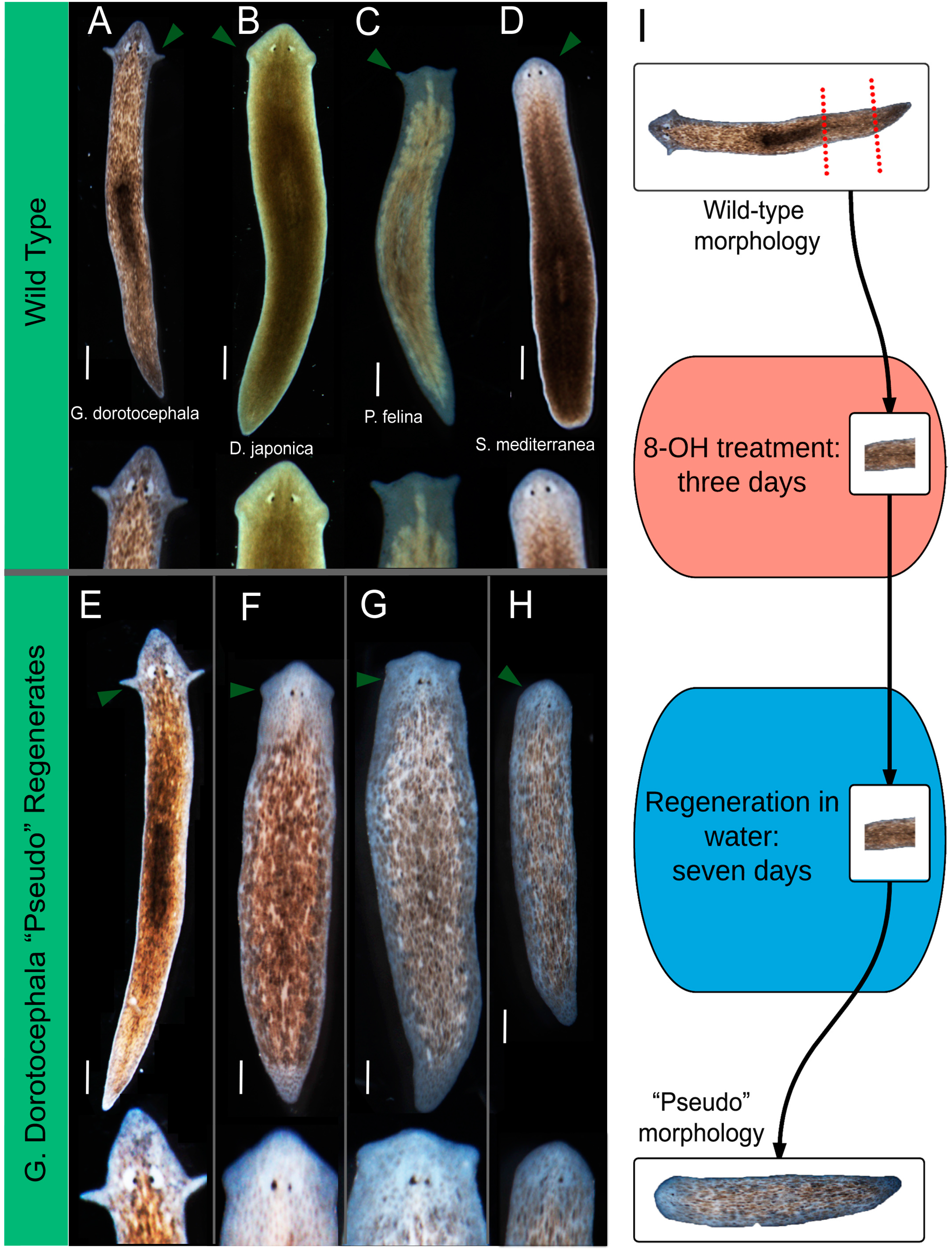
2.2. Quantitative Comparison of Induced vs. Genome-Specific Head Shapes
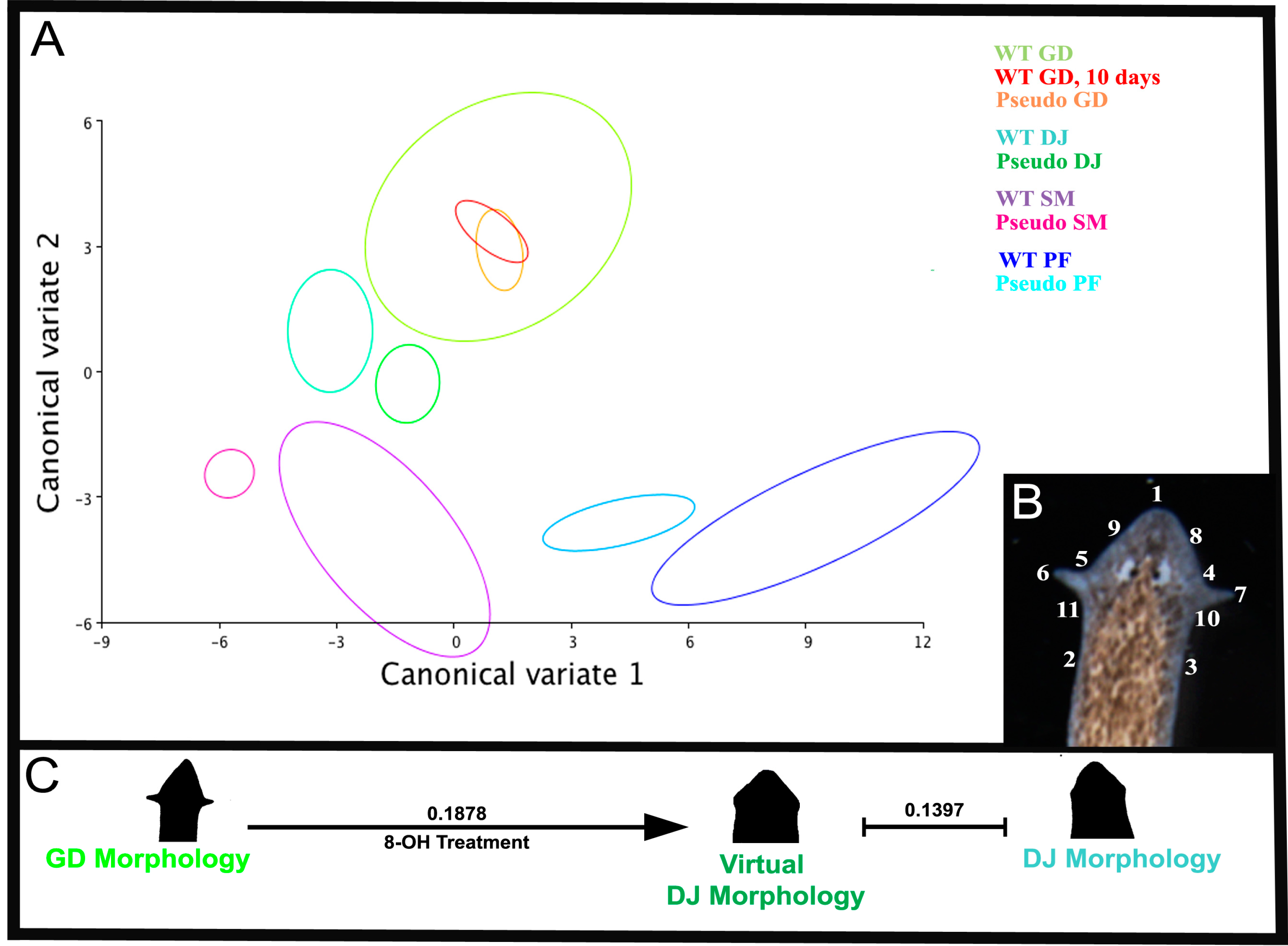
| Procrustes Distances between Morphologies | WT GD after 10 days in Water | Pseudo GD | WT GD | Pseudo DJ | WT DJ | Pseudo PF | WT PF | Pseudo SM |
|---|---|---|---|---|---|---|---|---|
| WT GD | 0.10 | 0.10 | ||||||
| Pseudo DJ | 0.14 | 0.16 | 0.19 | |||||
| WT DJ | 0.16 | 0.18 | 0.15 | 0.14 | ||||
| Pseudo PF | 0.38 | 0.38 | 0.40 | 0.27 | 0.33 | |||
| WT PF | 0.41 | 0.39 | 0.42 | 0.32 | 0.37 | 0.09 | ||
| Pseudo SM | 0.21 | 0.23 | 0.25 | 0.09 | 0.17 | 0.21 | 0.26 | |
| WT SM | 0.28 | 0.27 | 0.24 | 0.23 | 0.14 | 0.31 | 0.35 | 0.22 |
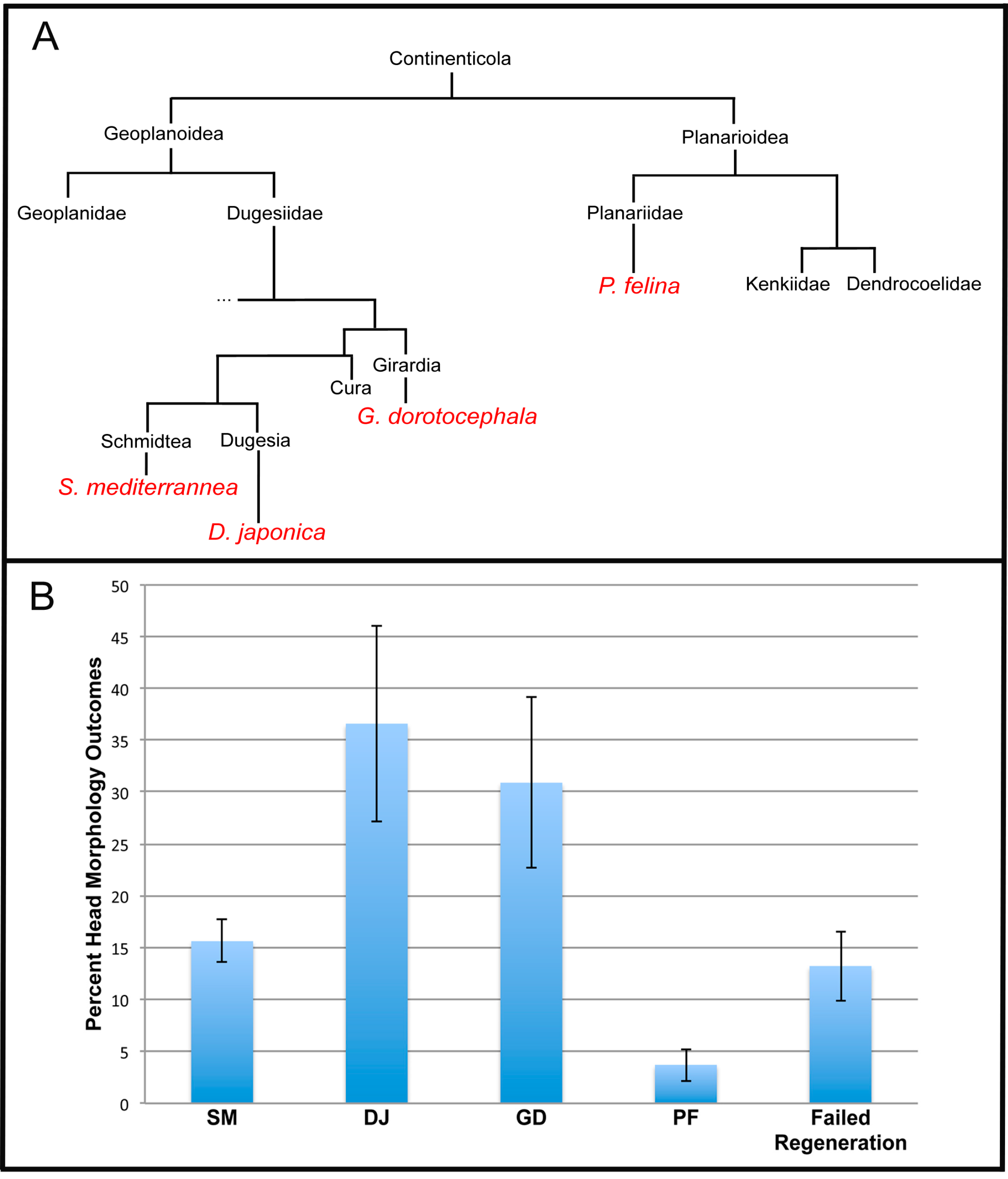
2.3. The Head Shape Changes Are Stochastic
2.4. Brain Structure of GJ-Perturbed Worms Is Also Altered to That of Other Species

2.5. Distribution of Neoblasts Is Altered to That of Other Species in GJ-Perturbed Worms
2.6. Patterns of Endogenous Relative Membrane Potential Are Altered in GJ-Perturbed Worms

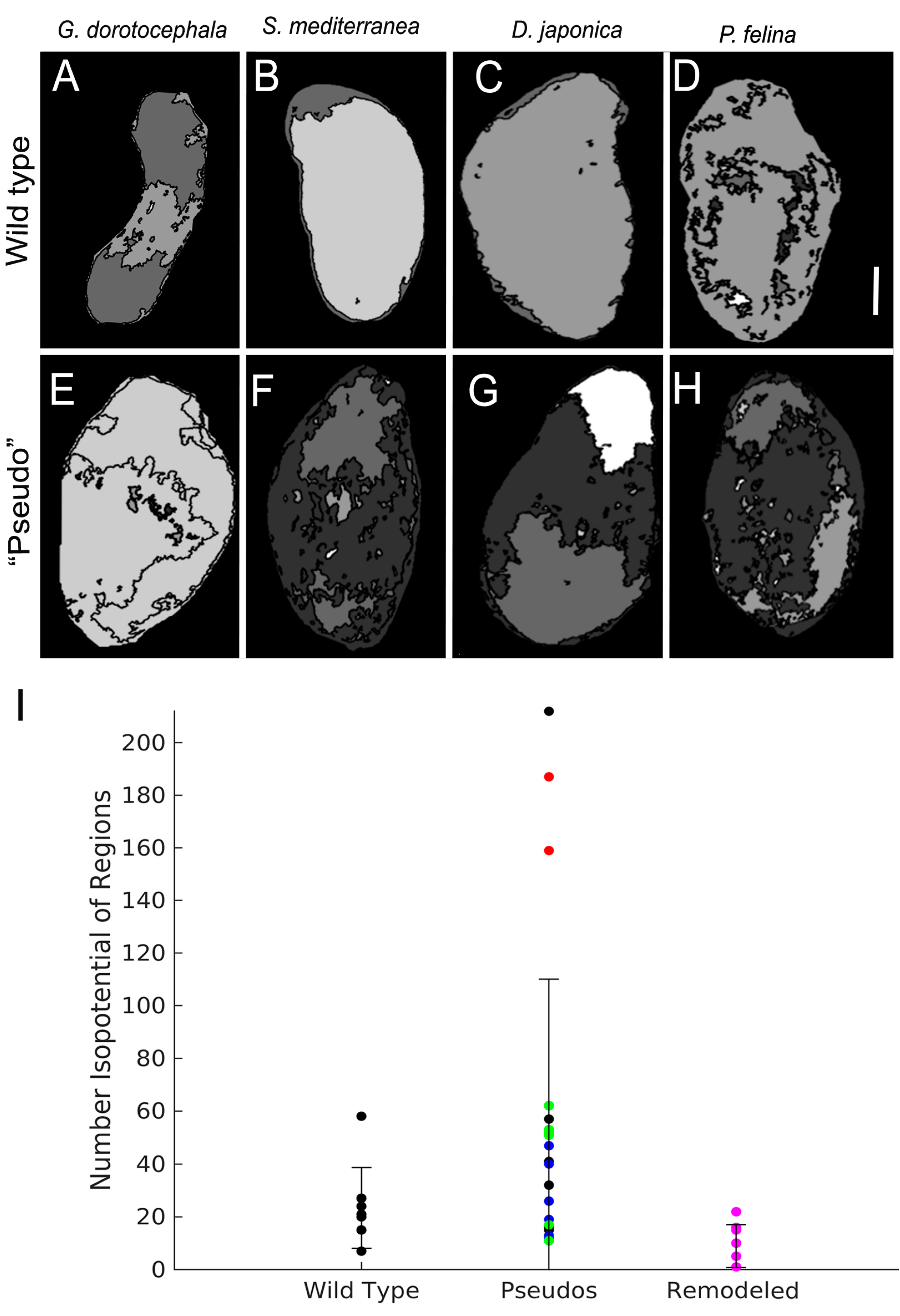
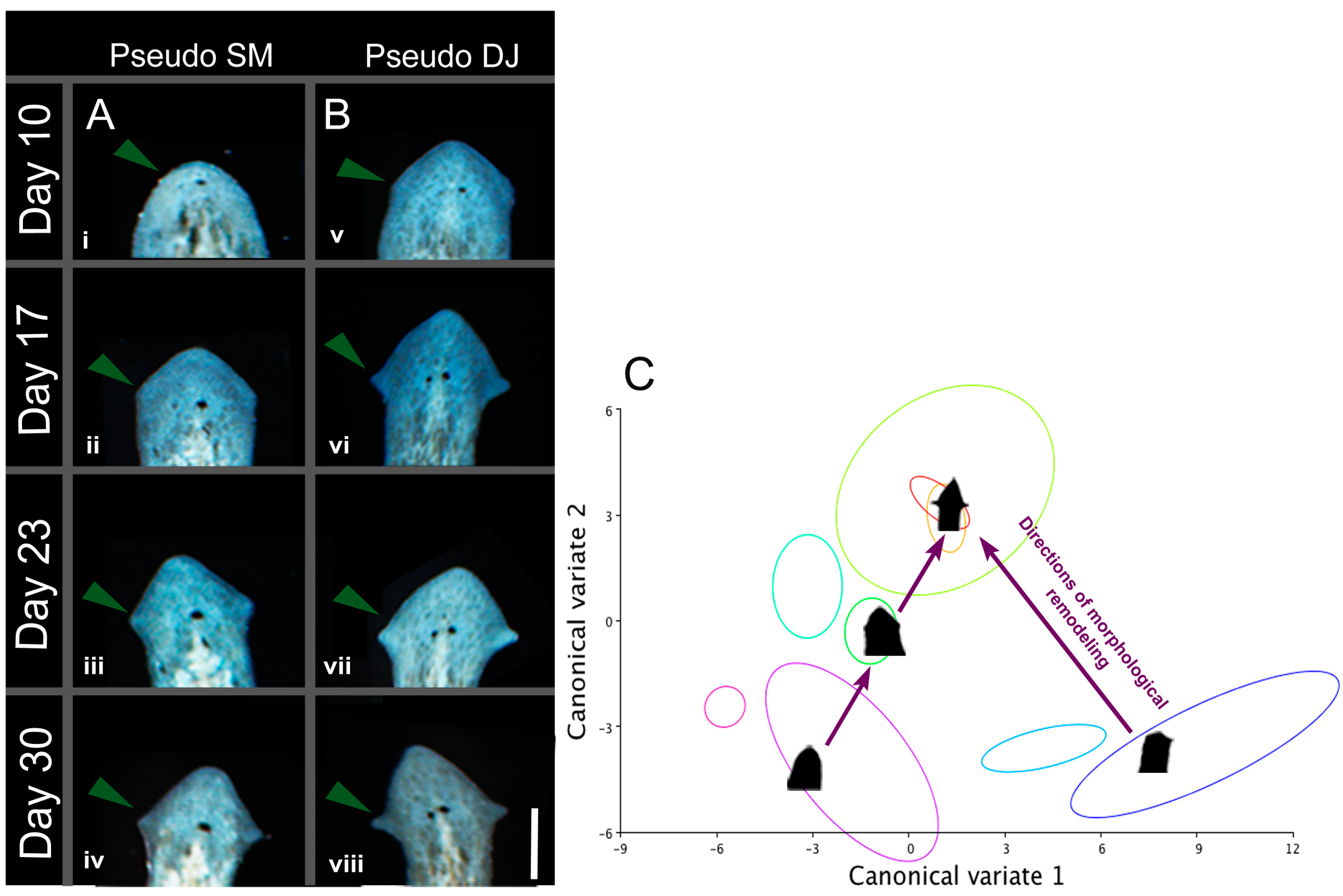
2.7. Induced Shape Changes Are Not Permanent
2.8. A Model of Planarian Head Shapes Arising from Cell Interactions
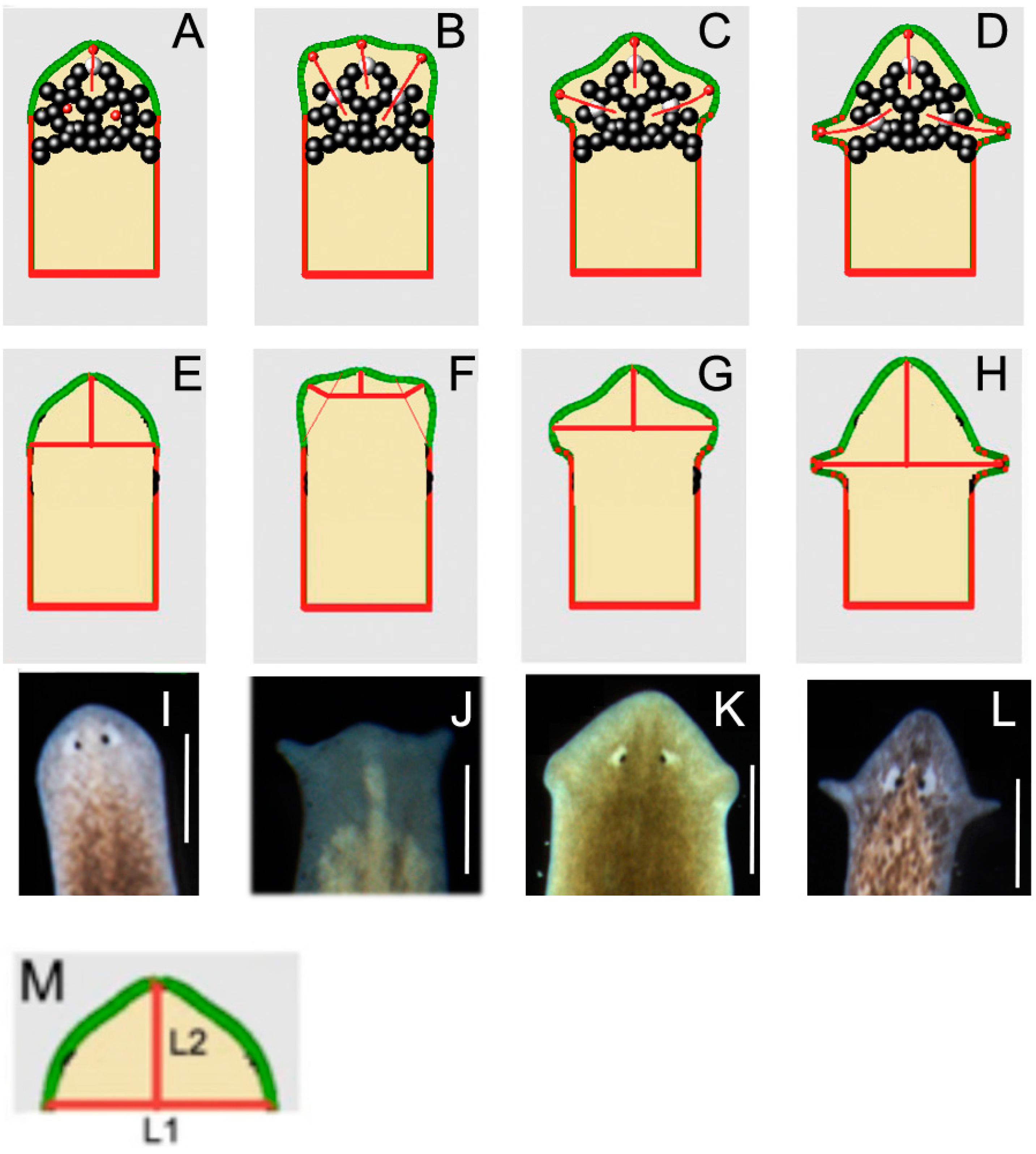
| In Silico | In Vivo | ||
|---|---|---|---|
| Worm Type | L1/L2 Ratio | Worm Type | L1/L2 Ratio |
| SM | 1.79 | SM | 1.92 |
| PF | 5.0 | PF | 7.40 |
| DJ | 2.46 | DJ | 2.11 |
| GD | 1.85 | GD | 2.06 |
3. Discussion
3.1. Perturbation of Bioelectric Networks Produces Inter-Species Morphological Change
3.2. Geometric Morphometrics Quantifies Planarian Shape Change
3.3. Morphological Change Is Not Just Skin-Deep
3.4. Bioelectric State of Tissues and Its Stable Alteration in Species-Specific Shape Change
3.5. Morphological Remodeling in 2 Phases: Head Shape Change without Traumatic Injury
3.6. Computational Modeling of Head Shape Formation
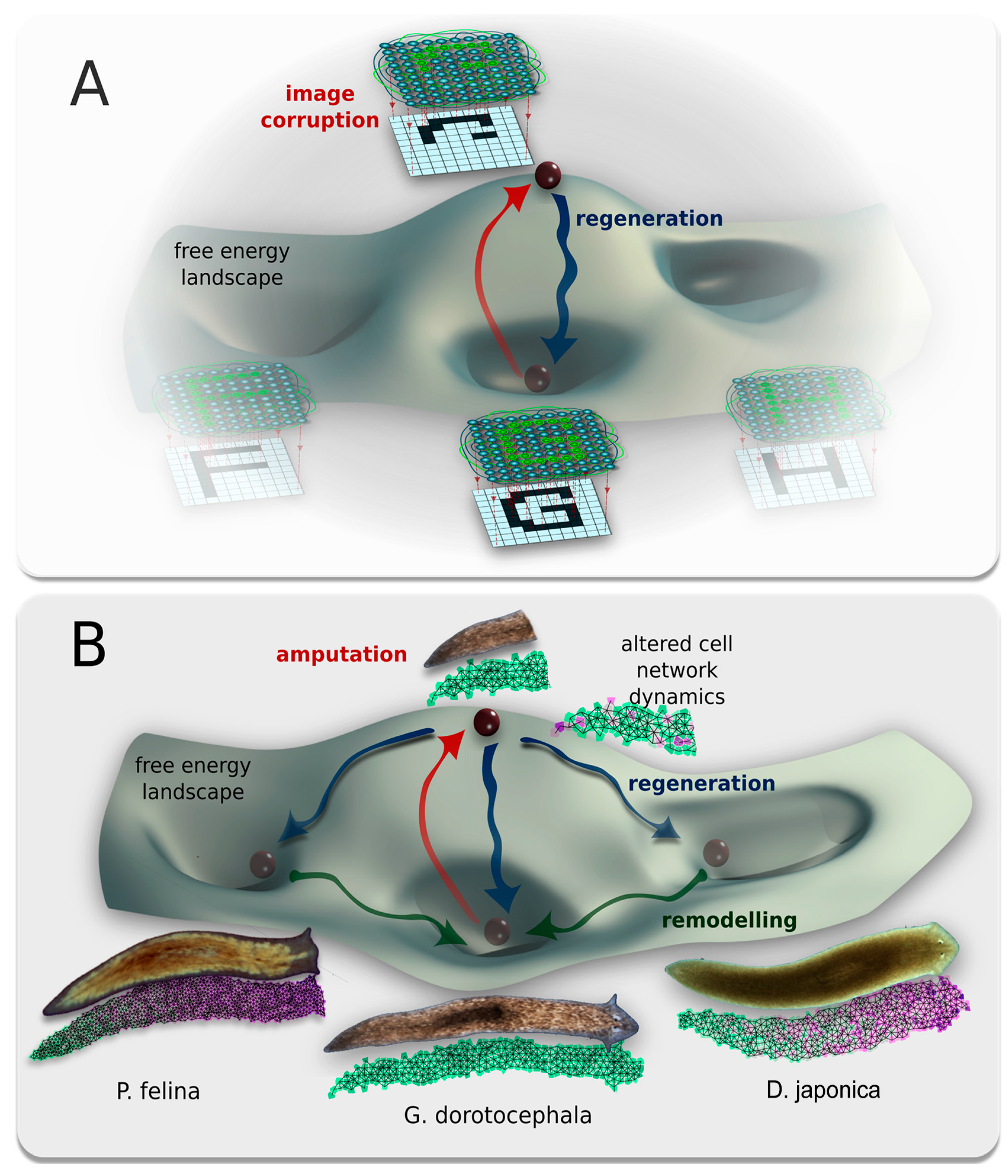
3.7. A hypothesis: Physiological Network Attractors Underlie Regions of Anatomical Morphospace
3.8. Stochastic Phenotypes and Evolutionary Implications
3.9. Limitations and Future Approaches
4. Materials and Methods
4.1. Husbandry and Pharmaceutical Treatment
4.2. Morphometrics
4.3. Visualization of Relative Membrane Potentials with DiBAC
4.4. Immunohistochemistry
4.5. Voltage Dye Image Analysis
4.6. Modeling
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mustard, J.; Levin, M. Bioelectrical Mechanisms for programming growth and form: taming physiological networks for soft body robotics. Soft Robot. 2014, 1, 169–191. [Google Scholar] [CrossRef]
- Levin, M. Morphogenetic fields in embryogenesis, regeneration, and cancer: Non-local control of complex patterning. BioSystems 2012, 109, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.A.; Müller, G.B. Epigenetic mechanisms of character origination. J. Exp. Zool. 2000, 288, 304–317. [Google Scholar] [CrossRef]
- Flueck, W.T.; Smith-Flueck, J.A.M. Intraspecific phenotypic variation in deer: The role of genetic and epigenetic processes. Anim. Prod. Sci. 2011, 51, 365–374. [Google Scholar] [CrossRef]
- Levin, M. The wisdom of the body: Future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regen. Med. 2011, 6, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Solano, M.; Bubenik, G.A.; Levin, M. A linear-encoding model explains the variability of the target morphology in regeneration. J. R. Soc. Interface 2014, 11, 20130918. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, E.R.; Balleza, E.; Benitez, M.; Espinosa-Soto, C.; Padilla-Longoria, P. Gene regulatory network models: A dynamic and integrative approach to development. SEB Exp. Biol. Ser. 2008, 61, 113–139. [Google Scholar] [PubMed]
- Davidson, E.H.; Rast, J.P.; Oliveri, P.; Ransick, A.; Calestani, C.; Yuh, C.H.; Minokawa, T.; Amore, G.; Hinman, V.; Arenas-Mena, C.; et al. A genomic regulatory network for development. Science 2002, 295, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Von Dassow, M.; Davidson, L.A. Physics and the canalization of morphogenesis: A grand challenge in organismal biology. Phys. Biol. 2011, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Beloussov, L.V. Mechanically based generative laws of morphogenesis. Phys. Biol. 2008, 5, 015009. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell. 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Reprogramming cells and tissue patterning via bioelectrical pathways: Molecular mechanisms and biomedical opportunities. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Rojas-Munoz, A.; Izpisua Belmonte, J.C. Bioelectricity and epimorphic regeneration. Bioessays 2007, 29, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Phys. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Lois, N.; Zhao, M.; McCaig, C. The spark of life: The role of electric fields in regulating cell behaviour using the eye as a model system. Ophthalmic Res. 2007, 39, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Levin, M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 2013, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays 2012, 34, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Beane, W.S.; Morokuma, J.; Adams, D.S.; Levin, M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem. Biol. 2011, 18, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Morokuma, J.; Walentek, P.; Kema, I.P.; Gu, M.B.; Ahn, J.M.; Hwang, J.S.; Gojobori, T.; Levin, M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 2010, 339, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Perathoner, S.; Daane, J.M.; Henrion, U.; Seebohm, G.; Higdon, C.W.; Johnson, S.L.; Nusslein-Volhard, C.; Harris, M.P. Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 2014, 10, e1004080. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Tseng, A.S.; Levin, M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: Sparking system-level controls in vivo. Biol. Open 2013, 2, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Beane, W.S.; Lemire, J.M.; Masi, A.; Levin, M. Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 2010, 30, 13192–13200. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Masi, A.; Levin, M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 2007, 134, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Beane, W.S.; Morokuma, J.; Lemire, J.M.; Levin, M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development 2013, 140, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Thorlin, T.; Robinson, K.R.; Nogi, T.; Mercola, M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 2002, 111, 77–89. [Google Scholar] [CrossRef]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Aw, S.; Koster, J.; Pearson, W.; Nichols, C.; Shi, N.Q.; Carneiro, K.; Levin, M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev. Biol. 2010, 346, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Morokuma, J.; Blackiston, D.; Levin, M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell. Physiol. Biochem. 2008, 21, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Nuckels, R.J.; Ng, A.; Darland, T.; Gross, J.M. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Aw, S.; Shomrat, T.; Lemire, J.M.; Levin, M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 2012, 139, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Lemire, J.M.; Pare, J.F.; Lin, G.; Chen, Y.; Levin, M. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J. Neurosci. 2015, 35, 4366–4385. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Stevenson, C.G. Regulation of cell behavior and tissue patterning by bioelectrical signals: Challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 2012, 14, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Levin, M. Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration. Anat. Rec. 2012, 295, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Anderson, G.M.; Rahman, N.; Bieck, C.; Levin, M. A novel method for inducing nerve growth via modulation of host resting potential: Gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics 2015, 12, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.; Adams, D.S.; Lemire, J.M.; Lobikin, M.; Levin, M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Model. Mech. 2011, 4, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Lobikin, M.; Chernet, B.; Lobo, D.; Levin, M. Resting potential, oncogene-induced tumorigenesis, and metastasis: The bioelectric basis of cancer in vivo. Phys. Biol. 2012, 9, 065002. [Google Scholar] [CrossRef] [PubMed]
- Chernet, B.T.; Levin, M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget 2014, 5, 3287–3306. [Google Scholar] [CrossRef] [PubMed]
- Chernet, B.T.; Fields, C.; Levin, M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front. Physiol. 2015, 5, 519. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 1889–1908. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2008, 3, e3737. [Google Scholar] [CrossRef] [PubMed]
- Chernet, B.; Levin, M. Endogenous voltage potentials and the microenvironment: Bioelectric signals that reveal, induce and normalize cancer. J. Clin. Exp. Oncol. 2013. [Google Scholar] [CrossRef]
- Chernet, B.T.; Levin, M. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis. Model. Mech. 2013, 6, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Pereda, A.E.; Curti, S.; Hoge, G.; Cachope, R.; Flores, C.E.; Rash, J.E. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim. Biophys. Acta 2013, 1828, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035. [Google Scholar] [PubMed]
- Palacios-Prado, N.; Bukauskas, F.F. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Bukauskas, F.F. Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis. Biochim. Biophys. Acta 2012, 1818, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 2004, 1662, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.A.; Kriegstein, A.R. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008, 31, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Levin, M. Gap junctions provide new links in left-right patterning. Cell 2007, 129, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 2007, 94, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Shih, M.F.; Lai, J.S.; Yang, H.T.; Turner, G.C.; Chen, L.; Chiang, A.S. Heterotypic gap junctions between two neurons in the Drosophila brain are critical for memory. Curr. Biol. 2011, 21, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Belousov, A.B. Deletion of neuronal gap junction protein connexin 36 impairs hippocampal LTP. Neurosci. Lett. 2011, 502, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Fuchs, E.C.; Jaschonek, H.; Bannerman, D.M.; Monyer, H. Gap Junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J. Neurosci. 2011, 31, 6542–6552. [Google Scholar] [CrossRef] [PubMed]
- Cebria, F. Regenerating the central nervous system: How easy for planarians! Dev. Genes Evol. 2007, 217, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Sanchez Alvarado, A. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 2004, 20, 725–757. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Cebria, F.; Bartscherer, K. The planarian flatworm: An in vivo model for stem cell biology and nervous system regeneration. Dis. Model. Mech. 2011, 4, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Newmark, P.A.; Sanchez Alvarado, A. Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev. Dyn. 2003, 226, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Aboobaker, A.A. Planarian stem cells: A simple paradigm for regeneration. Trends Cell Biol. 2011, 21, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Salo, E.; Abril, J.F.; Adell, T.; Cebria, F.; Eckelt, K.; Fernandez-Taboada, E.; Handberg-Thorsager, M.; Iglesias, M.; Molina, M.D.; Rodriguez-Esteban, G. Planarian regeneration: Achievements and future directions after 20 years of research. Int. J. Dev. Biol. 2009, 53, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Newmark, P.A. Opening a new can of worms: A large-scale RNAi screen in planarians. Dev. Cell 2005, 8, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Beane, W.S.; Levin, M. Modeling planarian regeneration: A primer for reverse-engineering the worm. PLoS Comput. Biol. 2012, 8, e1002481. [Google Scholar] [CrossRef] [PubMed]
- Mege, R.M.; Goudou, D.; Giaume, C.; Nicolet, M.; Rieger, F. Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhes. Commun. 1994, 2, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.L.; Petrino, T.R.; Wallace, R.A. Functional heterologous gap junctions in Fundulus ovarian follicles maintain meiotic arrest and permit hydration during oocyte maturation. Dev. Biol. 1993, 160, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bohrmann, J.; Haas-Assenbaum, A. Gap junctions in ovarian follicles of Drosophila melanogaster: Inhibition and promotion of dye-coupling between oocyte and follicle cells. Cell Tissue Res. 1993, 273, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.; Kempf, C.; Weingart, R. Electrical coupling between cells of the insect Aedes albopictus. J. Physiol. 1992, 448, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Santella, L.; Tosti, E. Gap-junctional permeability in early and cleavage-arrested ascidian embryos. Development 1991, 112, 153–160. [Google Scholar] [PubMed]
- Adler, E.L.; Woodruff, R.I. Varied effects of 1-octanol on gap junctional communication between ovarian epithelial cells and oocytes of Oncopeltus fasciatus, Hyalophora cecropia, and Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2000, 43, 22–32. [Google Scholar] [CrossRef]
- Weingart, R.; Bukauskas, F.F. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 1998, 435, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Levin, M. Smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development 2007, 134, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Nogi, T.; Levin, M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev. Biol. 2005, 287, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Soroker, V.; Harel, M.; Nakache, J.; Dunkelblum, E. Quantitative GC analysis of secondary alcohol pheromones: Determination of release rate of red palm weevil, Rhynchophorus ferrugineus, pheromone from lures. J. Chem. Ecol. 2002, 28, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Rink, J.C.; Riedel-Kruse, I.H.; Friedrich, B.M. Shape mode analysis exposes movement patterns in biology: Flagella and flatworms as case studies. PLoS ONE 2014, 9, e113083. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.; Sheets, H.D. A practical introduction to landmark-based geometric morphometrics. Quant. Methods Paleobiol. 2010, 16, 168–188. [Google Scholar]
- Riutort, M.; Field, K.G.; Raff, R.A.; Baguña, J. 18S rRNA sequences and phylogeny of Platyhelminthes. Biochem. System. Ecol. 1993, 21, 71–77. [Google Scholar] [CrossRef]
- Munoz, R.; Yarza, P.; Ludwig, W.; Euzéby, J.; Amann, R.; Schleifer, K.-H.; Glöckner, F.O.; Rosselló-Móra, R. Release LTPs104 of the all-species living tree. Syst. Appl. Microbiol. 2011, 34, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Salvetti, A.; Lena, A.; Batistoni, R.; Deri, P.; Pugliesi, C.; Loreti, E.; Gremigni, V. DjPiwi-1, a member of the PAZ-Piwi gene family, defines a subpopulation of planarian stem cells. Dev. Genes Evol. 2006, 216, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Newmark, P.A.; Sanchez Alvarado, A. Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev. Biol. 2000, 220, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Nicolas, C.L.; Adams, D.S.; Levin, M. Live Imaging of Planarian Membrane Potential Using DiBAC4(3). CSH Protoc. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Vu, A.T.; Naselaris, T.; Benjamini, Y.; Yu, B.; Gallant, J.L. Reconstructing visual experiences from brain activity evoked by natural movies. Curr. Biol. 2011, 21, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.; Rinzel, J. Model for synchronization of pancreatic beta-cells by gap junction coupling. Biophys. J. 1991, 59, 547–559. [Google Scholar] [CrossRef]
- Adams, D.S.; Levin, M. Endogenous voltage gradients as mediators of cell-cell communication: Strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013, 352, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Levin, M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, 2012, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Levin, M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb. Protoc. 2012, 2012, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Doursat, R.; Sayama, H.; Michel, O. Morphogenetic engineering: Reconciling self-organization and architecture. Morphog. Eng. 2012, 1–24. [Google Scholar]
- Doursat, R.; Sayama, H.; Michel, O. A review of morphogenetic engineering. Nat. Comput. 2013, 12, 517–535. [Google Scholar] [CrossRef]
- Thorne, B.C.; Bailey, A.M.; DeSimone, D.W.; Peirce, S.M. Agent-based modeling of multicell morphogenic processes during development. Birth Defects Res. C Embryo Today 2007, 81, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Slack, J. Establishment of spatial pattern. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.J.; Meinhardt, H. Biological Pattern-Formation—From basic mechanisms to complex structures. Rev. Mod. Phys. 1994, 66, 1481–1507. [Google Scholar] [CrossRef]
- Forsthoefel, D.J.; Newmark, P.A. Emerging patterns in planarian regeneration. Curr. Opin. Genet. Dev. 2009, 19, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Rouhana, L.; Agata, K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Dev. Growth Differ. 2010, 52, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, H. Beta-catenin and axis formation in planarians. Bioessays 2009, 31, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Stückemann, T.; Beirán Amigo, M.; Rink, J.C.; Jülicher, F.; Friedrich, B.M. Scaling and regeneration of self-organized patterns. Phys. Rev. Lett. 2015, 114, 138101. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Levin, M. Inferring regulatory networks from experimental morphological phenotypes: A computational method reverse-engineers planarian regeneration. PLoS Comput. Biol. 2015, 11, e1004295. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Davidson, L.A. The interplay between cell signalling and mechanics in developmental processes. Nat. Rev. Genet. 2013, 14, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Beloussov, L.V. Morphogenesis can be driven by properly parametrised mechanical feedback. Eur. Phys. J. E Soft Matter 2013, 36, 132. [Google Scholar] [CrossRef] [PubMed]
- Lander, A.D. Pattern, growth, and control. Cell 2011, 144, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Kotini, M.; Mayor, R. Connexins in migration during development and cancer. Dev. Biol. 2015, 401, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Yamanaka, H.; Kondo, S. Pigment pattern formation by contact-dependent depolarization. Science 2012, 335, 677. [Google Scholar] [CrossRef] [PubMed]
- Faniku, C.; Wright, C.; Martin, P. Connexins and pannexins in the integumentary system: The skin and appendages. Cell. Mol. Life Sci. 2015, 72, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Blackiston, D.J.; Rea, A.C.; Dore, T.M.; Levin, M. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. Int. J. Dev. Biol. 2014, 58, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.P.; Diez, T. Role for gap junctional intercellular communications in wound repair. Wound Repair Regen. 2003, 11, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Protas, M.; Grant, B.R.; Grant, P.R.; Tabin, C.J. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 2004, 305, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [PubMed]
- Garfinkel, M.D.; Sollars, V.E.; Lu, X.; Ruden, D.M. Multigenerational selection and detection of altered histone acetylation and methylation patterns. In Epigenetics Protocols; Springer: Berlin, Germany, 2004; pp. 151–168. [Google Scholar]
- Shoemaker, C.M.; Crews, D. Analyzing the coordinated gene network underlying temperature-dependent sex determination in reptiles. Semin. Cell Dev. Biol. 2009, 20, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Kuo, W.P.; Hartmann, C.; Grant, B.R.; Grant, P.R.; Tabin, C.J. The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 2006, 442, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Larimore, W.E. Canonical variate analysis in identification, filtering, and adaptive control. In Proceedings of the 29th IEEE Conference on Decision and Control, Honolulu, HI, USA, 5–7 December 1990; pp. 596–604.
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Mondia, J.P.; Levin, M.; Omenetto, F.G.; Orendorff, R.D.; Branch, M.R.; Adams, D.S. Long-distance signals are required for morphogenesis of the regenerating Xenopus tadpole tail, as shown by femtosecond-laser ablation. PLoS ONE 2011, 6, e24953. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, S.V.; Ghosh, J. On Hebbian-like adaptation in heart muscle: A proposal for “cardiac memory”. Biol. Cybern. 1997, 76, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, J.; Sachdeva, G.; Chakravarthy, V.S.; Radhakrishnan, S. Interpreting voltage-sensitivity of gap junctions as a mechanism of cardiac memory. Math. Biosci. 2008, 212, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Mehta, A. Learning theories reveal loss of pancreatic electrical connectivity in diabetes as an adaptive response. PLoS ONE 2013, 8, e70366. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.D.; Hulme, S.R.; Abraham, W.C. Purinergic receptor- and gap junction-mediated intercellular signalling as a mechanism of heterosynaptic metaplasticity. Neurobiol. Learn. Mem. 2013, 105, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Levin, M. Bioelectric memory: Modeling resting potential bistability in amphibian embryos and mammalian cells. Theor. Biol. Med. Model. 2015, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.E.; Nannapaneni, S.; Davidson, K.G.; Yasumura, T.; Bennett, M.V.; Rash, J.E.; Pereda, A.E. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, E573–E582. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Vargova, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Sykova, E.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef] [PubMed]
- Bissiere, S.; Zelikowsky, M.; Ponnusamy, R.; Jacobs, N.S.; Blair, H.T.; Fanselow, M.S. Electrical synapses control hippocampal contributions to fear learning and memory. Science 2011, 331, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wolszon, L.R.; Gao, W.Q.; Passani, M.B.; Macagno, E.R. Growth cone “collapse” in vivo: Are inhibitory interactions mediated by gap junctions? J. Neurosci. 1994, 14, 999–1010. [Google Scholar] [PubMed]
- Fukumoto, T.; Kema, I.P.; Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005, 15, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Vaney, D.I.; Nelson, J.C.; Pow, D.V. Neurotransmitter coupling through gap junctions in the retina. J. Neurosci. 1998, 18, 10594–10602. [Google Scholar] [PubMed]
- Chaturvedi, R.; Reddig, K.; Li, H.S. Long-distance mechanism of neurotransmitter recycling mediated by glial network facilitates visual function in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; de Pitta, M.; Volman, V.; Berry, H.; Ben-Jacob, E. Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks. PLoS Comput. Biol. 2010, 6, e1000909. [Google Scholar] [CrossRef] [PubMed]
- Ruckh, T.T.; Clark, H.A. Implantable nanosensors: Toward continuous physiologic monitoring. Anal. Chem. 2014, 86, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Ruckh, T.T.; Mehta, A.A.; Dubach, J.M.; Clark, H.A. Polymer-free optode nanosensors for dynamic, reversible, and ratiometric sodium imaging in the physiological range. Sci. Rep. 2013, 28, 3366. [Google Scholar] [CrossRef] [PubMed]
- Cash, K.J.; Clark, H.A. Phosphorescent nanosensors for in vivo tracking of histamine levels. Anal. Chem. 2013, 85, 6312–6318. [Google Scholar] [CrossRef] [PubMed]
- Naselaris, T.; Prenger, R.J.; Kay, K.N.; Oliver, M.; Gallant, J.L. Bayesian reconstruction of natural images from human brain activity. Neuron 2009, 63, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Morrie, R.D.; Adams, D.S. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev. Dyn. 2011, 240, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J. Physiol. 2014, 592, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Farinella-Ferruzza, N. The transformation of a tail into limb after xenoplastic transplantation. Experientia 1956, 12, 304–305. [Google Scholar] [CrossRef]
- Holtfreter, J. Transformation of a Tail into a Limb or Gill-Like Structures. J. Exp. Zool. 1955, 129, 623–648. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Shestopalov, V.I. The role of pannexin hemichannels in inflammation and regeneration. Front. Physiol. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Pera, M.F.; Pebay, A. Role of gap junctions in embryonic and somatic stem cells. Stem Cell Rev. 2008, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Joshi, S.D.; Kim, H.Y.; von Dassow, M.; Zhang, L.; Zhou, J. Emergent morphogenesis: Elastic mechanics of a self-deforming tissue. J. Biomech. 2010, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.W.; Schier, A.F. Morphogen gradients: From generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011, 27, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.J.; Scott, M.P. Incredible journey: How do developmental signals travel through tissue? Genes Dev. 2004, 18, 2985–2997. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Levin, M.; Sengupta, B.; Pezzulo, G. Knowing one’s place: A free-energy approach to pattern regulation. J. R. Soc. Interface 2015, 12, 20141383. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Rolls, E.T.; Romo, R. Stochastic dynamics as a principle of brain function. Prog. Neurobiol. 2009, 88, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Molnar, B.; Ercsey-Ravasz, M. Asymmetric continuous-time neural networks without local traps for solving constraint satisfaction problems. PLoS ONE 2013, 8, e73400. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, L.; Hu, L.; Wang, X.; Wang, E.; Wang, J. Nonequilibrium landscape theory of neural networks. Proc. Natl. Acad. Sci. USA 2013, 110, E4185–E4194. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Sengupta, B.; Auletta, G. Cognitive dynamics: From attractors to active inference. Proc. IEEE 2014, 102, 427–445. [Google Scholar] [CrossRef]
- Sengupta, B.; Stemmler, M.B.; Friston, K.J. Information and efficiency in the nervous system—A synthesis. PLoS Comput. Biol. 2013, 9, e1003157. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Schwartenbeck, P.; Fitzgerald, T.; Moutoussis, M.; Behrens, T.; Dolan, R.J. The anatomy of choice: Active inference and agency. Front. Hum. Neurosci. 2013, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Active inference and free energy. Behav. Brain Sci. 2013, 36, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Samothrakis, S.; Montague, R. Active inference and agency: Optimal control without cost functions. Biol. Cybern. 2012, 106, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Ao, P. Free energy, value, and attractors. Comput. Math. Methods Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Daunizeau, J.; Kilner, J.; Kiebel, S.J. Action and behavior: A free-energy formulation. Biol. Cybern. 2010, 102, 227–260. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Curti, S.; Hoge, G.; Nagy, J.I.; Pereda, A.E. Electrical transmission between mammalian neurons is supported by a small fraction of gap junction channels. J. Membr. Biol. 2012, 245, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A., Jr.; Furlan, F.A. Astrocytes and human cognition: Modeling information integration and modulation of neuronal activity. Prog. Neurobiol. 2010, 92, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R. The spirit of D’arcy Thompson dwells in empirical morphospace. Math. Biosci. 1997, 142, 13–30. [Google Scholar] [CrossRef]
- Prill, R.J.; Iglesias, P.A.; Levchenko, A. Dynamic properties of network motifs contribute to biological network organization. PLoS Biol. 2005, 3, e343. [Google Scholar] [CrossRef] [PubMed]
- Couzin, I.D. Collective cognition in animal groups. Trends Cogn. Sci. 2009, 13, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Minati, G.; Pessa, E. Emergence in Complex, Cognitive, Social, and Biological Systems; Kluwer Academic/Plenum: New York, NY, USA, 2002; p. 394. [Google Scholar]
- Kelso, J.S. Dynamic Patterns: The self-Organization of Brain and Behavior; MIT Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Hiscock, T.W.; Megason, S.G. Mathematically guided approaches to distinguish models of periodic patterning. Development 2015, 142, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.P. Intermanual coordination: From behavioural principles to neural-network interactions. Nat. Rev. Neurosci. 2002, 3, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Amit, D.J. The Hebbian Paradigm Reintegrated—Local Reverberations as Internal Representations. Behav. Brain Sci. 1995, 18, 617–626. [Google Scholar] [CrossRef]
- Levin, M.; Pezzulo, G. Re-Membering the body: Applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Annu. Rev. Biomed. Eng. 2015, 17. [Google Scholar]
- Loebrich, S.; Nedivi, E. The function of activity-regulated genes in the nervous system. Physiol. Rev. 2009, 89, 1079–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Levin, M. Particle tracking model of electrophoretic morphogen movement reveals stochastic dynamics of embryonic gradient. Dev. Dyn. 2009, 238, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Esser, A.T.; Smith, K.C.; Weaver, J.C.; Levin, M. Mathematical model of morphogen electrophoresis through gap junctions. Dev. Dyn. 2006, 235, 2144–2159. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, E.L.; van Eldik, L.J. Interaction of calmodulin and other calcium-modulated proteins with gap junctions. Methods Enzymol. 1987, 139, 445–454. [Google Scholar] [PubMed]
- Cooper, M.S.; Miller, J.P.; Fraser, S.E. Electrophoretic repatterning of charged cytoplasmic molecules within tissues coupled by gap junctions by externally applied electric fields. Dev. Biol. 1989, 132, 179–188. [Google Scholar] [CrossRef]
- Okamura, Y.; Dixon, J.E. Voltage-sensing phosphatase: Its molecular relationship with PTEN. Physiology 2011, 26, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Carneiro, K.; Lemire, J.M.; Levin, M. HDAC activity is required during Xenopus tail regeneration. PLoS ONE 2011, 6, e26382. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.S.; Steele, V.E. The mechanism of anterior-posterior polarity control in planarians. Differentiation 1978, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Decrock, E.; Krysko, D.V.; Vinken, M.; Kaczmarek, A.; Crispino, G.; Bol, M.; Wang, N.; de Bock, M.; de Vuyst, E.; Naus, C.C.; et al. Transfer of IP(3) through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ. 2011, 19, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Suadicani, S.O.; Flores, C.E.; Urban-Maldonado, M.; Beelitz, M.; Scemes, E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia 2004, 48, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Tosenberger, A.; Bessonov, N.; Levin, M.; Reinberg, N.; Volpert, V.; Morozova, N. A conceptual model of morphogenesis and regeneration. Acta Biotheor. 2015, 63, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bessonov, N.; Levin, M.; Morozova, N.; Reinberg, N.; Tosenberger, A.; Volpert, V. On a model of pattern regeneration based on cell memory. PLoS ONE 2015, 10, e0118091. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dexter, J.P.; Tamme, M.B.; Lind, C.H.; Collins, E.M. On-chip immobilization of planarians for in vivo imaging. Sci. Rep. 2014, 4, 6388. [Google Scholar] [CrossRef] [PubMed]
- Beane, W.S.; Tseng, A.S.; Morokuma, J.; Lemire, J.M.; Levin, M. Inhibition of planar cell polarity extends neural growth during regeneration, homeostasis, and development. Stem Cells Dev. 2012, 21, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- MATLAB and Image Processing Toolbox; Release 2012b; Software by The Mathworks, Inc.: Natick, MA, USA, 2012.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmons-Bell, M.; Durant, F.; Hammelman, J.; Bessonov, N.; Volpert, V.; Morokuma, J.; Pinet, K.; Adams, D.S.; Pietak, A.; Lobo, D.; et al. Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms. Int. J. Mol. Sci. 2015, 16, 27865-27896. https://doi.org/10.3390/ijms161126065
Emmons-Bell M, Durant F, Hammelman J, Bessonov N, Volpert V, Morokuma J, Pinet K, Adams DS, Pietak A, Lobo D, et al. Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms. International Journal of Molecular Sciences. 2015; 16(11):27865-27896. https://doi.org/10.3390/ijms161126065
Chicago/Turabian StyleEmmons-Bell, Maya, Fallon Durant, Jennifer Hammelman, Nicholas Bessonov, Vitaly Volpert, Junji Morokuma, Kaylinnette Pinet, Dany S. Adams, Alexis Pietak, Daniel Lobo, and et al. 2015. "Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms" International Journal of Molecular Sciences 16, no. 11: 27865-27896. https://doi.org/10.3390/ijms161126065
APA StyleEmmons-Bell, M., Durant, F., Hammelman, J., Bessonov, N., Volpert, V., Morokuma, J., Pinet, K., Adams, D. S., Pietak, A., Lobo, D., & Levin, M. (2015). Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms. International Journal of Molecular Sciences, 16(11), 27865-27896. https://doi.org/10.3390/ijms161126065