Morin, a Flavonoid from Moraceae, Induces Apoptosis by Induction of BAD Protein in Human Leukemic Cells
Abstract
:1. Introduction
2. Results
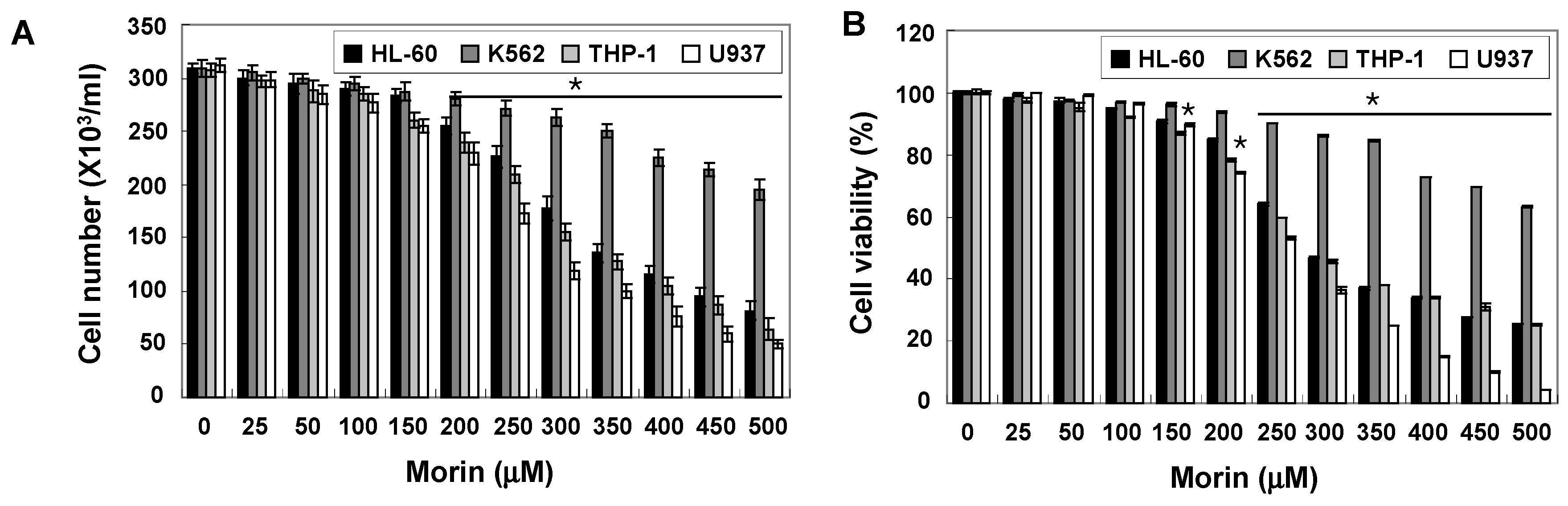
2.1. Morin Inhibited Proliferation and Induced Apoptosis of U937 Human Leukemic Cells
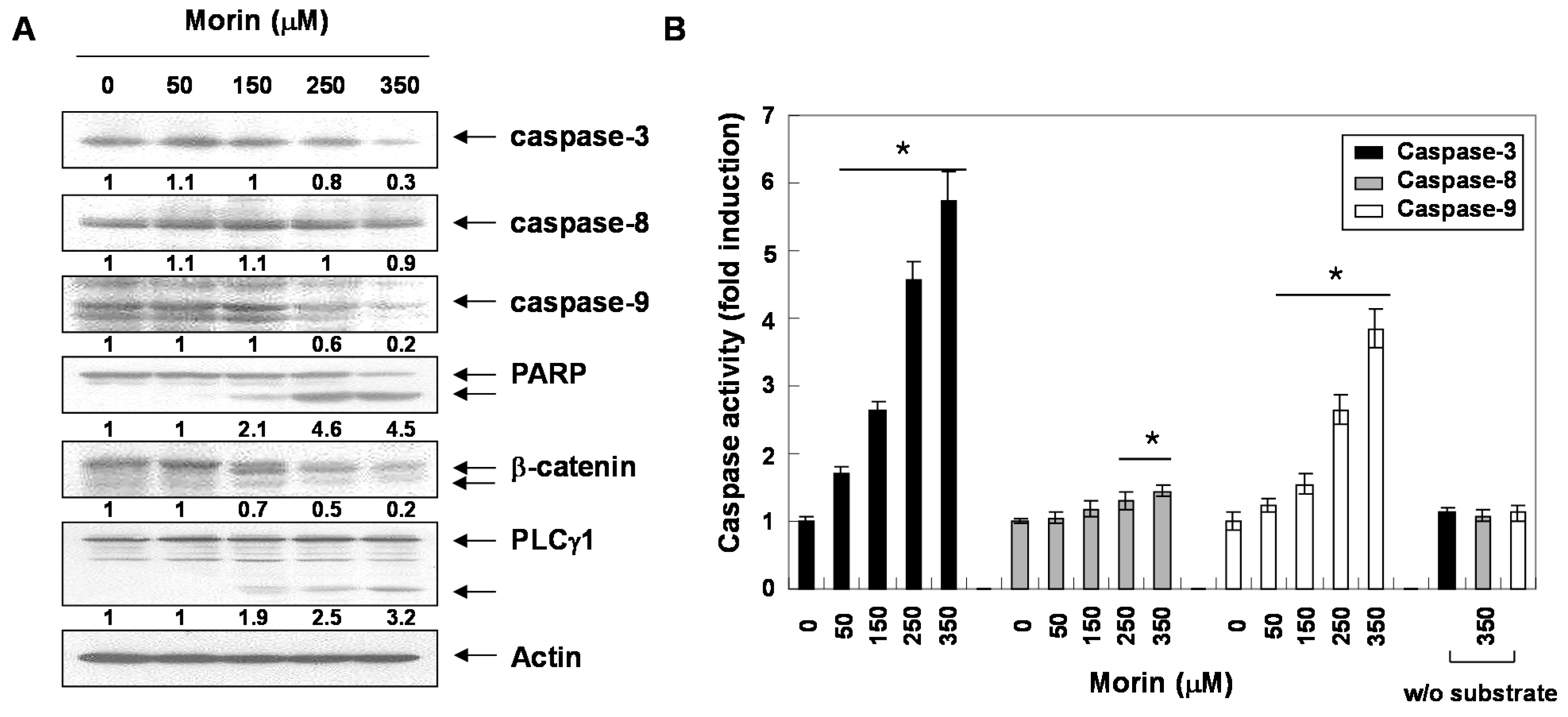
2.2. Morin Induces Caspase Activation and Subsequent Cleavage of Poly ADP Ribose Polymerase (PARP)

2.3. Morin Induced Loss of MMP (ΔΨm) Modulating Bcl-2 Family Members, BAD and Bcl-xL


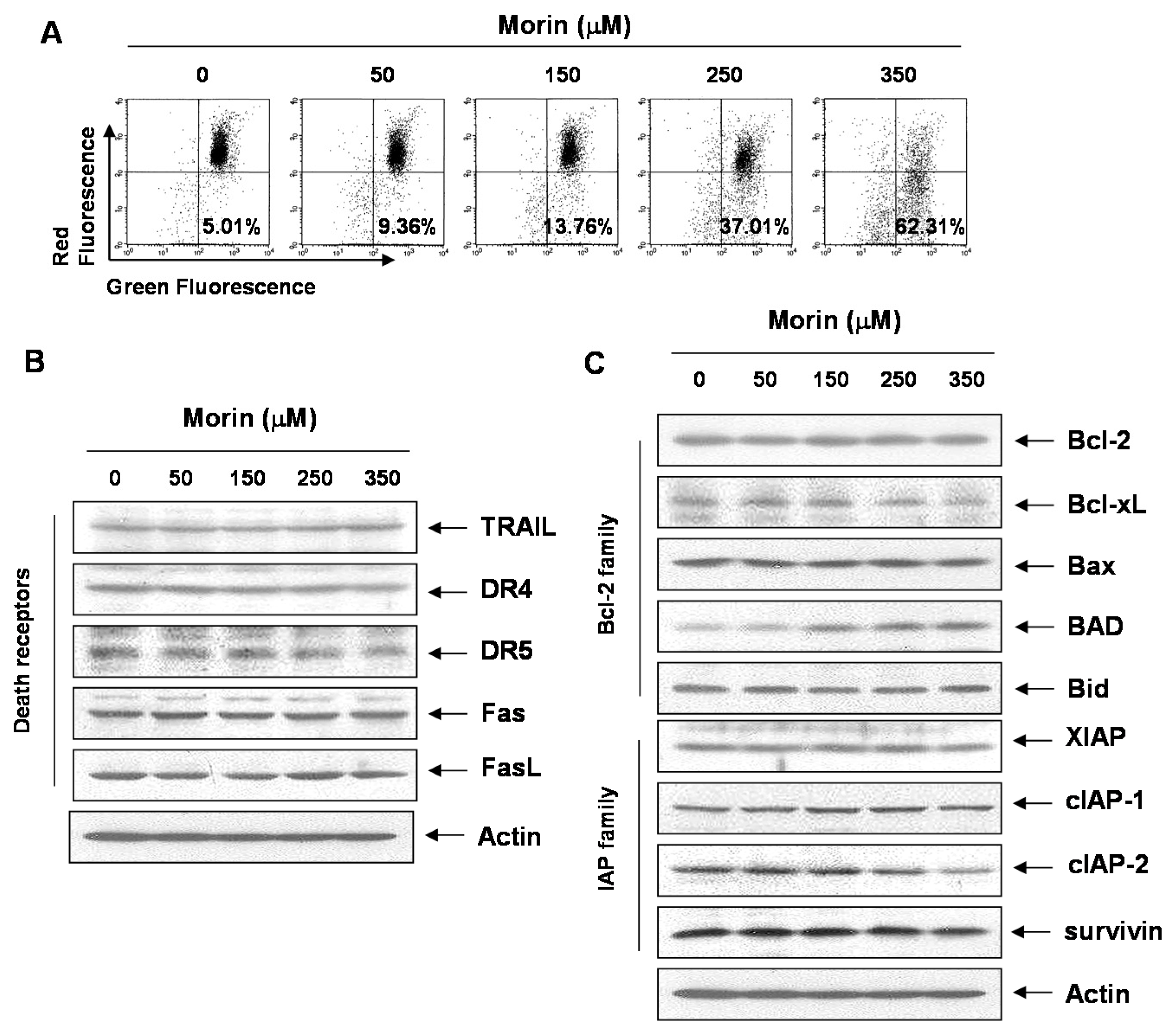
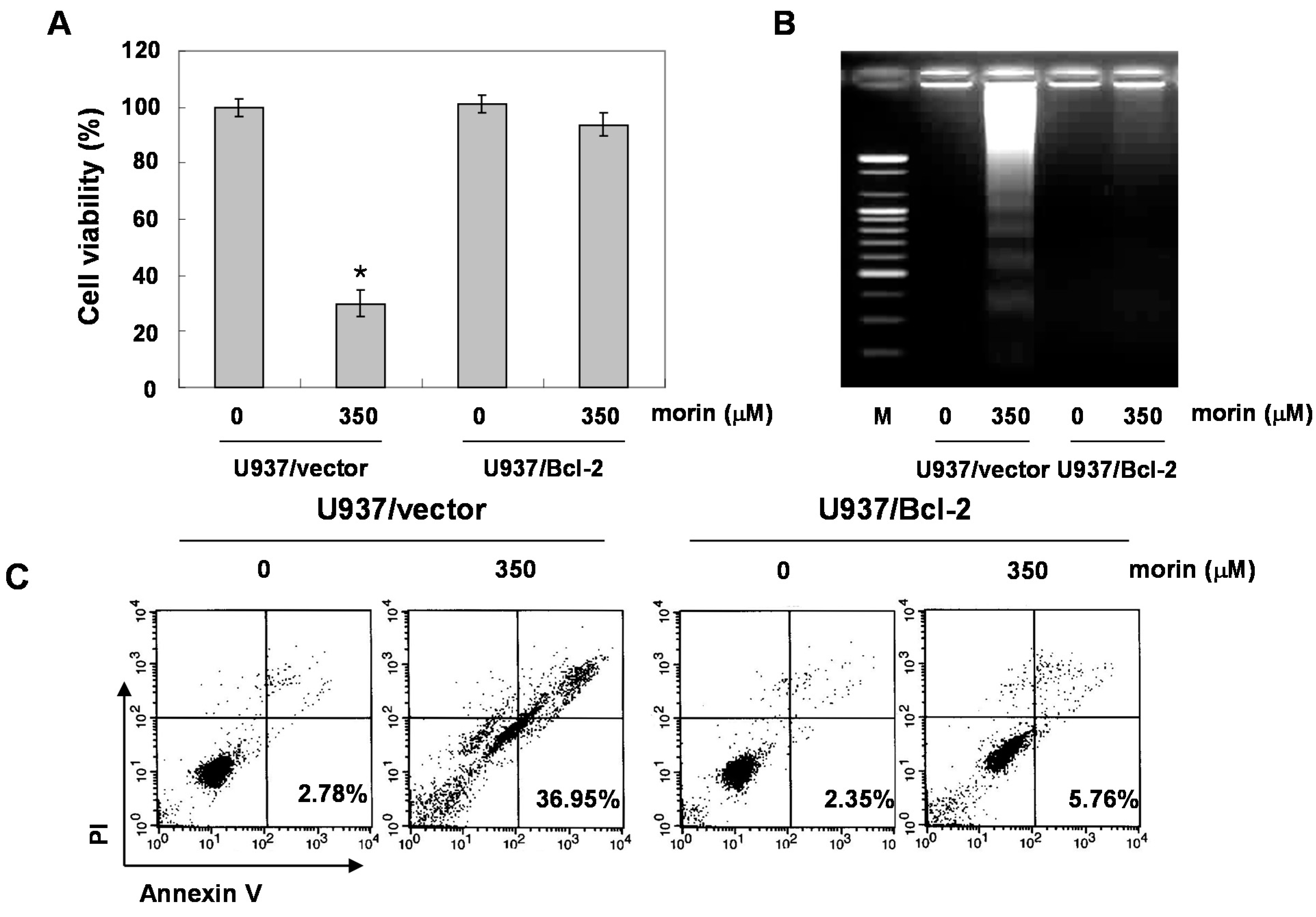
2.4. Bcl-2 Overexpression Suppressed Morin-Induced Apoptosis and Loss of MMP (ΔΨm) in U937 Cells

3. Discussion

4. Experimental Section
4.1. Cells and Reagents
4.2. Cell Viability Assay
4.3. DNA Fragmentation Test
4.4. Flow Cytometry Analysis for Cell Cycle Analysis and Annexin V Apoptosis Assay
4.5. Measurement of Mitochondrial Membrane Potential (MMP, ΔΨm) and Reactive Oxygen Species (ROS) Generation
4.6. Western Blot Analysis
4.7. Assay of Caspase Activity
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.E.; Mannisto, S.; Spiegelman, D.; Hunter, D.J.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Flood, A.; et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: A pooled analysis of 13 prospective studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1730–1739. [Google Scholar] [CrossRef]
- Gandini, S.; Merzenich, H.; Robertson, C.; Boyle, P. Meta-analysis of studies on breast cancer risk and diet: The role of fruit and vegetable consumption and the intake of associated micronutrients. Eur. J. Cancer 2000, 36, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Transcription factors in the cellular signaling network as prime targets of chemopreventive phytochemicals. Cancer Res. Treat. 2004, 36, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Tanaka, T.; Honjo, S.; Kakumoto, M.; Hara, A.; Makita, H.; Tatematsu, N.; Ushida, J.; Tsuda, H.; Mori, H. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int. J. Cancer 1999, 83, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; O’Prey, J.; Harrison, P.R. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the akt and stress kinase pathways. Carcinogenesis 2003, 24, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Buendia, B.; Santa-Maria, A.; Courvalin, J.C. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell Sci. 1999, 112 Pt 11, 1743–1753. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Zha, J.; Jockel, J.; Boise, L.H.; Thompson, C.B.; Korsmeyer, S.J. Bad, a heterodimeric partner for bcl-xl and bcl-2, displaces bax and promotes cell death. Cell 1995, 80, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, A.D.; Hedley, D.W.; Pham, N.A.; Chow, S.; Minden, M.D. Bad induces apoptosis in cells over-expressing bcl-2 or bcl-xl without loss of mitochondrial membrane potential. Leuk. Lymphoma 2001, 42, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Haller, D. Functional diversity of flavonoids in the inhibition of the proinflammatory nf-kappab, irf, and akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 2006, 136, 664–671. [Google Scholar] [PubMed]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line mda-mb231 partly through suppression of the akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [PubMed]
- 16. De Sousa, R.R.; Queiroz, K.C.; Souza, A.C.; Gurgueira, S.A.; Augusto, A.C.; Miranda, M.A.; Peppelenbosch, M.P.; Ferreira, C.V.; Aoyama, H. Phosphoprotein levels, mapk activities and nfkappab expression are affected by fisetin. J. Enzym. Inhib. Med. Chem. 2007, 22, 439–444. [Google Scholar]
- Liu, B.L.; Zhang, X.; Zhang, W.; Zhen, H.N. New enlightenment of french paradox: Resveratrol’s potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol. Ther. 2007, 6, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Kosmeder, J.W., 2nd; Sun, S.; Pezzuto, J.M.; Lotan, R.; Hong, W.K.; Lee, H.Y. Effects of deguelin on the phosphatidylinositol 3-kinase/akt pathway and apoptosis in premalignant human bronchial epithelial cells. J. Natl. Cancer Inst. 2003, 95, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.M.; Chang, L.S.; Lin, Y.L.; Lu, H.F.; Yang, J.S.; Lee, J.H.; Chung, J.G. Morin inhibits the growth of human leukemia hl-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res. 2007, 27, 395–405. [Google Scholar] [PubMed]
- Manna, S.K.; Aggarwal, R.S.; Sethi, G.; Aggarwal, B.B.; Ramesh, G.T. Morin (3,5,7,2',4'-pentahydroxyflavone) abolishes nuclear factor-kappab activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappab-regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. 2007, 13, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Park, C.; Cheong, J.; Choi, B.T.; Lee, T.H.; Lee, J.D.; Lee, W.H.; Kim, G.Y.; Ryu, C.H.; Choi, Y.H. Genistein sensitizes trail-resistant human gastric adenocarcinoma ags cells through activation of caspase-3. Cancer Lett. 2007, 257, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, V.; Cayla, X.; Garcia, A.; Roncal, F.; Fernandez, R.; Albar, J.P.; Martinez, C.; Rebollo, A. Bcl-2 targets protein phosphatase 1 alpha to bad. J. Immunol. 2001, 166, 7345–7352. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Sun, X.; Shen, H.Y.; Gao, F.; Fan, Y.M.; Sun, Z.J. Expression of the apoptosis-related genes bcl-2 and bad in human breast carcinoma and their associated relationship with chemosensitivity. J. Exp. Clin. Cancer Res. 2010, 29, 107. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yu, S.; Eder, A.; Mao, M.; Bast, R.C., Jr.; Boyd, D.; Mills, G.B. Regulation of bad phosphorylation at serine 112 by the ras-mitogen-activated protein kinase pathway. Oncogene 1999, 18, 6635–6640. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Lee, C.Y.; Hour, M.J.; Tsai, S.C.; Kuo, D.H.; Chen, F.A.; Shieh, P.C.; Yang, J.S. Curcumin-loaded nanoparticles enhance apoptotic cell death of u2os human osteosarcoma cells through the akt-bad signaling pathway. Int. J. Oncol. 2014, 44, 238–246. [Google Scholar] [PubMed]
- Gupta, S.C.; Phromnoi, K.; Aggarwal, B.B. Morin inhibits stat3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase shp1. Biochem. Pharmacol. 2013, 85, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, V.; Devaraj, S.N. Morin fosters apoptosis in experimental hepatocellular carcinogenesis model. Chem. Biol. Interact. 2010, 183, 284–292. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Lee, W.S.; Go, S.-I.; Nagappan, A.; Han, M.H.; Hong, S.H.; Kim, G.S.; Kim, G.Y.; Kwon, T.K.; Ryu, C.H.; et al. Morin, a Flavonoid from Moraceae, Induces Apoptosis by Induction of BAD Protein in Human Leukemic Cells. Int. J. Mol. Sci. 2015, 16, 645-659. https://doi.org/10.3390/ijms16010645
Park C, Lee WS, Go S-I, Nagappan A, Han MH, Hong SH, Kim GS, Kim GY, Kwon TK, Ryu CH, et al. Morin, a Flavonoid from Moraceae, Induces Apoptosis by Induction of BAD Protein in Human Leukemic Cells. International Journal of Molecular Sciences. 2015; 16(1):645-659. https://doi.org/10.3390/ijms16010645
Chicago/Turabian StylePark, Cheol, Won Sup Lee, Se-Il Go, Arulkumar Nagappan, Min Ho Han, Su Hyun Hong, Gon Sup Kim, Gi Young Kim, Taeg Kyu Kwon, Chung Ho Ryu, and et al. 2015. "Morin, a Flavonoid from Moraceae, Induces Apoptosis by Induction of BAD Protein in Human Leukemic Cells" International Journal of Molecular Sciences 16, no. 1: 645-659. https://doi.org/10.3390/ijms16010645





