Synthesis and Bioactivity of 5-Substituted-2-furoyl Diacylhydazide Derivatives with Aliphatic Chain
Abstract
:1. Introduction
2. Results and Discussion
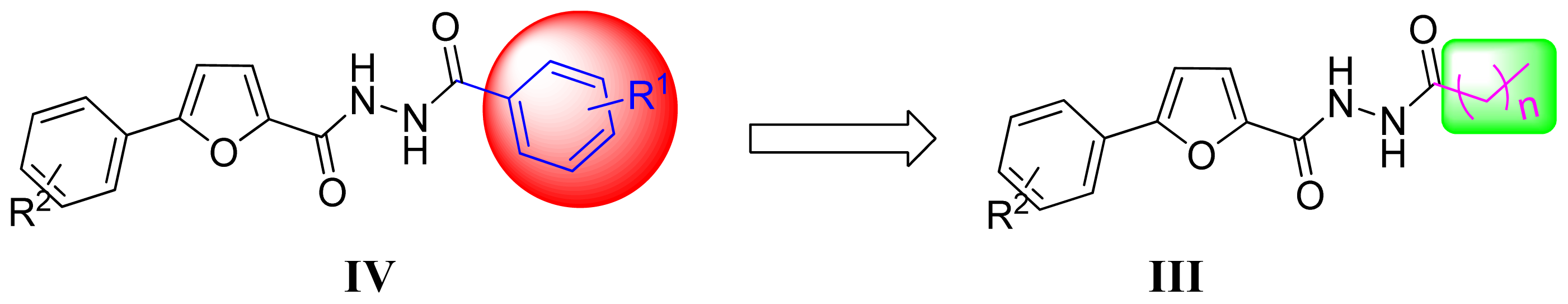
2.1. Synthesis and Structure Elucidation
2.2. Bioassay
2.2.1. Insecticidal Activity
2.2.2. Fungicidal Activity
2.2.3. Anti-Tumor Activity
3. Experimental Section
3.1. General Information
3.2. Synthetic Procedures
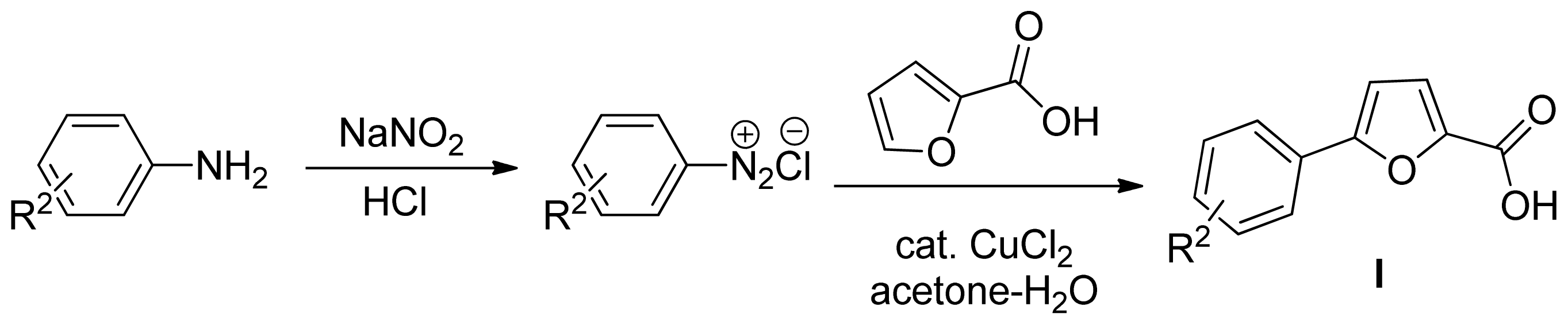
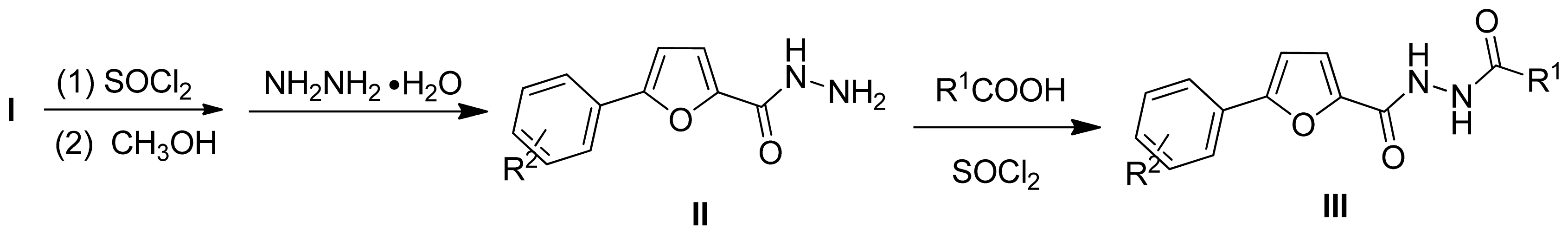
3.2.1. General Synthetic Procedure for the Key Intermediates
3.2.2. General Synthetic Procedure for the Title Compounds III
3.3. Crystallography
3.4. Bioassay
3.4.1. Insecticidal Activity
3.4.2. Fungicidal Activity
3.4.3. Anti-Tumor Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsConceived and designed the experiments: Z.C. and X.Y. Performed the experiments: Z.C., X.L., and X.Y. Analyzed the data: Z.C. and F.T. Wrote the paper: Z.C.
References
- Fox, H.H. Chemical approach to the control of tuberculosis. Science 1952, 116, 129–134. [Google Scholar]
- Magliozzo, R.S.; Marcinkeviciene, J.A. Evidence for isoniazid oxidation by oxyferrous mycobacterial catalase-peroxidase. J. Am. Chem. Soc 1996, 118, 11303–11304. [Google Scholar]
- Joshi, S.D.; More, Y.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S.; Kulkarni, V.H. Synthesis of new 4-(2,5-dimethylpyrrol-1-yl)/4-pyrrol-1-ylbenzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial, antifungal and antitubercular agents. Med. Chem. Res 2013, 22, 1073–1089. [Google Scholar]
- Vigorita, M.G.; Maccari, R.; Ottana, R.; Monforte, F. Lipophilic analogs of isoniazid with antiproliferative in vitro activity–VIII. Med. Chem. Res 1999, 9, 306–321. [Google Scholar]
- Sechi, M.; Azzena, U.; Delussu, M.P.; Dallocchio, R.; Dessi, A.; Cosseddu, A.; Pala, N.; Neamati, N. Design and synthesis of bis-amide and hydrazide-containing derivatives of malonic acid as potential HIV-1 integrase inhibitors. Molecules 2008, 13, 2442–2461. [Google Scholar]
- Arvadia, P.; Narwaley, M.; Whittal, R.M.; Siraki, A.G. 4-Aminobenzoic acid hydrazide inhibition of microperoxidase-11: Catalytic inhibition by reactive metabolites. Arch. Biochem. Biophys 2011, 515, 120–126. [Google Scholar]
- Watson, K.A.; Mitchell, E.P.; Johnson, L.N.; Son, J.C.; Bichard, C.J.F.; Orchard, M.G.; Fleet, G.W.J.; Oikonomakos, N.G.; Leonidas, D.D.; Kontou, M.; et al. Design of inhibitors of glycogen phosphorylase: A study of α- and β-C-glucosides and 1-thio-β-d-glucose compounds. Biochemistry 1994, 33, 5745–5758. [Google Scholar]
- Wing, K.D. RH5849, a nonsteroidal ecdysone agonist: Effects on a Drosophila cell line. Science 1988, 241, 467–469. [Google Scholar]
- Wing, K.D.; Slawecki, R.A.; Carlson, G.R. RH 5849, a nonsteroidal ecdysone agonist: Effects on larval. Lepidoptera. Science 1988, 241, 470–472. [Google Scholar]
- Cowles, R.S.; Villani, M.G. Susceptibility of Japanese beetle, Oriental beetle, and European chafer (Coleoptera: Scarabaeidae) to halofenozide, an insect growth regulator. J. Econ. Entomol 1996, 89, 1356–1365. [Google Scholar]
- Moulin, A.; Bibian, M.; Blayo, A.-L.; El Habnouni, S.; Martinez, J.; Fehrentz, J.-A. Synthesis of 3,4,5-trisubstituted-1,2,4-triazoles. Chem. Rev 2010, 110, 1809–1827. [Google Scholar]
- Cui, Z.N.; Shi, Y.X.; Zhang, L.; Ling, Y.; Li, B.J.; Nishida, Y.; Yang, X.L. Synthesis and fungicidal activity of novel 2,5-disubstituted-1,3,4-oxadiazole derivatives. J. Agric. Food Chem 2012, 60, 11649–11656. [Google Scholar]
- Cui, Z.N.; Yang, L.; Li, X.C.; Wang, Z.; Yang, X.L. Progress in the study on the synthesis and the activities as insect growth regulators of 2,5-disubstituted 1,3,4-oxadiazoles. Chin. J. Org. Chem 2006, 26, 1647–1656. [Google Scholar]
- Nagendra, G.; Lamani, R.S.; Narendra, N.; Sureshbabu, V.V. A convenient synthesis of 1,3,4-thiadiazole and 1,3,4-oxadiazole based peptidomimetics employing diacylhydrazines derived from amino acids. Tetrahedron Lett 2010, 51, 6338–6341. [Google Scholar]
- Saracoglu, N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. Tetrahedron 2007, 63, 4199–4236. [Google Scholar]
- Cui, Z.N.; Yang, X.L.; Shi, Y.X.; Uzawa, H.; Cui, J.R.; Dohi, H.; Nishida, Y. Molecular design, synthesis and bioactivity of glycosyl hydrazine and hydrazone derivatives: Notable effects of the sugar moiety. Bioorg. Med. Chem. Lett 2011, 21, 7193–7196. [Google Scholar]
- Cui, Z.N.; Zhang, L.; Huang, J.; Yang, X.L.; Ling, Y. Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(III). Chin. J. Chem 2010, 28, 1257–1266. [Google Scholar]
- Cui, Z.N.; Zhang, L.; Huang, J.; Ling, Y.; Yang, X.L. Synthesis, insecticidal activity and 3D-QSAR studies on diacylhydrazine derivatives containing furan. Chin. J. Org. Chem 2010, 30, 1482–1491. [Google Scholar]
- Zhang, L.; Cui, Z.N.; Yin, B.; Yang, G.F.; Ling, Y.; Yang, X.L. QSAR and 3D-QSAR studies of the diacyl-hydrazine derivatives containing furan rings based on the density functional theory. Sci. China Chem 2010, 53, 1322–1331. [Google Scholar]
- Li, X.C.; Yang, X.L.; Cui, Z.N.; Li, Y.; He, H.W.; Ling, Y. Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(II). Chin. J. Chem 2010, 28, 1233–1239. [Google Scholar]
- Cui, Z.N.; Huang, J.; Li, Y.; Ling, Y.; Yang, X.L.; Chen, F.H. Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(I). Chin. J. Chem 2008, 26, 916–922. [Google Scholar]
- Cui, Z.N.; Wang, Z.; Li, Y.; Zhou, X.Y.; Ling, Y.; Yang, X.L. Synthesis of 5-(chlorophenyl)- 2-furancarboxylic acid 2-(benzoyl)hydrazide derivatives and determination of their insecticidal activity. Chin. J. Org. Chem 2007, 27, 1300–1304. [Google Scholar]
- Cui, Z.N.; Li, Y.; Huang, J.; Ling, Y.; Cui, J.R.; Wang, R.Q.; Yang, X.L. New class of potent antitumor acylhydrazone derivatives containing furan. Eur. J. Med. Chem 2010, 45, 5576–5584. [Google Scholar]
- Li, X.H.; Cui, Z.N.; Chen, X.Y.; Wu, D.C.; Qi, Z.Q.; Ji, M.S. Synthesis of 2-acyloxycyclohexyl sulfonamides and evaluation on their fungicidal activity. Int. J. Mol. Sci 2013, 14, 22544–22557. [Google Scholar]
- Li, X.H.; Pan, Q.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo-and 2-hydroxycycloalkylsulfonamides. Lett. Drug Des. Discov 2013, 10, 353–359. [Google Scholar]
- Cui, Z.N.; Shi, Y.X.; Cui, J.R.; Ling, Y.; Li, B.J.; Yang, X.L. Synthesis and bioactivities of novel pyrazole and triazole derivatives containing 5-phenyl-2-furan. Chem. Biol. Drug Des 2012, 79, 121–127. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst 1991, 83, 757–766. [Google Scholar]

| Empirical formula | C14H13ClN2O3 |
|---|---|
| Formula weight | 292.71 |
| T | 113(2) K |
| Wavelength | 0.71070 Å |
| Crystal system | Orthorhombic |
| Space group | C 2 2 21 |
| Unit cell dimensions | a = 8.4488(4) Å, α = 90° b = 20.1074(13) Å, β = 90° c = 16.1363(10) Å, γ = 90° |
| Volume | 2741.3(3) Å3 |
| Z | 8 |
| Dx | 1.418 mg·m−3 |
| Absorption coefficient | 0.287 mm−1 |
| F (0 0 0) | 1216 |
| Crystal dimensions | 0.22 × 0.20 × 0.20 mm |
| θ range for data collection | 2.03 to 27.85 |
| Completeness to θ = 27.85 | 99.9% |
| Limiting indices | −11 ≤ h ≤ 10, −26 ≤ k ≤ 23,−21 ≤ l ≤ 21 |
| Reflection collected/unique | 12970/3269 [R(int) = 0.0486] |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9448 and 0.9395 |
| Data/restraints/parameters | 3269/0/184 |
| Goodness-of-fit on F2 | 1.101 |
| Final R indices [I > 2σ (I)] | R1 = 0.0693, wR2 = 0.1949 |
| 2θmax | 55.7° with Mo Kα |
| (Δρ)max | 1.204 eÅ−3 |
| (Δρ)min | −0.406 eÅ−3 |
| Program system | SHELXS-97, SHELXL-97 |
| Structure determination | Direct method |
| Refinement | Full-matrix least-squares on F2 |
| CCDC No. | 935116 |
| Lengths | (Å) | Angles | (°) | Torsion angles | (°) |
|---|---|---|---|---|---|
| Cl(1)–C(1) | 1.825(5) | C(11)–N(1)–N(2) | 118.4(4) | C(11)–N(1)–N(2)–C(12) | 102.3(5) |
| N(1)–C(11) | 1.355(6) | C(12)–N(2)–N(1) | 120.2(4) | C(6)–C(1)–C(2)–C(3) | 2.3(7) |
| N(1)–N(2) | 1.396(5) | C(10)–O(1)–C(7) | 106.8(4) | Cl(1)–C(1)–C(2)–C(3) | −177.6(3) |
| N(2)–C(12) | 1.343(6) | C(2)–C(1)–Cl(1) | 119.1(4) | O(1)–C(7)–C(8)–C(9) | 2.5(5) |
| O(1)–C(10) | 1.364(5) | O(1)–C(7)–C(4) | 117.2(4) | N(2)–N(1)–C(11)–O(2) | −1.6(7) |
| O(2)–C(11) | 1.238(5) | O(2)–C(11)–N(1) | 123.5(4) | N(2)–N(1)–C(11)–C(10) | 176.9(4) |
| O(3)–C(12) | 1.218(5) | O(3)–C(12)–N(2) | 124.2(4) | O(1)–C(10)–C(11)–O(2) | 171.7(3) |
| C(12)–C(13) | 1.531(6) | N(2)–C(12)–C(13) | 112.8(3) | N(1)–N(2)–C(12)–O(3) | 5.8(7) |
| C(8)–C(9) | 1.386(7) | C(14)–C(13)–C(12) | 109.3(5) | N(1)–N(2)–C(12)–C(13) | −175.2(4) |
| C(9)–C(10) | 1.368(7) | O(3)–C(12)–C(13) | 123.0(4) | O(3)–C(12)–C(13)–C(14) | −70.8(6) |
| C(10)–C(11) | 1.465(6) | C(6)–C(1)–C(2) | 122.5(4) | N(2)–C(12)–C(13)–C(14) | 110.2(5) |
| C(4)–C(7) | 1.455(6) | O(1)–C(7)–C(8) | 109.1(4) | C(10)–O(1)–C(7)–C(4) | 177.6(3) |
| Different planes | Dihedral angles (°) | Defination of the planes | The mean deviation of the plane (Å) |
|---|---|---|---|
| Plane I and plane II | 10.0 | Plane I (C1 to C6) | 0.0153 |
| Plane I and plane III | 12.4 | Plane II (C7 to C10, O1) | 0.0150 |
| Plane I and plane IV | 56.9 | Plane III (O2, C11, N1, N2) | 0.0047 |
| Plane II and plane III | 6.8 | Plane IV (O3, C12, C13, C14) | 0.3708 |
| Plane II and plane IV | 47.7 | ||
| Plane III and plane IV | 50.6 |
| Compd. | R1 | R2 | Plutella xylostella | Mythimna separata | Culex pipiens pallens |
|---|---|---|---|---|---|
| Larvicidal activity (%) at 200 mg·L−1 | Larvicidal activity (%) at 200·mg L−1 | Larvicidal activity (%) at 10 mg·L−1 | |||
| III-3-1 | CH3 | 4-Cl | 45 | 30 | 20 |
| III-3-2 | CH2CH3 | 4-Cl | 40 | 30 | 20 |
| III-3-3 | n-C3H7 | 4-Cl | 55 | 40 | 30 |
| III-3-4 | i-C3H7 | 4-Cl | 30 | 45 | 45 |
| III-3-5 | n-C5H11 | 4-Cl | 35 | 30 | 20 |
| III-3-6 | n-C6H13 | 4-Cl | 25 | 35 | 10 |
| III-3-7 | n-C7H15 | 4-Cl | 30 | 30 | 10 |
| III-3-8 | n-C8H17 | 4-Cl | 10 | 15 | 20 |
| III-3-9 | n-C9H19 | 4-Cl | 15 | 10 | 30 |
| III-1-1 | CH3 | 2-Cl | 45 | 35 | 40 |
| III-2-1 | CH3 | 3-Cl | 20 | 20 | 10 |
| III-4-1 | CH3 | 2-F | 35 | 30 | 45 |
| III-5-1 | CH3 | 3-F | 10 | 10 | 20 |
| III-6-1 | CH3 | 4-F | 35 | 30 | 40 |
| III-7-1 | CH3 | 2,4-di-F | 40 | 30 | 30 |
| III-8-1 | CH3 | 2,6-di-F | 60 | 50 | 60 |
| III-9-1 | CH3 | 2-NO2 | 25 | 20 | 30 |
| III-10-1 | CH3 | 3-NO2 | 35 | 10 | 20 |
| III-11-1 | CH3 | 4-NO2 | 20 | 30 | 20 |
| III-12-1 | CH3 | H | 10 | 35 | 35 |
| III-13-1 | CH3 | 4-CH3 | 35 | 30 | 25 |
| III-14-1 | CH3 | 4-OCH3 | 30 | 25 | 20 |
| III-15-1 | CH3 | 4-Br | 10 | 20 | 25 |
| IV-1 | 4-CH3 | 4-Cl | 88 | 85 | 90 |
| RH-5849 | 100 | 100 | 100 |
| Compd. | R1 | R2 | Control efficacy (%) | |||
|---|---|---|---|---|---|---|
| F. oxysporum | C. cassiicola | B. cinerea | R. solanii | |||
| III-3-1 | CH3 | 4-Cl | 33.41 ± 1.22 | 19.32 ± 0.93 | 84.23 ± 2.43 | 93.43 ± 1.63 |
| III-3-2 | CH2CH3 | 4-Cl | 34.23 ± 0.84 | 32.43 ± 1.23 | 61.23 ± 0.51 | 62.42 ± 1.61 |
| III-3-3 | n-C3H7 | 4-Cl | 16.32 ± 1.12 | 41.32 ± 1.31 | 52.34 ± 1.23 | 51.32 ± 1.52 |
| III-3-4 | i-C3H7 | 4-Cl | 15.76 ± 0.42 | 36.43 ± 1.49 | 55.13 ± 1.02 | 48.32 ± 0.81 |
| III-3-5 | n-C5H11 | 4-Cl | 9.32 ± 0.65 | 12.43 ± 0.91 | 11.35 ± 0.80 | 43.32 ± 1.41 |
| III-3-6 | n-C6H13 | 4-Cl | 33.62 ± 1.03 | 28.23 ± 0.54 | 27.53 ± 1.43 | 52.32 ± 0.97 |
| III-3-7 | n-C7H15 | 4-Cl | 11.42 ± 0.52 | 12.61 ± 0.61 | 25.12 ± 0.91 | 32.21 ± 0.72 |
| III-3-8 | n-C8H17 | 4-Cl | 21.34 ± 0.53 | 22.34 ± 0.72 | 30.52 ± 0.79 | 44.62 ± 0.72 |
| III-3-9 | n-C9H19 | 4-Cl | 19.43 ± 0.63 | 12.33 ± 0.59 | 32.39 ± 0.53 | 52.52 ± 1.02 |
| III-1-1 | CH3 | 2-Cl | 35.85 ± 0.55 | 28.24 ± 2.00 | 82.38 ± 3.02 | 84.13 ±1.16 |
| III-2-1 | CH3 | 3-Cl | 25.73 ± 1.35 | 38.62 ± 1.45 | 33.54 ± 2.12 | 48.63 ± 1.63 |
| III-4-1 | CH3 | 2-F | 18.61 ± 0.62 | 12.63 ± 1.27 | 62.36 ± 1.72 | 65.62 ± 1.82 |
| III-5-1 | CH3 | 3-F | 9.32 ± 0.24 | 13.72 ± 0.85 | 35.43 ± 1.53 | 25.53 ± 1.63 |
| III-6-1 | CH3 | 4-F | 18.52 ± 1.34 | 34.43 ± 1.62 | 48.45 ± 2.04 | 54.56 ± 1.23 |
| III-7-1 | CH3 | 2,4-di-F | 9.53 ± 0.53 | 23.83 ± 1.11 | 63.32 ± 2.21 | 51.42 ± 1.51 |
| III-8-1 | CH3 | 2,6-di-F | 13.21 ± 2.12 | 23.22 ± 1.42 | 36.13 ± 1.23 | 26.32 ± 1.26 |
| III-9-1 | CH3 | 2-NO2 | 62.34 ± 1.41 | 51.52 ± 2.34 | 82.62 ± 2.21 | 88.62 ± 1.62 |
| III-10-1 | CH3 | 3-NO2 | 47.34 ± 1.03 | 43.53 ± 1.34 | 37.43 ± 1.62 | 36.62 ± 1.63 |
| III-11-1 | CH3 | 4-NO2 | 34.62 ± 1.23 | 32.53 ± 1.72 | 92.52 ± 2.71 | 38.43 ± 0.53 |
| III-12-1 | CH3 | H | 32.61 ± 1.34 | 63.72 ± 1.23 | 66.62 ± 2.52 | 39.53 ± 2.32 |
| III-13-1 | CH3 | 4-CH3 | 12.82 ± 1.01 | 35.33 ± 1.73 | 27.63 ± 1.73 | 49.34 ± 1.35 |
| III-14-1 | CH3 | 4-OCH3 | 31.72 ± 1.72 | 28.92 ± 1.45 | 12.42 ± 1.43 | 52.54 ± 2.62 |
| III-15-1 | CH3 | 4-Br | 40.42 ± 2.51 | 18.62 ± 0.82 | 32.23 ± 0.66 | 23.72 ± 1.32 |
| IV-1 | 4-CH3 | 4-Cl | 19.78 ± 0.84 | 48.56 ± 1.21 | 21.45 ± 0.97 | 20.78 ± 1.06 |
| Acetone (blank control) | 1.92 ± 0.82 | 2.25 ± 0.72 | 1.83 ± 0.53 | 2.62 ± 0.84 | ||
| Fungicides # | 94.17 ± 1.80 a | 95.21 ± 1.94 b | 89.57 ± 2.15 c | 92.21 ± 2.41 d | ||
| Compd. | R1 | R2 | IC50 values (μM) | |||
|---|---|---|---|---|---|---|
| HL-60 | BGC-823 | Bel-7402 | KB | |||
| III-3-1 | CH3 | 4-Cl | 21.6 | 32.5 | 15.2 | 38.5 |
| III-3-2 | CH2CH3 | 4-Cl | 35.8 | 25.9 | 56.7 | 32.1 |
| III-3-3 | n-C3H7 | 4-Cl | 42.5 | 125.8 | 89.7 | 56.7 |
| III-3-4 | i-C3H7 | 4-Cl | 45.8 | 56.8 | 56.9 | 198.4 |
| III-3-5 | n-C5H11 | 4-Cl | 56.8 | 78.9 | 128.2 | 56.9 |
| III-3-6 | n-C6H13 | 4-Cl | 65.4 | 198.4 | 98.7 | 95.7 |
| III-3-7 | n-C7H15 | 4-Cl | 156.2 | 268.1 | 56.9 | 346.7 |
| III-3-8 | n-C8H17 | 4-Cl | 286.5 | 96.8 | 389.8 | 156.8 |
| III-3-9 | n-C9H19 | 4-Cl | 483.5 | 185.3 | 149.8 | 81.5 |
| III-1-1 | CH3 | 2-Cl | 25.3 | 29.4 | 28.7 | 59.7 |
| III-2-1 | CH3 | 3-Cl | 56.8 | 189.5 | 56.8 | 125.4 |
| III-4-1 | CH3 | 2-F | 59.4 | 48.2 | 45.1 | 198.7 |
| III-5-1 | CH3 | 3-F | 254.3 | 156.2 | 45.9 | 65.4 |
| III-6-1 | CH3 | 4-F | 52.4 | 23.5 | 59.7 | 89.4 |
| III-7-1 | CH3 | 2,4-di-F | 53.6 | 45.2 | 49.2 | 98.4 |
| III-8-1 | CH3 | 2,6-di-F | 25.9 | 10.8 | 18.9 | 29.7 |
| III-9-1 | CH3 | 2-NO2 | 59.3 | 56.8 | 158.2 | 286.4 |
| III-10-1 | CH3 | 3-NO2 | 159.5 | 256.4 | 98.7 | 45.8 |
| III-11-1 | CH3 | 4-NO2 | 102.3 | 152.1 | 94.1 | 56.4 |
| III-12-1 | CH3 | H | 56.8 | 98.5 | 59.2 | 184.6 |
| III-13-1 | CH3 | 4-CH3 | 63.1 | 98.4 | 65.1 | 187.5 |
| III-14-1 | CH3 | 4-OCH3 | 45.5 | 158.4 | 108.9 | 204.2 |
| III-15-1 | CH3 | 4-Br | 526.7 | 254.2 | 125.9 | 253.4 |
| IV-1 | 4-CH3 | 4-Cl | 598.7 | 421.8 | 261.5 | 398.7 |
| doxorubicin | 35.6 | 10.2 | 9.7 | 15.8 | ||
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, Z.; Li, X.; Tian, F.; Yan, X. Synthesis and Bioactivity of 5-Substituted-2-furoyl Diacylhydazide Derivatives with Aliphatic Chain. Int. J. Mol. Sci. 2014, 15, 8941-8958. https://doi.org/10.3390/ijms15058941
Cui Z, Li X, Tian F, Yan X. Synthesis and Bioactivity of 5-Substituted-2-furoyl Diacylhydazide Derivatives with Aliphatic Chain. International Journal of Molecular Sciences. 2014; 15(5):8941-8958. https://doi.org/10.3390/ijms15058941
Chicago/Turabian StyleCui, Zining, Xinghai Li, Fang Tian, and Xiaojing Yan. 2014. "Synthesis and Bioactivity of 5-Substituted-2-furoyl Diacylhydazide Derivatives with Aliphatic Chain" International Journal of Molecular Sciences 15, no. 5: 8941-8958. https://doi.org/10.3390/ijms15058941




