Age-Dependent Changes in the Inflammatory Nociceptive Behavior of Mice
Abstract
:1. Introduction
2. Results
2.1. Old Mice Show Reduced Nociceptive Behavior in Inflammatory Models
 ) (n = 8 mice/group) after injection of formalin (5%, 20 µL) into the hind paw. Formalin was injected at time “0”, and the time spent licking the injected paw was measured at 5 min intervals for 45 min; (B) Statistical analysis of total licking time, phase I (0–10 min) and phase II (11–45 min) between young (black column) and old (grey column) mice. *** p < 0.001, significant mean difference between young and old mice concerning the total licking time and the licking time in the second phase of the formalin test, respectively.
) (n = 8 mice/group) after injection of formalin (5%, 20 µL) into the hind paw. Formalin was injected at time “0”, and the time spent licking the injected paw was measured at 5 min intervals for 45 min; (B) Statistical analysis of total licking time, phase I (0–10 min) and phase II (11–45 min) between young (black column) and old (grey column) mice. *** p < 0.001, significant mean difference between young and old mice concerning the total licking time and the licking time in the second phase of the formalin test, respectively.
 ) (n = 8 mice/group) after injection of formalin (5%, 20 µL) into the hind paw. Formalin was injected at time “0”, and the time spent licking the injected paw was measured at 5 min intervals for 45 min; (B) Statistical analysis of total licking time, phase I (0–10 min) and phase II (11–45 min) between young (black column) and old (grey column) mice. *** p < 0.001, significant mean difference between young and old mice concerning the total licking time and the licking time in the second phase of the formalin test, respectively.
) (n = 8 mice/group) after injection of formalin (5%, 20 µL) into the hind paw. Formalin was injected at time “0”, and the time spent licking the injected paw was measured at 5 min intervals for 45 min; (B) Statistical analysis of total licking time, phase I (0–10 min) and phase II (11–45 min) between young (black column) and old (grey column) mice. *** p < 0.001, significant mean difference between young and old mice concerning the total licking time and the licking time in the second phase of the formalin test, respectively.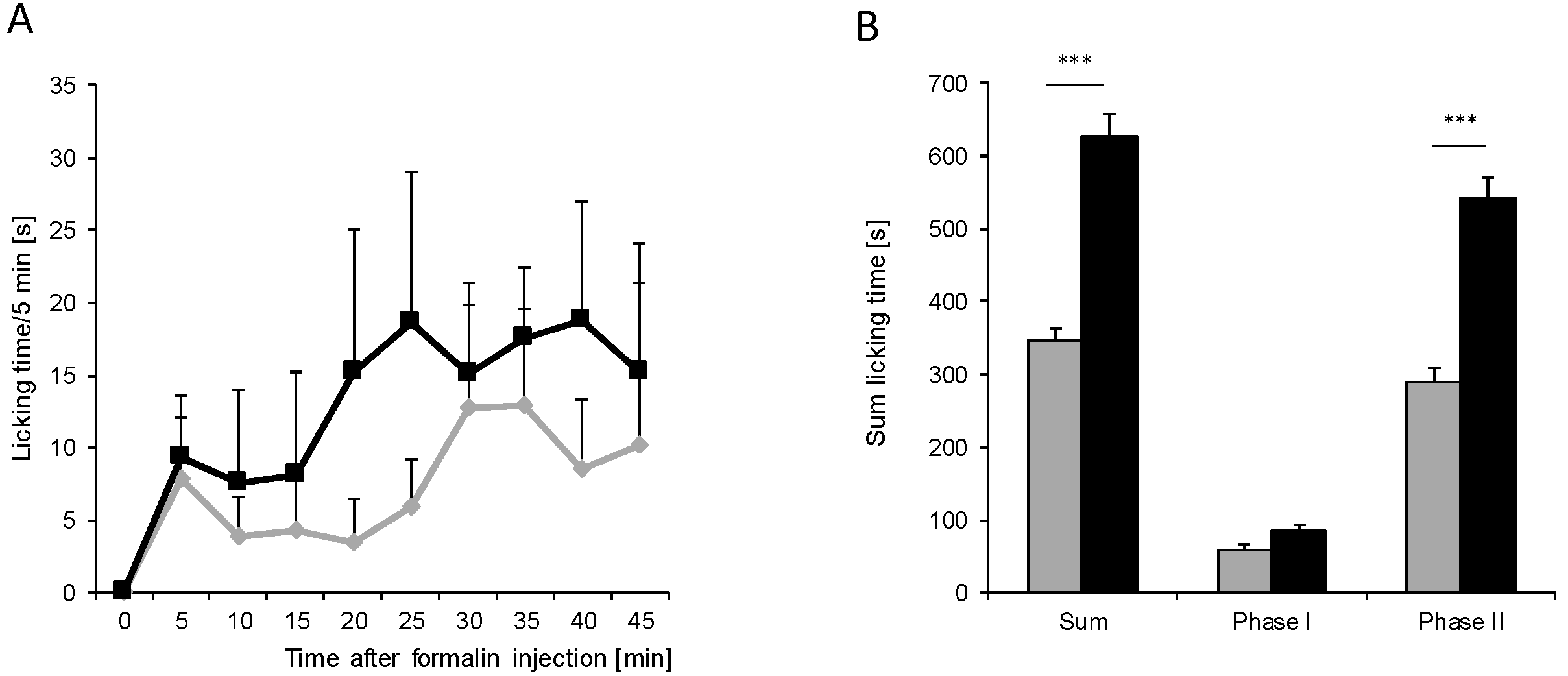
 ) after injection of 10 mg/mL (20 µL) zymosan A into a hind paw. The diagram shows the delta paw withdrawal latencies (ΔPWL) in response to mechanical stimulation, as assessed with a Dynamic Plantar Aesthesiometer; (B) Comparison of the area under the paw withdrawal latency versus time curve between young (black column) and old (grey column) mice 0 to 8 h after zymosan A injection. (n = 6 mice/group).
) after injection of 10 mg/mL (20 µL) zymosan A into a hind paw. The diagram shows the delta paw withdrawal latencies (ΔPWL) in response to mechanical stimulation, as assessed with a Dynamic Plantar Aesthesiometer; (B) Comparison of the area under the paw withdrawal latency versus time curve between young (black column) and old (grey column) mice 0 to 8 h after zymosan A injection. (n = 6 mice/group).
 ) after injection of 10 mg/mL (20 µL) zymosan A into a hind paw. The diagram shows the delta paw withdrawal latencies (ΔPWL) in response to mechanical stimulation, as assessed with a Dynamic Plantar Aesthesiometer; (B) Comparison of the area under the paw withdrawal latency versus time curve between young (black column) and old (grey column) mice 0 to 8 h after zymosan A injection. (n = 6 mice/group).
) after injection of 10 mg/mL (20 µL) zymosan A into a hind paw. The diagram shows the delta paw withdrawal latencies (ΔPWL) in response to mechanical stimulation, as assessed with a Dynamic Plantar Aesthesiometer; (B) Comparison of the area under the paw withdrawal latency versus time curve between young (black column) and old (grey column) mice 0 to 8 h after zymosan A injection. (n = 6 mice/group).
| Test/Age | Old | Young |
|---|---|---|
| Hargreaves paw withdrawal latencies (PWL) [s] ± SD | 11.5 ± 3.4 (n = 8) | 10.5 ± 2.8 (n = 6) |
| Dynamic Plantar PWL [s] ± SD | 8.2 ± 1.3 (n = 8) | 8.2 ± 0.6 (n = 6) |
| Hot Plate PWL [s] ± SD | 21 ± 2.7 (n = 8) | 18.2 ± 3.8 (n = 4) |
| Acetone Reaction time/2 min [s] ± SD | 2.9 ± 2.7 (n = 8) | 2.1 ± 1.9 (n = 6) |
2.2. Effect of Age on Central and Peripheral TRP Channels
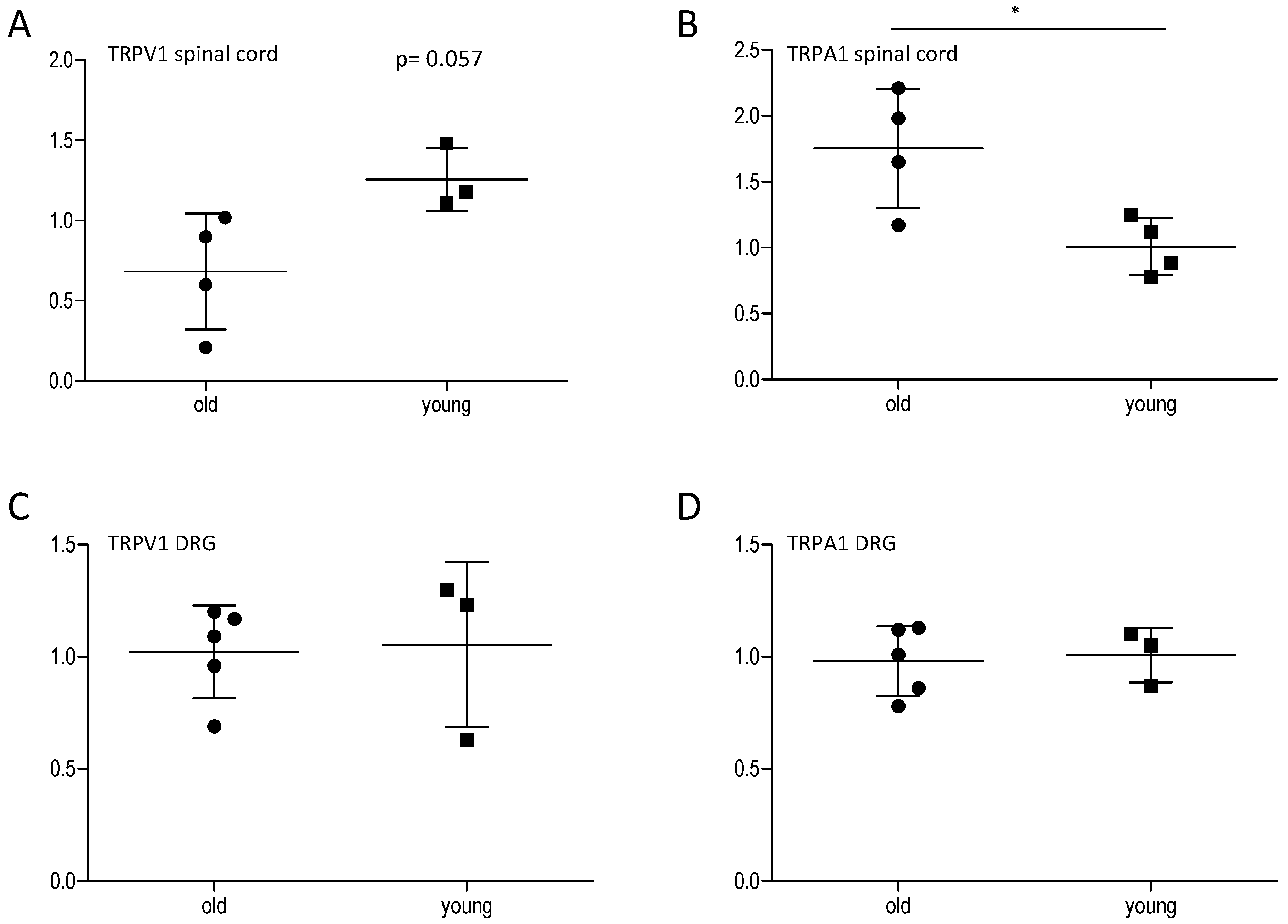
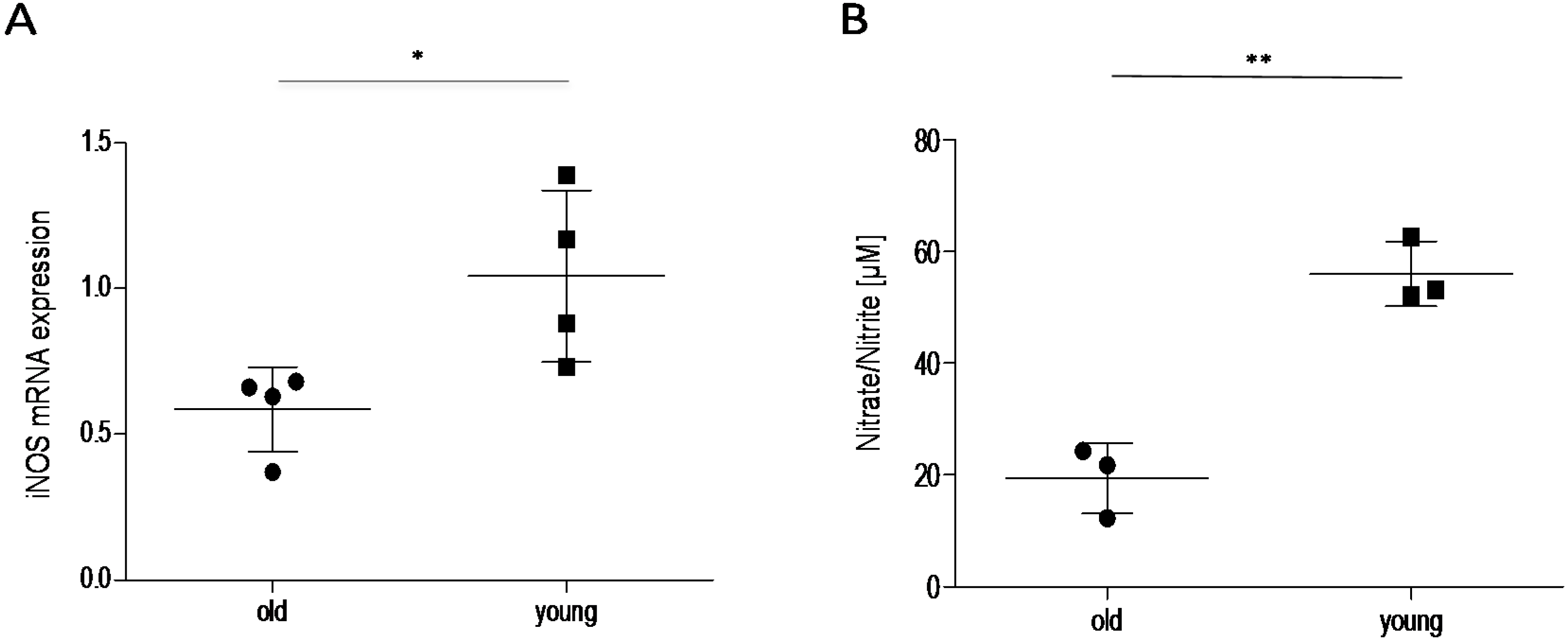
2.3. Effect of Age on Cortisol and Downstream Targets
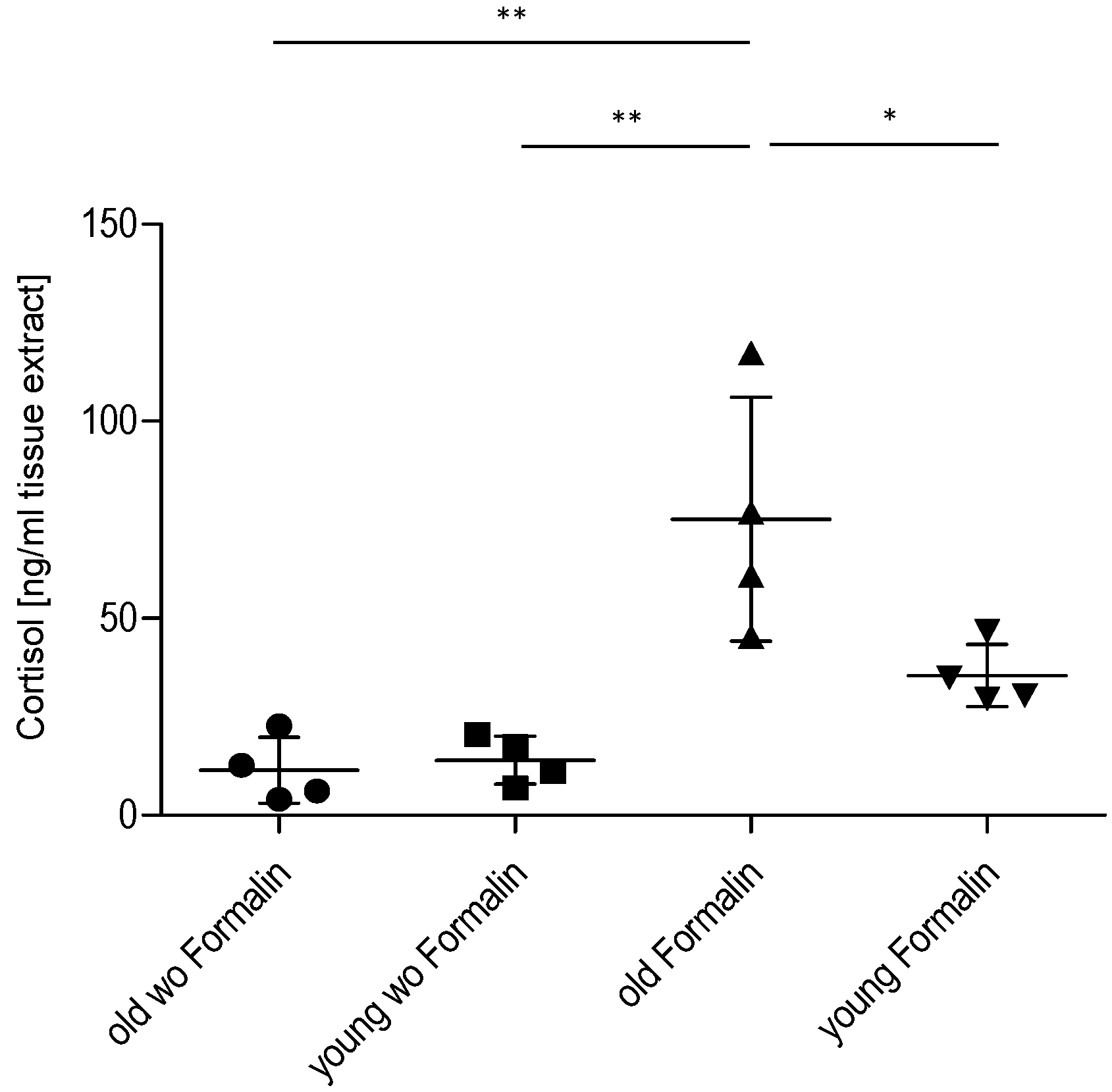
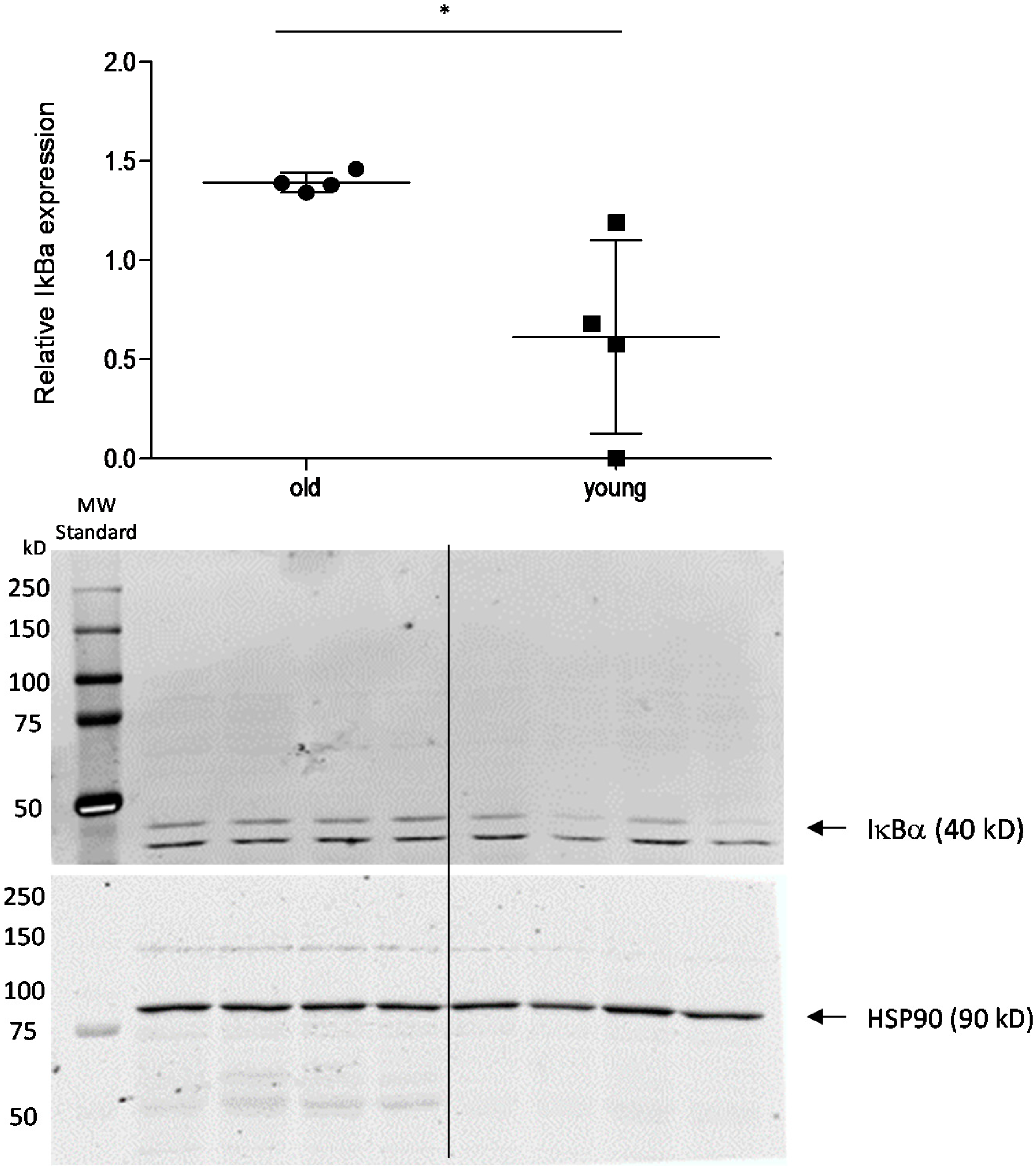
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Behavioral Testing
4.2.1. Motor Coordination (Hanging Wire Test) (Adapted to [31])
4.2.2. Mechanical Sensitivity (Dynamic Plantar Test)
4.2.3. Thermal Sensitivity (Hargreaves Test) [33]
4.2.4. Hot-Plate Test [34]
4.2.5. Acetone Test
4.2.6. Formalin Test
4.2.7. Zymosan-Induced Paw Inflammation, Mechanical Hypersensitivity
4.3. Assessment of Cortisol Levels
4.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
| FW | 5′-CCAAGCCCTCACCTACTTCC-3′ |
| RV | 5′-CTCTGAGGGCTGACACAAGG-3′ |
4.5. Analysis of Nitrite/Nitrate
4.6. Western Blot Analysis
4.7. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.; Davis, B.M.; Zwick, M.; Waxman, S.G.; Albers, K.M. Reduced thermal sensitivity and nav1.8 and trpv1 channel expression in sensory neurons of aged mice. Neurobiol. Aging 2006, 27, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.J.; Knisely, J.S. Environmentally induced analgesia: An age-related decline in an endogenous opioid system. J. Gerontol. 1985, 40, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.J.; Knisely, J.S. Environmentally induced analgesia: Age-related decline in a neurally mediated, nonopioid system. Psychol. Aging 1986, 1, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.J.; Farrell, M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin. J. Pain 2004, 20, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Gagliese, L.; Melzack, R. Age differences in nociception and pain behaviours in the rat. Neurosci. Biobehav. Rev. 2000, 24, 843–854. [Google Scholar] [CrossRef]
- Vierck, C.J.; Yezierski, R.P. Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci. Biobehav. Rev. 2015, 51, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Cruz-Almeida, Y.; Fillingim, R.B.; Riley, J.L., 3rd. Offset analgesia is reduced in older adults. Pain 2013, 154, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Cruz-Almeida, Y.; Vierck, C.J.; Mauderli, A.P.; Riley, J.L., 3rd. Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav. Brain Res. 2015, 289, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gibbison, B.; Angelini, G.D.; Lightman, S.L. Dynamic output and control of the hypothalamic-pituitary-adrenal axis in critical illness and major surgery. Br. J. Anaesth. 2013, 111, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 1983, 289, 235–240. [Google Scholar] [CrossRef]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C.A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Scheinman, R.I.; Gualberto, A.; Jewell, C.M.; Cidlowski, J.A.; Baldwin, A.S., Jr. Characterization of mechanisms involved in transrepression of nf-kappa b by activated glucocorticoid receptors. Mol. Cell. Biol. 1995, 15, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Heck, S.; Bender, K.; Kullmann, M.; Gottlicher, M.; Herrlich, P.; Cato, A.C. I kappab alpha-independent downregulation of nf-kappab activity by glucocorticoid receptor. EMBO J. 1997, 16, 4698–4707. [Google Scholar] [CrossRef] [PubMed]
- Cortright, D.N.; Krause, J.E.; Broom, D.C. Trp channels and pain. Biochim. Biophys. Acta 2007, 1772, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; He, L.; Lai, W.; Shen, T.; Herkenham, M. Induction of ikappabalpha mrna expression in the brain by glucocorticoids: A negative feedback mechanism for immune-to-brain signaling. J. Neurosci. 2000, 20, 6473–6477. [Google Scholar] [PubMed]
- Liu, W.; Fan, Z.; Han, Y.; Xu, L.; Wang, M.; Zhang, D.; Mao, Y.; Li, J.; Wang, H. Activation of nf-kappab signaling pathway in hsv-1-induced mouse facial palsy: Possible relation to therapeutic effect of glucocorticoids. Neuroscience 2015, 289, 251–261. [Google Scholar] [CrossRef] [PubMed]
- De Vera, M.E.; Taylor, B.S.; Wang, Q.; Shapiro, R.A.; Billiar, T.R.; Geller, D.A. Dexamethasone suppresses inos gene expression by upregulating i-kappa b alpha and inhibiting nf-kappa b. Am. J. Physiol. 1997, 273, G1290–G1296. [Google Scholar] [PubMed]
- Niederberger, E.; Tegeder, I.; Vetter, G.; Schmidtko, A.; Schmidt, H.; Euchenhofer, C.; Brautigam, L.; Grosch, S.; Geisslinger, G. Celecoxib loses its anti-inflammatory efficacy at high doses through activation of nf-kappab. FASEB J. 2001, 15, 1622–1624. [Google Scholar] [PubMed]
- Ghirardi, O.; Caprioli, A.; Ramacci, M.T.; Angelucci, L. Effect of long-term acetyl-l-carnitine on stress-induced analgesia in the aging rat. Exp. Gerontol. 1994, 29, 569–574. [Google Scholar] [CrossRef]
- Hamm, R.J.; Knisely, J.S.; Watson, A. Environmentally-induced analgesia: Age-related differences in a hormonally-mediated, nonopioid system. J. Gerontol. 1986, 41, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Ghirardi, O.; Caprioli, A.; di Serio, S.; Ramacci, M.T.; Angelucci, L. Multivariate analysis of behavioral aging highlights some unexpected features of complex systems organization. Behav. Neural Biol. 1994, 61, 110–122. [Google Scholar] [CrossRef]
- Kramer, E.; Bodnar, R.J. Age-related decrements in the analgesic response to cold-water swims. Physiol. Behav. 1986, 36, 875–880. [Google Scholar] [CrossRef]
- Yezierski, R.P. The effects of age on pain sensitivity: Preclinical studies. Pain Med. 2012, 13 (Suppl. 2), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, A.C.; Clark, A.K.; Malcangio, M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age (Dordr) 2015, 37, 9792. [Google Scholar] [CrossRef] [PubMed]
- Gagliese, L.; Melzack, R. Age differences in the response to the formalin test in rats. Neurobiol. Aging 1999, 20, 699. [Google Scholar] [CrossRef]
- Scheinman, R.I.; Cogswell, P.C.; Lofquist, A.K.; Baldwin, A.S., Jr. Role of transcriptional activation of i kappa b alpha in mediation of immunosuppression by glucocorticoids. Science 1995, 270, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.D.; Chavis, J.A. Inhibition of microglial cell activation by cortisol. Brain Res. Bull. 2000, 52, 391–396. [Google Scholar] [CrossRef]
- Barger, S.W.; Chavis, J.A.; Drew, P.D. Dehydroepiandrosterone inhibits microglial nitric oxide production in a stimulus-specific manner. J. Neurosci. Res. 2000, 62, 503–509. [Google Scholar] [CrossRef]
- Aktan, F. Inos-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Ye, X.; Malik, A.B. In vivo inhibition of nuclear factor-kappa b activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J. Immunol. 1997, 159, 3976–3983. [Google Scholar] [PubMed]
- Hashemi, E.; Sahbaie, P.; Davies, M.F.; Clark, J.D.; DeLorey, T.M. Gabrb3 gene deficient mice exhibit increased risk assessment behavior, hypotonia and expansion of the plexus of locus coeruleus dendrites. Brain Res. 2007, 1129, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.; Haussler, A.; Vannoni, E.; Wolfer, D.P.; Tegeder, I. Learning and memory with neuropathic pain: Impact of old age and progranulin deficiency. Front. Behav. Neurosci. 2013, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. Ii. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar] [PubMed]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of nociception i: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Meller, S.T.; Gebhart, G.F. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur. J. Pain 1997, 1, 43–52. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534, 536–537. [Google Scholar] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Sanden, S.; Tripmacher, R.; Weltrich, R.; Rohde, W.; Hiepe, F.; Burmester, G.R.; Buttgereit, F. Glucocorticoid dose dependent downregulation of glucocorticoid receptors in patients with rheumatic diseases. J. Rheumatol. 2000, 27, 1265–1270. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Olbrich, K.; Geisslinger, G.; Niederberger, E. Age-Dependent Changes in the Inflammatory Nociceptive Behavior of Mice. Int. J. Mol. Sci. 2015, 16, 27508-27519. https://doi.org/10.3390/ijms161126041
King-Himmelreich TS, Möser CV, Wolters MC, Olbrich K, Geisslinger G, Niederberger E. Age-Dependent Changes in the Inflammatory Nociceptive Behavior of Mice. International Journal of Molecular Sciences. 2015; 16(11):27508-27519. https://doi.org/10.3390/ijms161126041
Chicago/Turabian StyleKing-Himmelreich, Tanya S., Christine V. Möser, Miriam C. Wolters, Katrin Olbrich, Gerd Geisslinger, and Ellen Niederberger. 2015. "Age-Dependent Changes in the Inflammatory Nociceptive Behavior of Mice" International Journal of Molecular Sciences 16, no. 11: 27508-27519. https://doi.org/10.3390/ijms161126041






