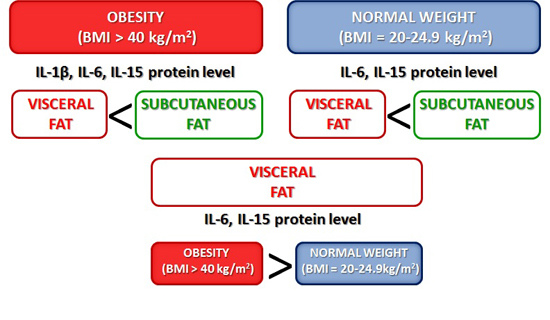
Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots
Abstract
:1. Introduction
2. Results
2.1. Expression of Cytokines in Adipose Tissues from Obese and Normal-Weight Individuals
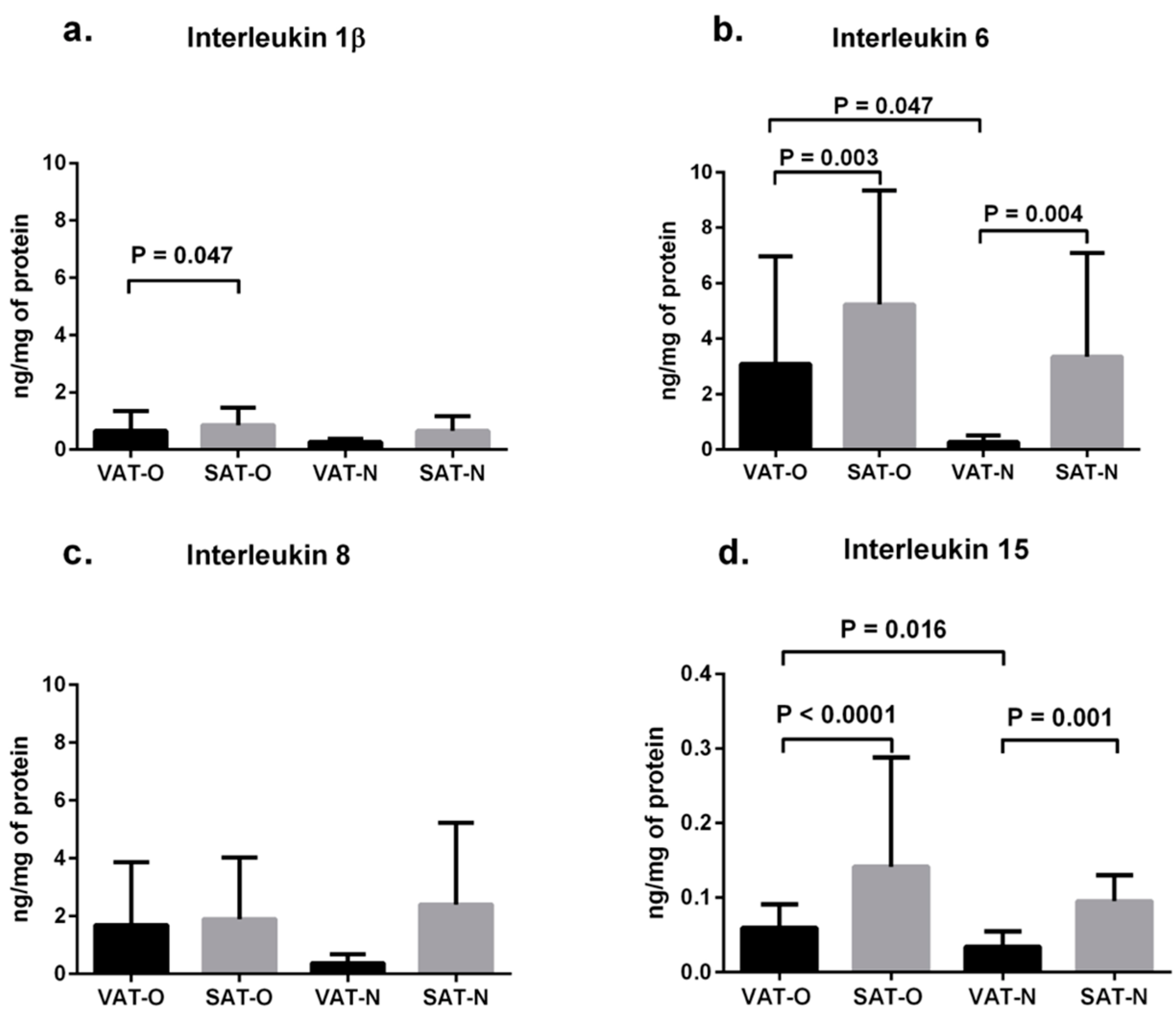
2.2. Expression of Cytokine mRNA Levels in Adipose Tissues from Obese and Normal-Weight Individuals

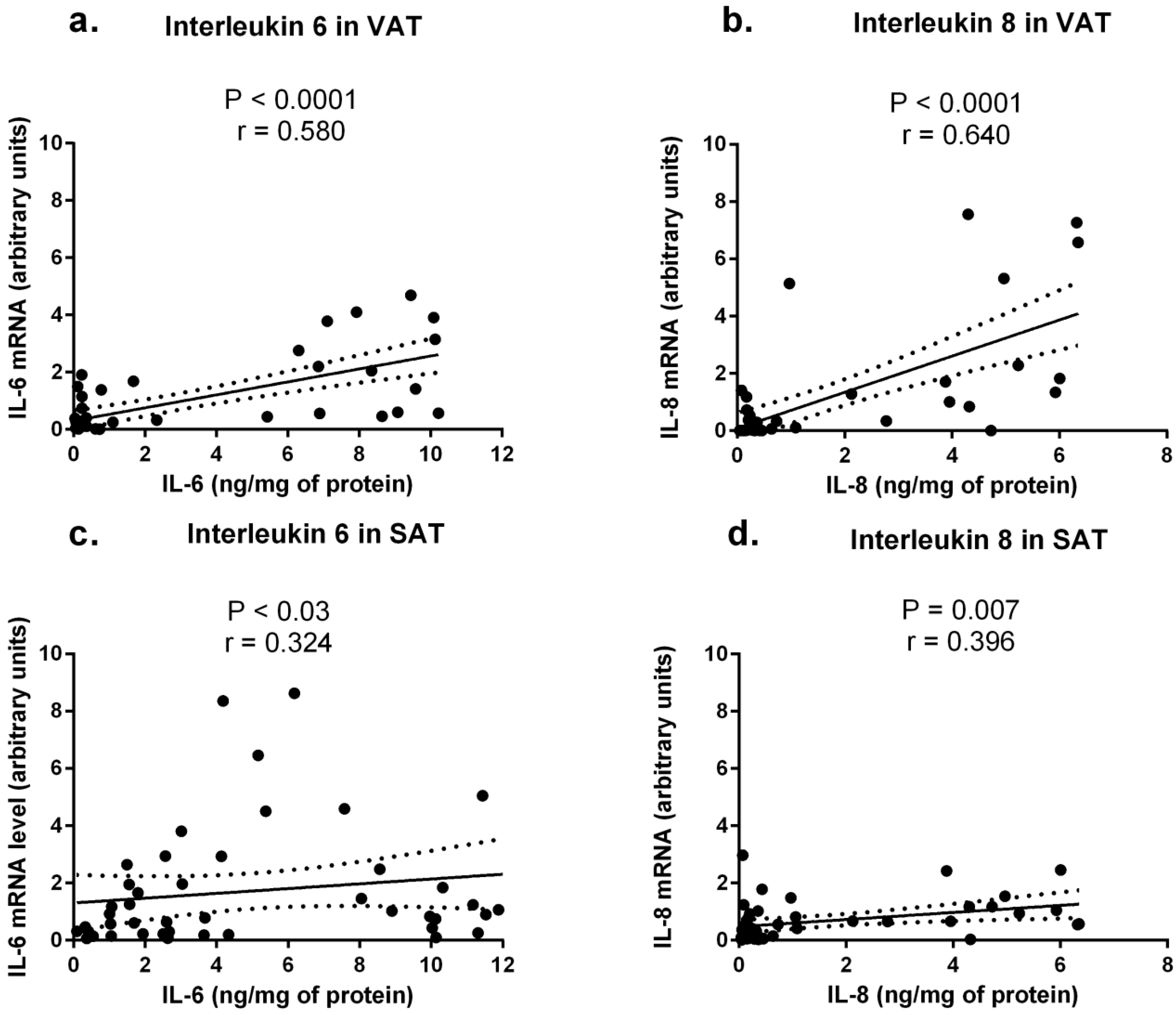
2.3. Cytokine Concentrations in Sera from Obese and Normal-Weight Individuals and Their Correlation with Protein Levels in Adipose Tissues
2.4. Correlation between Cytokine Concentrations and Clinical and Biochemical Parameters in Obese Individuals
3. Discussion
4. Material and Methods
4.1. Studied Groups
| Parameter | Obese Individuals | Normal-Weight Controls | p | ||
|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||
| Age (years) | 42.02 ± 10.33 | 20–59 | 44.08 ± 13.50 | 26–63 | 0.623 |
| Weight (kg) | 132.17 ± 18.61 | 100–198.6 | 67.92 ± 10.48 | 54–90 | <0.0001 |
| BMI (kg/m2) | 47.02 ± 4.91 | 40.48–59.26 | 23.06 ± 1.77 | 20.07-24.93 | <0.0001 |
| Adipose tissue (% body mass) | 48.55 ± 4.10 | 36.30–57.23 | – | – | |
| Waist circumference (cm) | 124.47 ± 18.78 | 97–167 | – | – | |
| CRP (nmol/L) | 123.15 ± 114 | 11.43–657.16 | 30.86 ± 16.38 | 6.67–49.52 | 0.04 |
| Glucose (mmol/L) | 5.65 ± 1.53 | 3.36–10.08 | 5.17 ± 0.21 | 4.77–5.44 | 0.314 |
| Total cholesterol (mmol/L) | 5.20 ± 1.03 | 3.13–7.86 | 4.80 ± 0.20 | 4.62–4.92 | 0.623 |
| LDL (mmol/L) | 3.28 ± 1.06 | 1.25–5.64 | 2.62 ± 0.08 | 2.75–2.80 | 0.547 |
| HDL (mmol/L) | 1.24 ± 0.23 | 0.78–1.78 | 1.47 ± 0.27 | 1.24–1.76 | 0.136 |
| Triglycerides (mmol/L) | 1.44 ± 0.73 | 0.52–2.92 | 1.33 ± 0.09 | 1.10–1.46 | 0.797 |
| Co-morbidities | |||||
| Type 2 DM/IGT | 24 (49.0%) | none | |||
| Hypertension | 29 (59.2%) | none | |||
| Hyperlipidemia | 30 (61.2%) | none | |||
4.2. Adipose Tissues
4.3. Isolation of a Protein Fraction from Adipose Tissue
4.4. Measurement of Cytokine Concentrations
4.5. Isolation of Total RNA, Reverse Transcription, and Real-Time PCR
| Gene | Gene Description | Gene Bank | Primers | Annealing (°C) | |
|---|---|---|---|---|---|
| IL1B | interleukin 1β | NM_000576 | F | 5ʹ-CACCAAGCTTTTTTGCTGTGAGT-3ʹ | 60 |
| R | 5ʹ-GCACGATGCACCTGTACGAT-3ʹ | ||||
| IL6 | interleukin 6 | NM_000600 | F | 5ʹ-CCTTCGGTCCAGTTGCCTTC-3ʹ | 60 |
| R | 5ʹ-GTGGGGCGGCTACATCTTTG-3ʹ | ||||
| IL8 | interleukin 8 | NM_000584 | F | 5ʹ-CACCGGAAGAACCATCTCACT-3ʹ | 60 |
| R | 5ʹ-TCAGCCCTCTTCAAAAACTTCTCC-3ʹ | ||||
| IL15 | interleukin 15 | NM_172175 | F | 5ʹ-GGATTTACCGTGGCTTTGAGTAATGAG-3ʹ | 55 |
| R | 5ʹ-GAATCAATTGCAATCAAGAAGTG-3ʹ | ||||
| ACTB | β-actin | NM_001101 | F | 5ʹ-CAGCCTGGATAGCAACGTAC-3ʹ | 61 |
| R | 5ʹ-TTCTACAATGAGCTGCGTGTG-3ʹ | ||||
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Lapice, E.; Maione, S.; Patti, L.; Cipriano, P.; Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care 2009, 32, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef]
- Poirier, P. Exploring the spectrum of diseases influenced by excess adiposity. Transl. Res. 2014, 164, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A., Jr.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [PubMed]
- Fujita, H.; Yagishita, N.; Aratani, S.; Saito-Fujita, T.; Morota, S.; Yamano, Y.; Hansson, M.J.; Inazu, M.; Kokuba, H.; Sudo, K.; et al. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1β. EMBO J. 2015, 34, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Chen, Q.; Shah, S.; Du, J.; Tao, B.; Tzameli, I.; Yan, W.; Xu, H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: Involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 2009, 58, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Tanaka, M.; Ogawa, Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr. J. 2012, 59, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Campbell, L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 2008, 14, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F., Jr.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.; Haffner, S.; Davidson, M.H.; D’Agostino, R.B., Sr.; Feinstein, S.; Kondos, G.; Perez, A.; Mazzone, T. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 2009, 32, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Alvehus, M.; Burén, J.; Sjöström, M.; Goedecke, J.; Olsson, T. The human visceral fat depot has a unique inflammatory profile. Obesity 2010, 18, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Stout, M.B.; Giorgadze, N.; Wang, L.; Li, P.W.; Heppelmann, C.J.; Bouloumié, A.; Jensen, M.D.; Bergen, H.R., 3rd; et al. Inflammation and the depot-specific secretome of human preadipocytes. Obesity 2015, 23, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Auguet, T.; Aragonès, G.; Guiu-Jurado, E.; Berlanga, A.; Martinez, S.; Martí, A.; Sabench, F.; Hernandez, M.; Aguilar, C.; et al. Interleukin-17A gene expression in morbidly obese women. Int. J. Mol. Sci. 2015, 16, 17469–17481. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Böni-Schnetzler, M. IL-1β activation as a response to metabolic disturbances. Cell Metab. 2010, 12, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Nov, O.; Shapiro, H.; Ovadia, H.; Tarnovscki, T.; Dvir, I.; Shemesh, E.; Kovsan, J.; Shelef, I.; Carmi, Y.; Voronov, E.; et al. Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 2013, 8, e53626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Hojman, P.; Erikstrup, C.; Fischer, C.P.; Plomgaard, P.; Mounier, R.; Mortensen, O.H.; Broholm, C.; Taudorf, S.; Krogh-Madsen, R.; et al. Association between interleukin-15 and obesity: Interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 2008, 93, 4486–4493. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.S.; Anderson, B.G. Interleukin-15, IL-15 receptor-alpha, and obesity: Concordance of laboratory animal and human genetic studies. J. Obes. 2011, 2011, 456347. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Jardel, C.; Bruckert, E.; Blondy, P.; Capeau, J.; Laville, M.; Vidal, H.; Hainque, B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 2000, 85, 3338–3342. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stepien, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.R.; Maples, J.M.; Hickner, R.C. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: local effects of IL-15 on adipose tissue lipolysis. Am. J. Physiol. Endocrinol. Metab. 2015, 308, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ayers, C.R.; Rohatgi, A.K.; Turer, A.T.; Berry, J.D.; Das, S.R.; Vega, G.L.; Khera, A.; McGuire, D.K.; Grundy, S.M.; et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity 2013, 21, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Poirier, P.; Pibarot, P.; Lemieux, I.; Després, J.P. Visceral obesity: The link among inflammation, hypertension, and cardiovascular disease. Hypertension 2009, 53, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 745–751. [Google Scholar]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Antunes, T.T.; Gagnon, A.; Chen, B.; Pacini, F.; Smith, T.J.; Sorisky, A. Interleukin-6 release from human abdominal adipose cells is regulated by thyroid-stimulating hormone: Effect of adipocyte differentiation and anatomic depot. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Koenen, T.B.; Stienstra, R.; van Tits, L.J.; Joosten, L.A.; van Velzen, J.F.; Hijmans, A.; Pol, J.A.; van der Vliet, J.A.; Netea, M.G.; Tack, C.J.; et al. The inflammasome and caspase-1 activation: A new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 2011, 152, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Pitombo, C.; Araújo, E.P.; de Souza, C.T.; Pareja, J.C.; Geloneze, B.; Velloso, L.A. Amelioration of diet-induced diabetes mellitus by removal of visceral fat. J. Endocrinol. 2006, 191, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Porramatikul, S.; Al-Ozairi, E. Metabolic obesity: The paradox between visceral and subcutaneous fat. Curr. Diabetes Rev. 2006, 2, 367–373. [Google Scholar] [PubMed]
- Loh, N.Y.; Neville, M.J.; Marinou, K.; Hardcastle, S.A.; Fielding, B.A.; Duncan, E.L.; McCarthy, M.I.; Tobias, J.H.; Gregson, C.L.; Karpe, F.; et al. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015, 21, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Koutsari, C.; Snozek, C.L.; Jensen, M.D. Plasma NEFA storage in adipose tissue in the postprandial state: Sex-related and regional differences. Diabetologia 2008, 51, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.M.; de Jong, S.; Teerlink, T.; Asscheman, H.; Seidell, J.C.; Gooren, L.J. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism 1999, 48, 1371–1377. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [PubMed]
- Liu, A.; McLaughlin, T.; Liu, T.; Sherman, A.; Yee, G.; Abbasi, F.; Lamendola, C.; Morton, J.; Cushman, S.W.; Reaven, G.M.; et al. Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes. Surg. 2009, 19, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.S.; Styer, A.M.; Strodel, W.E.; Roesch, S.L.; Yavorek, A.; Carey, D.J.; Wood, G.C.; Petrick, A.T.; Gabrielsen, J.; Ibele, A.; et al. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int. J. Obes. 2014, 38, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Beneficial metabolic activities of inflammatory cytokine interleukin 15 in obesity and type 2 diabetes. Front. Med. 2014, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Ting, J.P.; O’Neill, L.A. A role for the NLRP3 inflammasome in metabolic diseases—Did Warburg miss inflammation? Nat. Immunol. 2012, 13, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Pedersen, S.B.; Richelsen, B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J. Clin. Endocrinol. Metab. 2001, 86, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Available online: http://idf.org (accessed on 15 September 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817-25830. https://doi.org/10.3390/ijms161025817
Jonas MI, Kurylowicz A, Bartoszewicz Z, Lisik W, Jonas M, Wierzbicki Z, Chmura A, Pruszczyk P, Puzianowska-Kuznicka M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. International Journal of Molecular Sciences. 2015; 16(10):25817-25830. https://doi.org/10.3390/ijms161025817
Chicago/Turabian StyleJonas, Marta Izabela, Alina Kurylowicz, Zbigniew Bartoszewicz, Wojciech Lisik, Maurycy Jonas, Zbigniew Wierzbicki, Andrzej Chmura, Piotr Pruszczyk, and Monika Puzianowska-Kuznicka. 2015. "Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots" International Journal of Molecular Sciences 16, no. 10: 25817-25830. https://doi.org/10.3390/ijms161025817
APA StyleJonas, M. I., Kurylowicz, A., Bartoszewicz, Z., Lisik, W., Jonas, M., Wierzbicki, Z., Chmura, A., Pruszczyk, P., & Puzianowska-Kuznicka, M. (2015). Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. International Journal of Molecular Sciences, 16(10), 25817-25830. https://doi.org/10.3390/ijms161025817