Detection of a Specific Biomarker for Epstein-Barr Virus Using a Polymer-Based Genosensor
Abstract
:1. Introduction
2. Results and Discussion
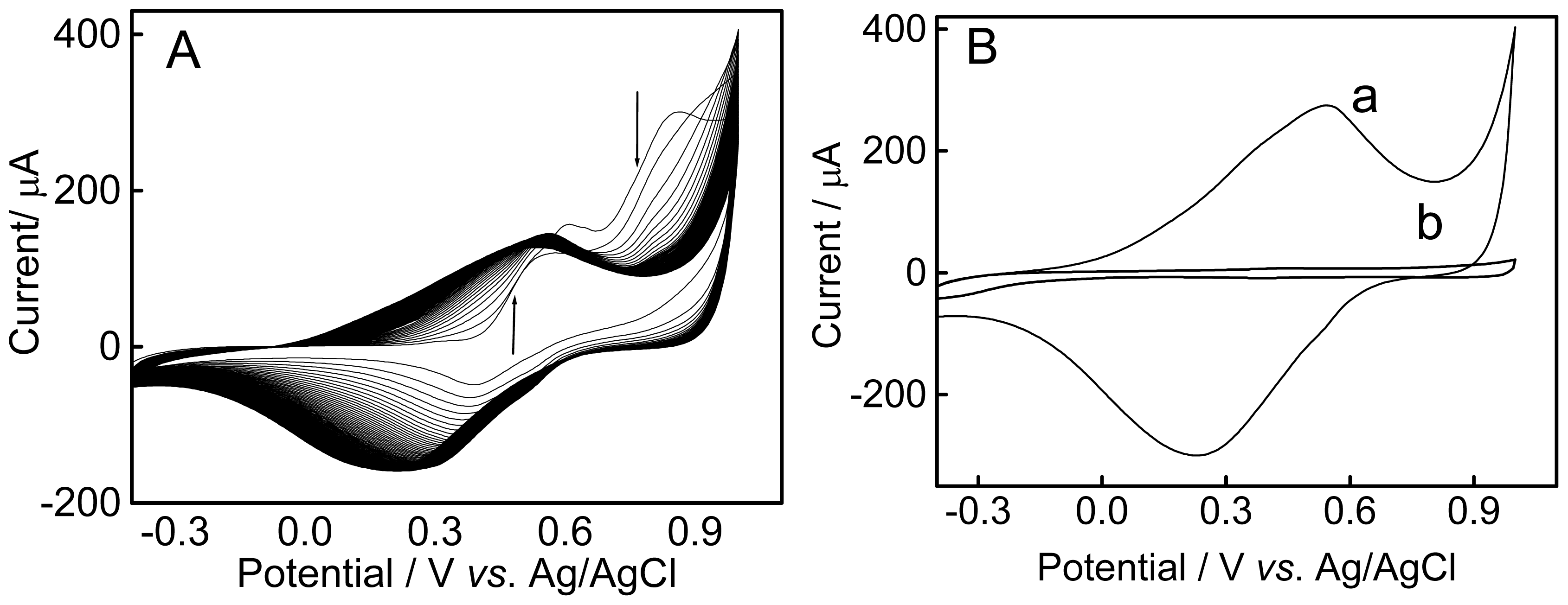
2.1. Electrochemical Behavior of 4-Aminothiophenol (4-ATP)
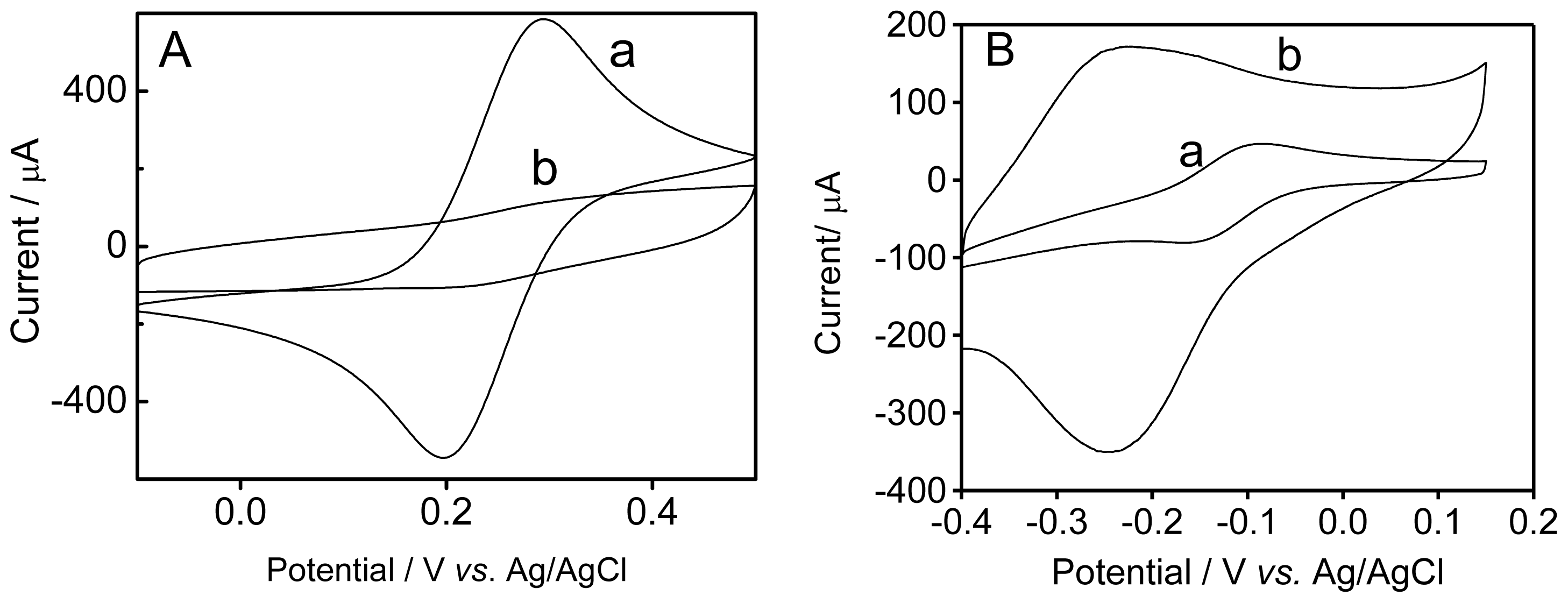
2.2. Electrochemical Characterization of the Modified Electrodes
2.3. Immobilization and Hybridization
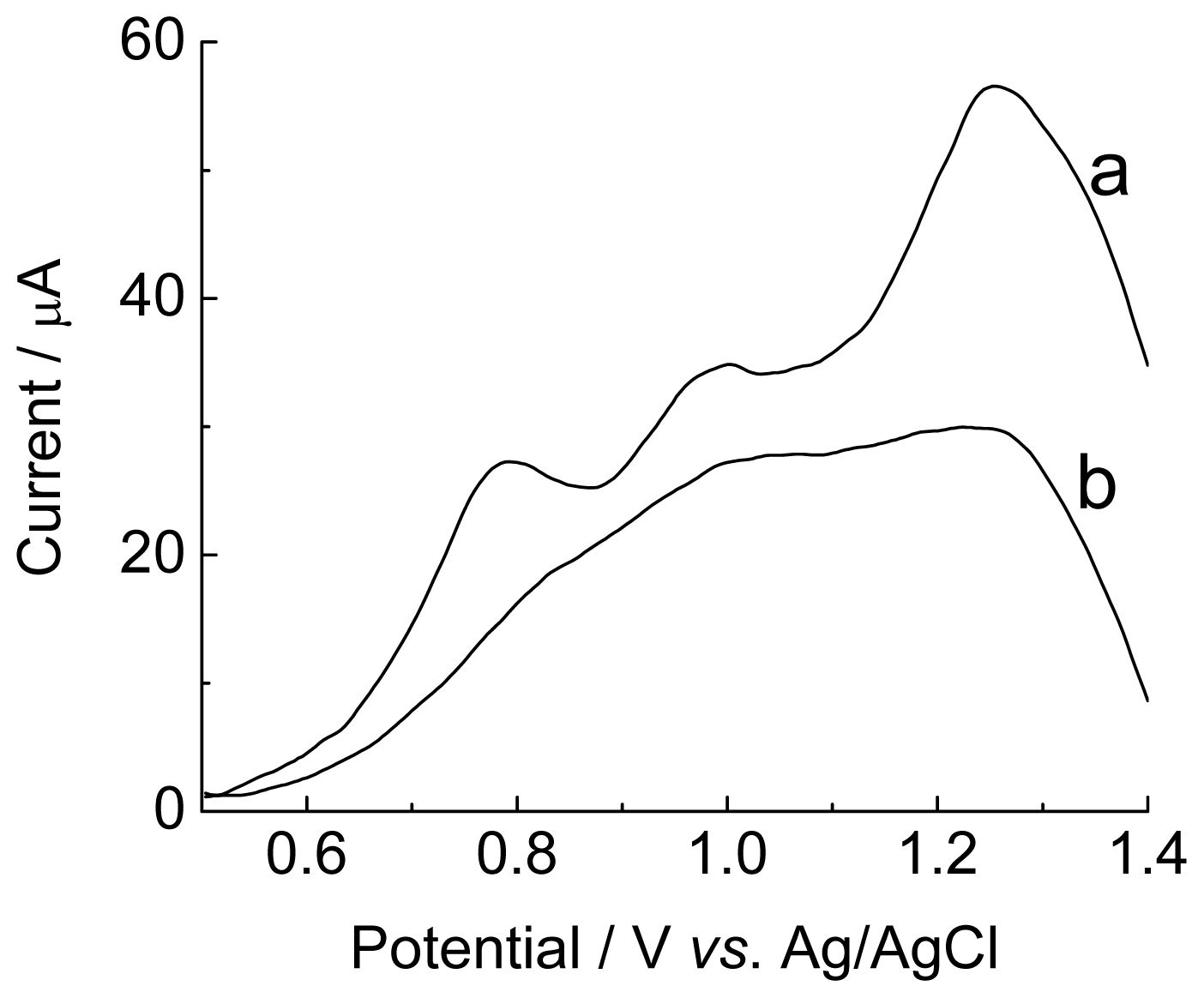
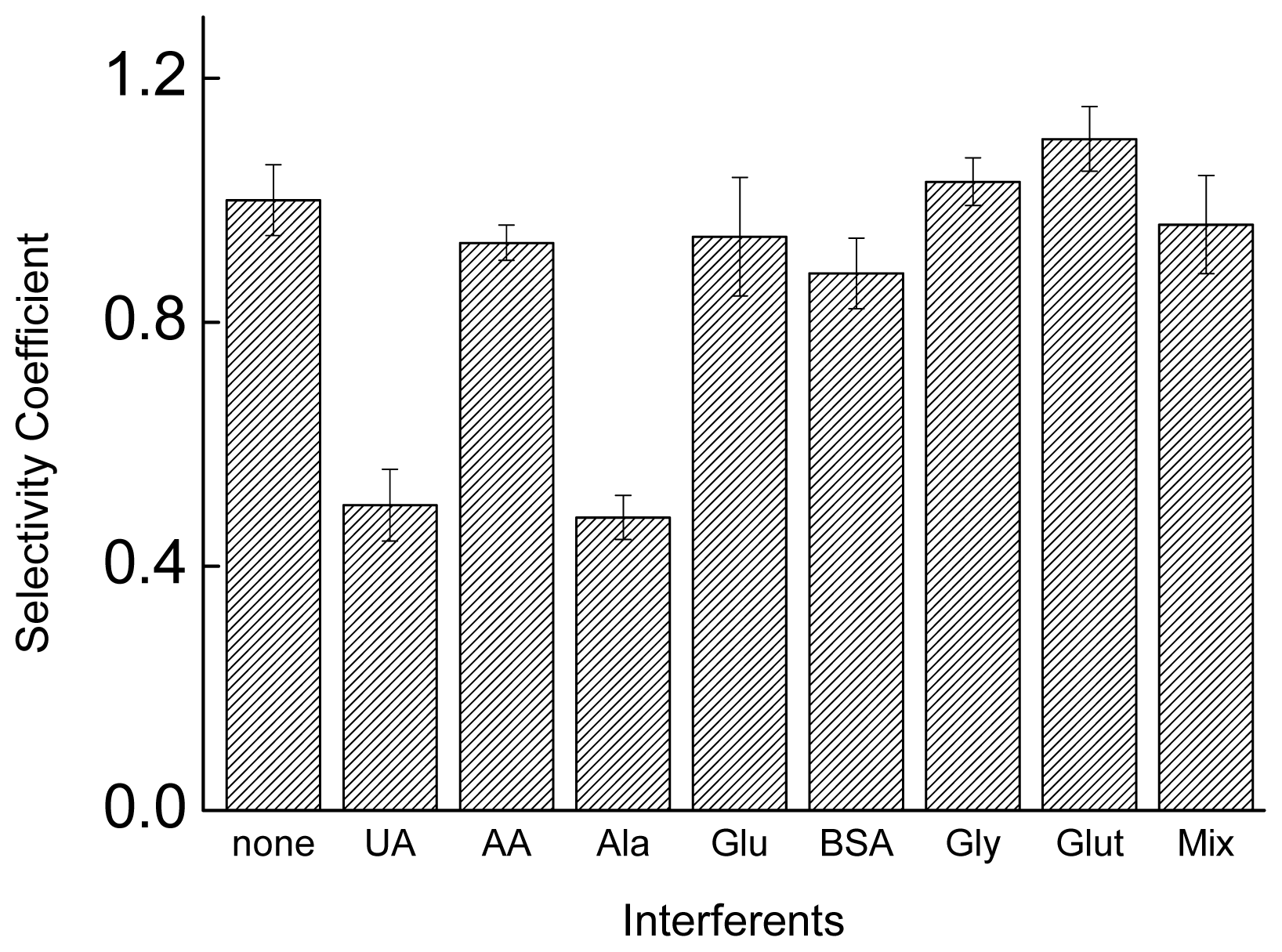
2.4. Interference Studies
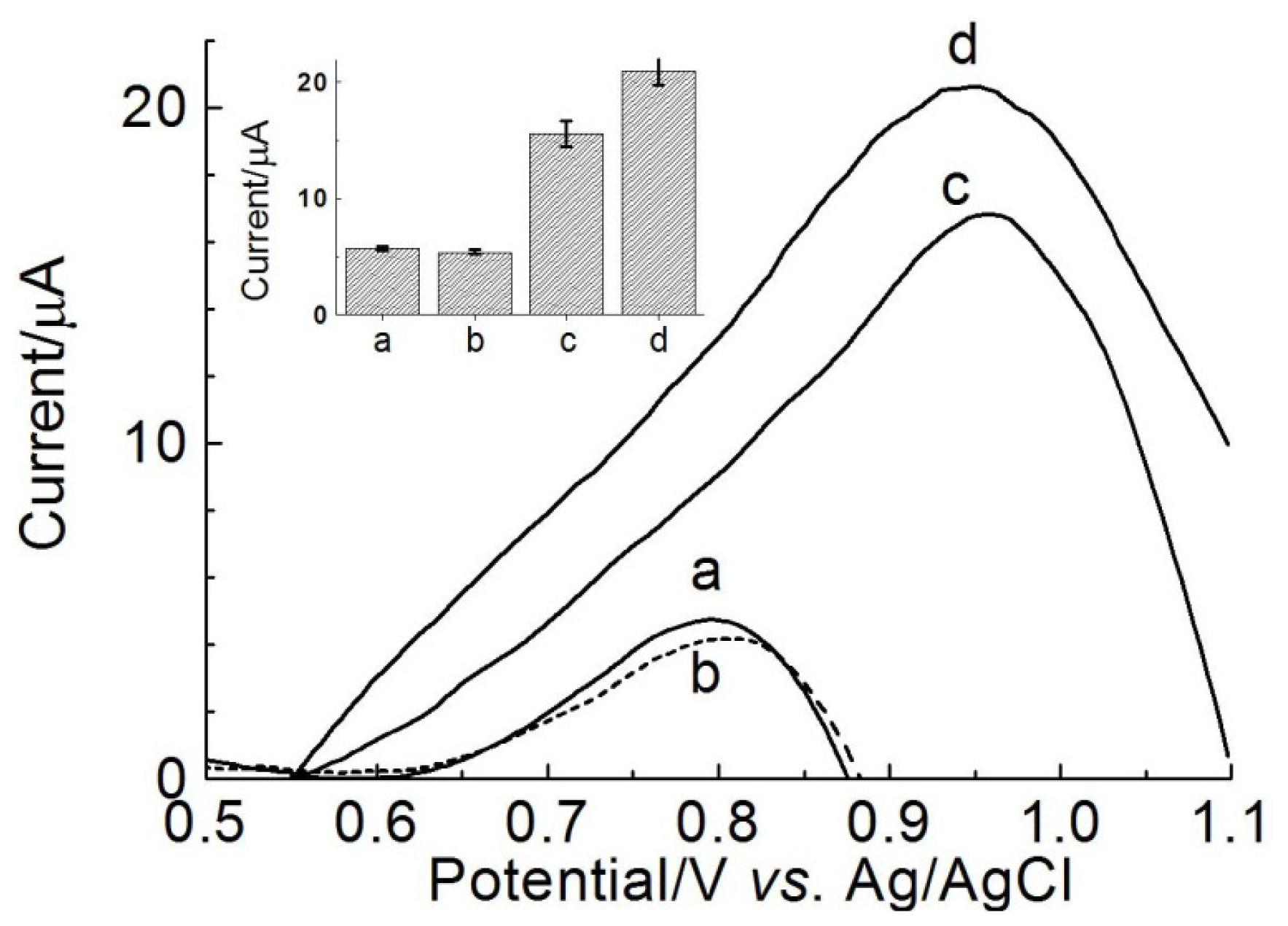
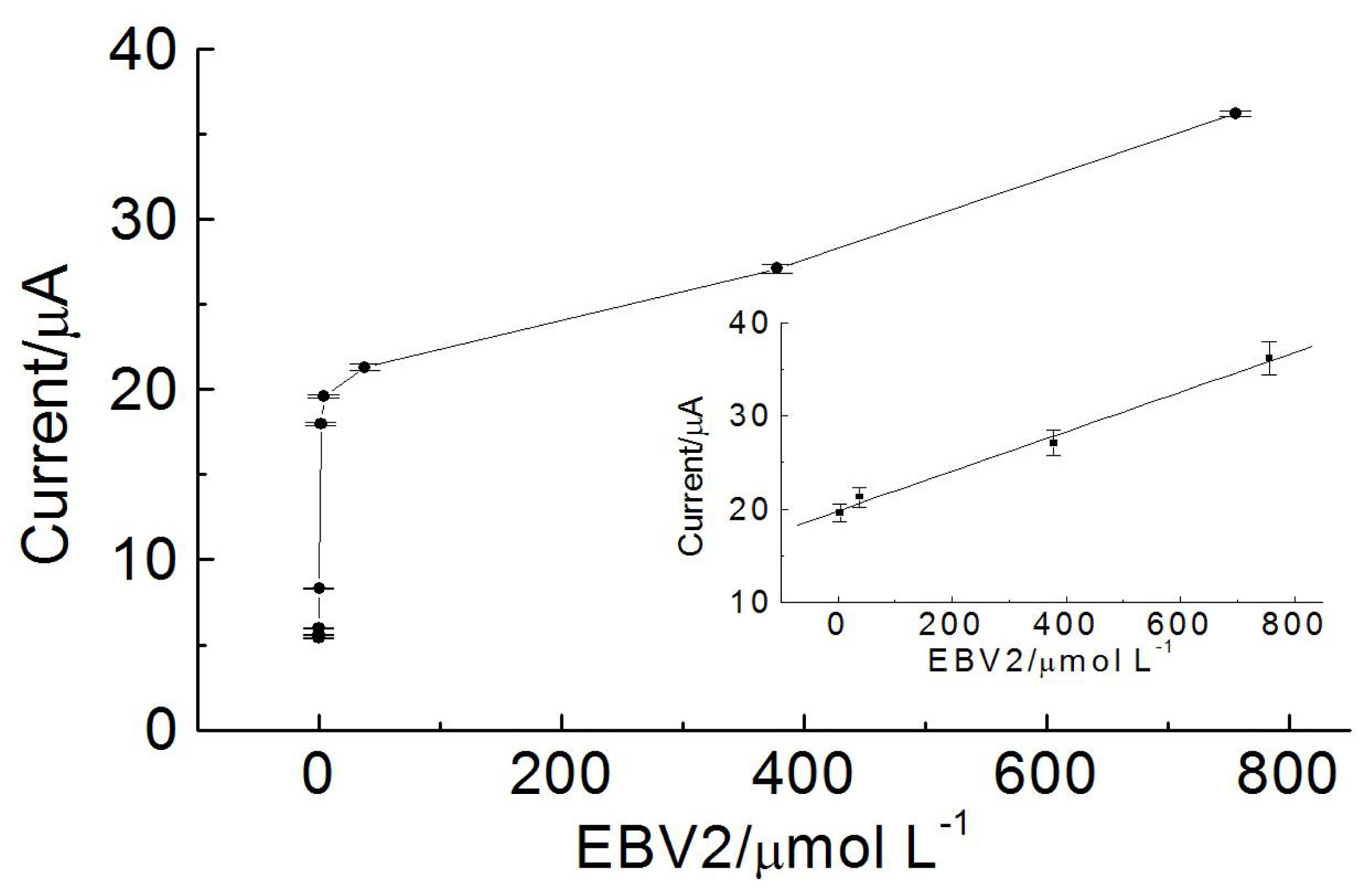
2.5. Calibration Curve
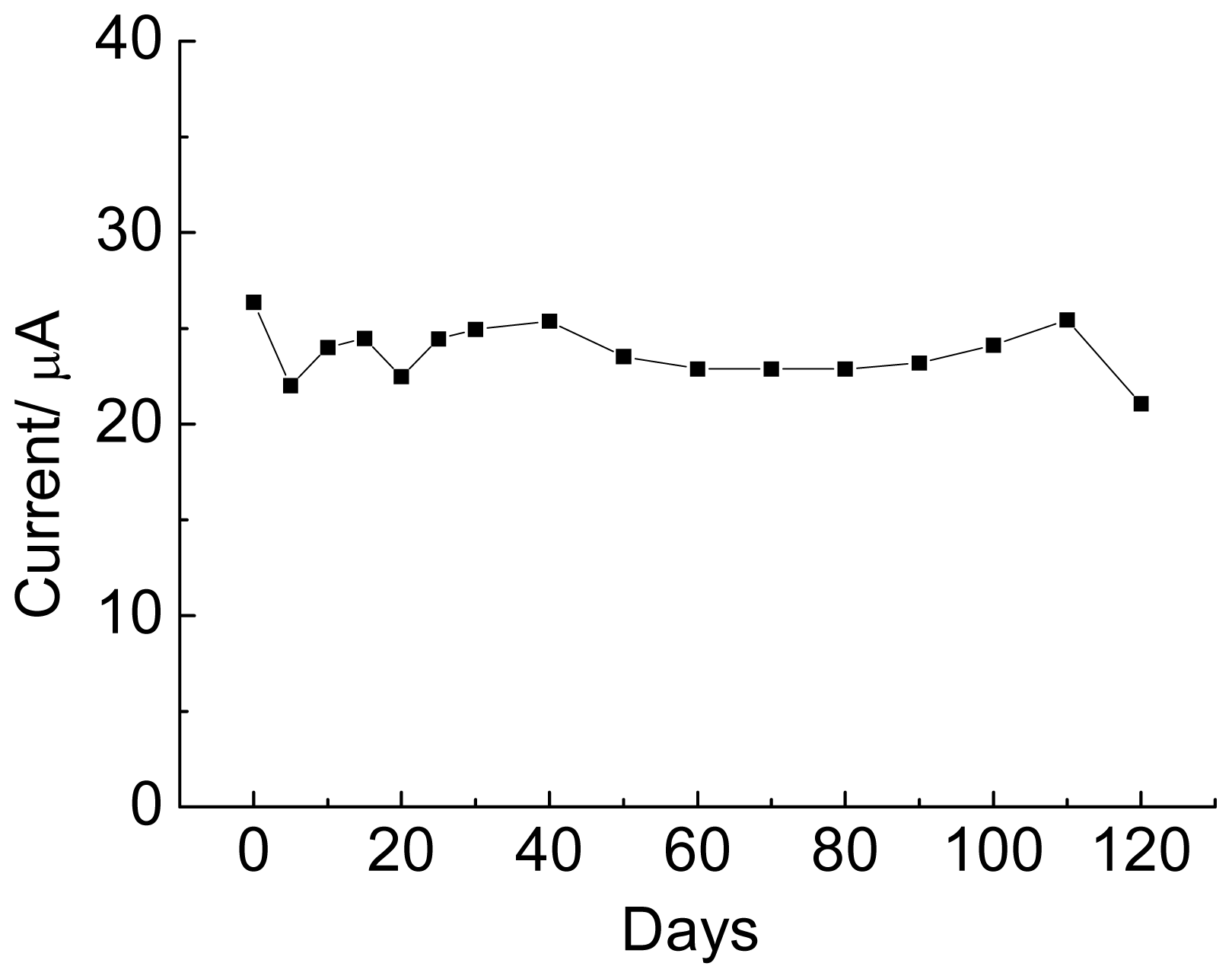
2.6. Genosensor Stability
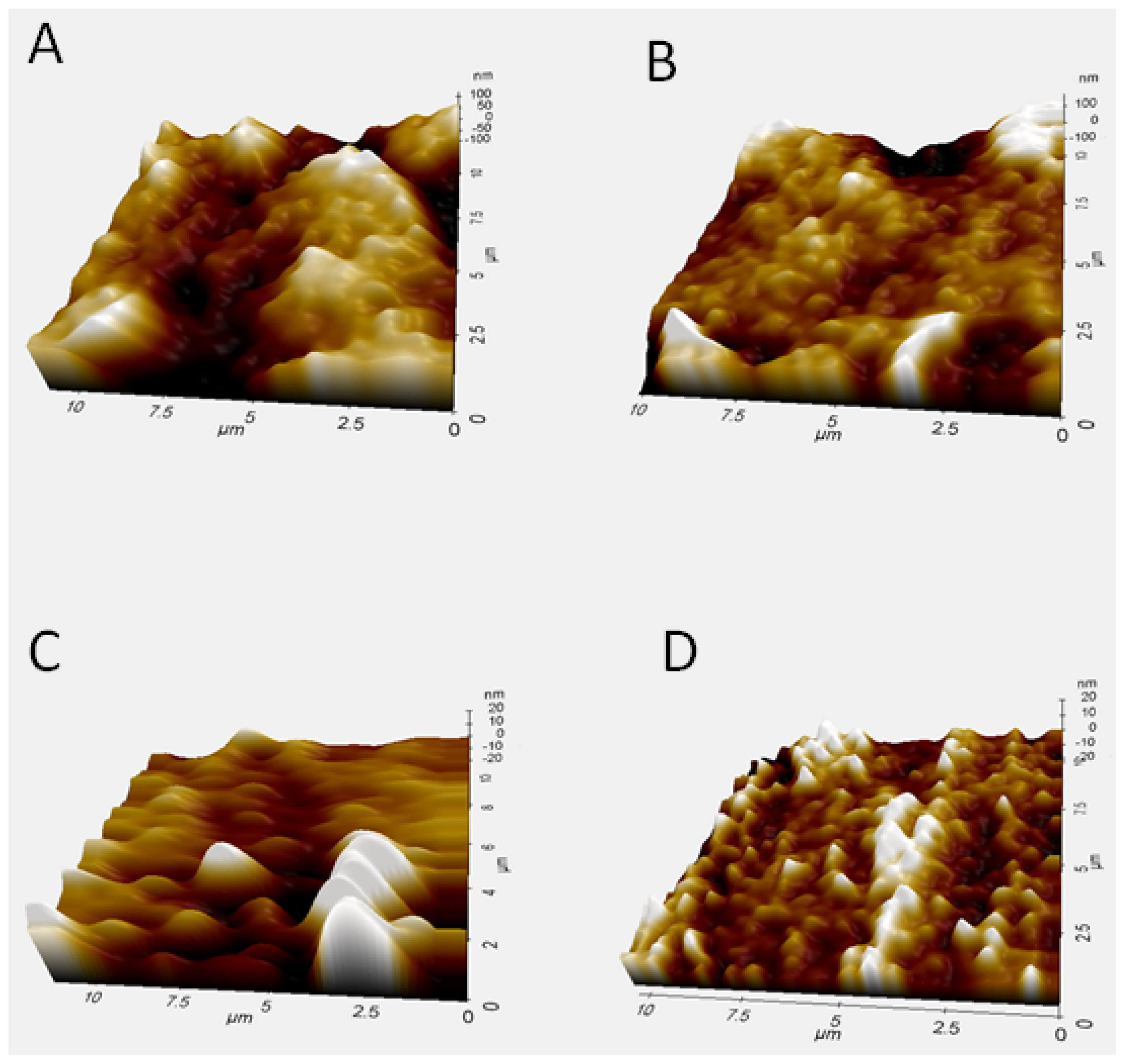
2.7. Morphological Characterization of the Genosensor Using Atomic Force Microscopy
3. Experimental Section
3.1. Reagents
3.2. Apparatus
3.3. Electrochemical Polymerization
3.4. Electrochemical Characterization
3.5. Oligonucleotide Immobilization onto Graphite Electrode/Poly(4-ATP)
3.6. Investigation on the Hybridization of Oligonucleotide Immobilized onto Poly(4-Atp) Using Guanine and Adenine Monitoring or Ethidium Bromide as Redox Indicator
3.7. Specificity of the Biosensor
3.8. Analysis of Interfering Compounds
3.9. Stability Studies
3.10. Calibration Curve
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsAll authors contributed equally to this work.
References
- Cohen, J.I. Epstein-Barr virus infection. N. Engl. J. Med 2000, 343, 481–492. [Google Scholar]
- González, X.; Correnti, M.; Rivera, H.; Perrone, M. Epstein-Barr virus detection and latent membrane protein 1 in oral hairy leukoplakia in HIV+ venezuelan patients. Med. Oral Patol. Oral Cir. Bucal 2010, 15, 297–302. [Google Scholar]
- Dolcetti, R.; Masucci, M.G. Epstein-Barr virus: Induction and control of cell transformation. J. Cell. Physiol 2003, 196, 207–218. [Google Scholar]
- Al Moustafa, A.E.; Chen, D.; Ghabreau, L.; Akil, N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med. Hypotheses 2009, 73, 184–186. [Google Scholar]
- Middeldorp, J.M.; Brink, A.A.T.P.; van den Brule, A.J.C.; Meijer, C.J.L.M. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit. Rev. Oncol. Hematol 2003, 45, 1–36. [Google Scholar]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar]
- Niller, H.H.; Wolf, H.; Ay, E.; Minarovits, J. Epigenetic deregulation of Epstein-Barr virus latency and development of autoimmune disease. Adv. Exp. Med. Biol 2011, 711, 82–102. [Google Scholar]
- Toussirot, E.; Roudier, J. Epstein-Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol 2008, 22, 883–896. [Google Scholar]
- Dai, X.Z.; Chen, S.H.; Ge, J.; Han, X.Q.; Zhou, X.H.; Wu, Z.Q.; Zhao, T. Expression pattern of HSAo-miR-9 and its association with BCL6 in EBV-positive and EBV-negative Burkitt’s lymphoma cell lines. Nan Fang Yi Ke Da Xue Xue Bao 2013, 33, 661–666. (In Chinese) [Google Scholar]
- Weiss, L.M.; Chen, Y.Y. EBER in situ hybridization for Epstein-Barr virus. Methods Mol. Biol 2013, 999, 223–230. [Google Scholar]
- Calore, E.E.; Pérez, N.M.; Martins, J.F.; Cárdenas, P.G. Immunohistochemistry in a case of EBV encephalitis. Rev. Chil. Infectol 2012, 29, 687–688. [Google Scholar]
- Strong, M.J.; Xu, G.; Coco, J.; Baribault, C.; Vinay, D.S.; Lacey, M.R.; Strong, A.L.; Lehman, T.A.; Seddon, M.B.; Lin, Z.; et al. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: Implications for possible immune adjuvant therapy. PLoS Pathog 2013, 9, e1003341. [Google Scholar]
- Goulart, L.R.; Vieira, C.U.; Freschi, A.P.; Capparelli, F.E.; Fujimura, P.T.; Almeida, J.F.; Ferreira, L.F.; Goulart, I.M.; Brito-Madurro, A.G.; Madurro, J.M. Biomarkers for serum diagnosis of infectious diseases and their potential application in novel sensor platforms. Crit. Rev. Immunol 2010, 30, 201–222. [Google Scholar]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensor. Nat. Biotechnol 2003, 21, 1192–1199. [Google Scholar]
- Kerman, K.; Kobayashi, M.; Tamiya, E. Recent trends in electrochemical DNA biosensor technology. Meas. Sci. Technol 2003, 15, R1–R11. [Google Scholar]
- Wang, J. Survey and summary from DNA biosensor to gene chip. Nucleic Acids Res 2000, 28, 3011–3016. [Google Scholar]
- Popovich, N.D.; Eckhardt, A.E.; Mikulecky, J.C.; Napier, M.E.; Thomas, R.S. Electrochemical sensor for detection of unmodified nucleic acids. Talanta 2002, 56, 821–828. [Google Scholar]
- Wang, J.; Palecek, E.; Nielsen, P.E.; Rivas, G.; Cai, X.; Shiraishi, H.; Dontha, N.; Luo, D.; Farias, P.A.M. Peptide nucleic acid probes for sequence-specific DNA biosensors. J. Am. Chem. Soc 1996, 118, 7667–7670. [Google Scholar]
- Liu, L.; Shen, B.; Shi, J.; Liu, F.; Lu, G.-Y.; Zhu, J.-J. A novel mediator-free biosensor based on co-intercalation of DNA and hemoglobin in the interlayer galleries of [α]-zirconium phosphate. Biosens. Bioelectron 2010, 25, 2627–2632. [Google Scholar]
- Wang, J.; Rivas, G.; Fernandes, J.R.; Paz, J.L.L.; Jiang, M.; Waymire, R. Indicator-free electrochemical DNA hybridization biosensor. Anal. Chim. Acta 1998, 375, 197–203. [Google Scholar]
- Chen, Z.-W.; Balamurugan, A.; Chen, S.-M. Detection of DNA by using bio-conducting polymer-Nile blue composite electrode; Nile blue as an indicator. Bioelectrochemistry 2009, 75, 13–18. [Google Scholar]
- Niu, S.; Zhao, M.; Hu, L.; Zhang, S. Carbon nanotube-enhanced DNA biosensor for DNA hybridization detection using rutin-Mn as electrochemical indicator. Sens. Actuators B 2008, 135, 200–205. [Google Scholar]
- Budnikov, H.C.; Evtugyn, G.A.; Porfireva, A.V. Electrochemical DNA sensors based on electropolymerized materials. Talanta 2012, 102, 137–155. [Google Scholar]
- Nambiar, S.; Yeow, J.T. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron 2011, 26, 1825–1832. [Google Scholar]
- Silva, F.B.; Vieira, S.N.; Filho, L.R.G.; Boodts, J.F.C.; Brito-Madurro, A.G.; Madurro, J.M. Electrochemical investigation of oligonucleotide-DNA hybridization on poly(4-methoxy phenethylamine). Int. J. Mol. Sci 2008, 9, 1173–1187. [Google Scholar]
- Ahuja, T.; Mir, I.A.; Kumar, D. Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 2007, 28, 791–805. [Google Scholar]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci 2007, 32, 876–921. [Google Scholar]
- Ferreira, L.F.; Boodts, J.F.C.; Brito-Madurro, A.G.; Madurro, J.M. Gold electrodes modified with poly(4-aminophenol): Incorporation of nitrogenated bases and an oligonucleotide. Polym. Int 2008, 57, 644–650. [Google Scholar]
- Franco, D.L.; Afonso, A.S.; Ferreira, L.F.; Gonçalves, R.A.; Boodts, J.F.C.; Brito-Madurro, A.G.; Madurro, J.M. Electrodes modified with polyaminophenols: Immobilization of purines and pyrimidines. Polym. Eng. Sci 2008, 48, 2043–2050. [Google Scholar]
- Brito-Madurro, A.G.; Ferreira, L.F.; Vieira, S.N.; Goulart, L.R.; Madurro, J.M. Immobilization of purine bases in poly-4-aminophenol matrix. J. Mater. Sci 2007, 42, 3238–3243. [Google Scholar]
- Vieira, S.N.; Ferreira, L.F.; Franco, D.L.; Afonso, A.S.; Gonçalves, R.A.; Brito-Madurro, A.G.; Madurro, J.M. Electrochemical modification of graphite electrodes with poly(4-aminophenol). Macromol. Symp 2006, 245–246, 236–242. [Google Scholar]
- Castro, C.M.; Vieira, S.N.; Gonçalves, R.A.; Madurro, A.G.B.; Madurro, J.M. Electrochemical and morphologic studies of nickel incorporation on graphite electrodes modified with polytyramine. J. Mater. Sci 2008, 43, 475–482. [Google Scholar]
- Ferreira, L.F.; Souza, L.M.; Franco, D.L.; Castro, A.C.H.; Oliveira, A.A.; Boodts, J.F.C.; Brito-Madurro, A.G.; Madurro, J.M. Formation of novel polymeric films derived from 4-hydroxybenzoic acid. Mater. Chem. Phys 2011, 129, 46–52. [Google Scholar]
- Oliveira, R.M.L.; Vieira, S.N.; Alves, H.C.; França, E.G.; Franco, D.L.; Ferreira, L.F.; Brito-Madurro, A.G.; Madurro, J.M. Voltammetric determination of all DNA nucleotides. J. Mater. Sci 2010, 45, 475–482. [Google Scholar]
- Santos, O.S.; Nascimento, R.; Rodrigues, L.P.; Santos, F.A.A.; Faria, P.C.B.; Martins, J.R.S.; Brito-Madurro, A.G.; Madurro, J.M.; Goulart, L.R. Functional epitope core motif of the Anaplasma marginale major surface protein 1a and its incorporation onto bioelectrodes for antibody detection. PLoS One 2012, 7, e33045. [Google Scholar]
- Silva, T.A.R.; Ferreira, L.F.; Boodts, J.F.C.; Eiras, S.P.; Madurro, J.M.; Brito-Madurro, A.G. Poly(4-hydroxyphenylacetic acid): A new material for immobilization of biomolecules. Polym. Eng. Sci 2008, 48, 1963–1970. [Google Scholar]
- Coelho, N.M.; Melo, E.I.; Rezende, H.C.; Madurro, A.G.B.; Madurro, J.M. Surface properties of sensors based on aminophenol-polymerized film. J. Solid State Electrochem 2012, 16, 945–951. [Google Scholar]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanism of pyrrole electropolymerization. Chem. Soc. Rev 2000, 29, 283–293. [Google Scholar]
- Oh, E.J.; Jang, K.S.; MacDiarmid, A.G. High molecular weight soluble polypyrrole. Synth. Met 2002, 125, 267–272. [Google Scholar]
- Sonmez, G.; Schottland, P.; Reynolds, J.R. PEDOT/PAMPS: An electrically conductive polymer composite with electrochromic and cation exchange properties. Synth. Met 2005, 155, 130–137. [Google Scholar]
- La-Scalea, M.A.; Serrano, S.H.P.; Gutz, I.G.R. Eletrodos modificados com DNA: Uma nova alternativa em eletroanálise. Quím. Nova 1999, 22, 417–424. [Google Scholar]
- Tran, L.D.; Piro, B.; Pham, M.C.; Ledoam, T.; Angiari, C.; Dao, L.H.; Teston, F. A polytyramine film for covalent immobilization of oligonucleotides and hybridization. Synth. Met 2003, 139, 251–262. [Google Scholar]
- Oliveira-Brett, A.M.; Piedade, J.A.; Silva, L.A.; Diculescu, V.C. Voltammetric determination of all DNA nucleotides. Anal. Biochem 2004, 332, 321–329. [Google Scholar]
- Yang, M.; McGovern, M.E.; Thompson, M. Genosensor technology and the detection of interfacial nucleic acid chemistry. Anal. Chem. Acta 1997, 346, 259–275. [Google Scholar]
- Watkins, T.I.; Woolfe, G. Effect of changing the quaternizing group on the trypanocidal activity of dimidium bromide. Nature 1952, 169, 506–507. [Google Scholar]
- Bugs, M.R.; Cornelio, M.L. Analysis of the ethidium bromide bound to DNA by photoacoustic and FTIR spectroscopy. Photochem. Photobiol 2001, 74, 512–520. [Google Scholar]
- Minasyan, S.H.; Tavadyan, L.A.; Antonyan, A.P.; Davtyan, H.G.; Parsadanyan, M.A.; Vardevanyan, P.O. Differential pulse voltammetric studies of ethidium bromide binding to DNA. Bioelectrochemistry 2006, 68, 48–55. [Google Scholar]
- Oliveira, C.B.; Nascimento, V.B. Electrochemical oxidation mechanism of ethidium bromide at a glassy carbon electrode. Electroanalysis 2013, 25, 2117–2123. [Google Scholar]
- Hu, X.; Wang, Q.; He, P.; Fang, Y. Spectroelectrochemical study on the electrochemical reduction of ethidium bromide. Anal. Sci 2002, 18, 645–650. [Google Scholar]
- Zhang, C.; Liu, L.; Wang, J.; Rong, F.; Fu, D. Electrochemical degradation of ethidium bromide using boron-doped diamond. Sep. Purif. Technol 2013, 107, 91–101. [Google Scholar]
- Wei, Y.; Liu, Z.; Gao, C.; Wang, L.; Liu, J.; Huang, X. Electrochemical sensors and biosensors based on nanomaterials: A new approach for detection of organic micropollutants. Prog. Chem 2010, 24, 616–627. [Google Scholar]
- Waring, M.; Makoff, A. Breakdown of pulse-labeled ribonucleic acid and polysomes in bacillus megaterium: Actions of streptolydigin, echinomycin, and triostins. Mol. Pharmacol 1974, 10, 214–224. [Google Scholar]
- Saxena, U.; Chakraborty, M.; Goswami, P. Covalent immobilization of cholesterol oxidase on self-assembled gold nanoparticles for highly sensitive amperometric detection of cholesterol in real samples. Biosens. Bioelectron 2011, 26, 3037–3043. [Google Scholar]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar]
- Hsu, C.T.; Hsiao, H.C.; Lee, M.S.; Chang, S.F.; Lee, T.C.; Tsai, Y.S.; Zen, J.M. Assessing the quality of bionime self-monitoring blood glucose system rightest GM110: A critical evaluation of interference and ambient circumstances. Clin. Chim. Acta 2009, 402, 119–123. [Google Scholar]
- Gokel, G.W. Dean’s Handbook of Organic Chemistry, 2nd ed; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Rubio-Retama, J.; Lopez-Cabarcos, E.; Lopez-Ruiz, B. High stability amperometric biosensor based on enzyme entrapment in Microgels. Talanta 2005, 68, 99–107. [Google Scholar]
- Medard, C.; Morin, M. Chemisorption of aromatic thiols onto a glassy carbon surface. J. Electroanal. Chem 2009, 632, 120–126. [Google Scholar]
- Lassale, N.; Mailley, P.; Vieil, E.; Vilache, T.; Roget, A.; Correia, J.P.; Abrantes, L.M. Electronically conductive polymer grafted with oligonucleotides as electrosensors of DNA. Preliminary study of real time monitoring by in situ techniques. J. Electroanal. Chem 2001, 509, 48–57. [Google Scholar]
| Organism | BLASTn | |||
|---|---|---|---|---|
| Query cover | E value | Max ident | Access number | |
| Epstein-Barr virus (EBV) genome, strain B95-8 | 100% | 0.079 | 100% | V01555.2 |
| Epstein-Barr virus (B95-8 isolate) | 100% | 0.079 | 100% | K03333.1 |
| Epstein-Barr virus (AG876 isolate) | 100% | 0.079 | 100% | K03332.1 |
| Epstein-Barr virus, artifactual joining of B95-8 | 100% | 0.079 | 100% | M80517.1 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Balvedi, R.P.A.; Castro, A.C.H.; Madurro, J.M.; Brito-Madurro, A.G. Detection of a Specific Biomarker for Epstein-Barr Virus Using a Polymer-Based Genosensor. Int. J. Mol. Sci. 2014, 15, 9051-9066. https://doi.org/10.3390/ijms15059051
Balvedi RPA, Castro ACH, Madurro JM, Brito-Madurro AG. Detection of a Specific Biomarker for Epstein-Barr Virus Using a Polymer-Based Genosensor. International Journal of Molecular Sciences. 2014; 15(5):9051-9066. https://doi.org/10.3390/ijms15059051
Chicago/Turabian StyleBalvedi, Renata P. A., Ana C. H. Castro, João M. Madurro, and Ana G. Brito-Madurro. 2014. "Detection of a Specific Biomarker for Epstein-Barr Virus Using a Polymer-Based Genosensor" International Journal of Molecular Sciences 15, no. 5: 9051-9066. https://doi.org/10.3390/ijms15059051




