A New Pepstatin-Insensitive Thermopsin-Like Protease Overproduced in Peptide-Rich Cultures of Sulfolobus solfataricus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Growth and Analysis of Extracellular Protease Activities
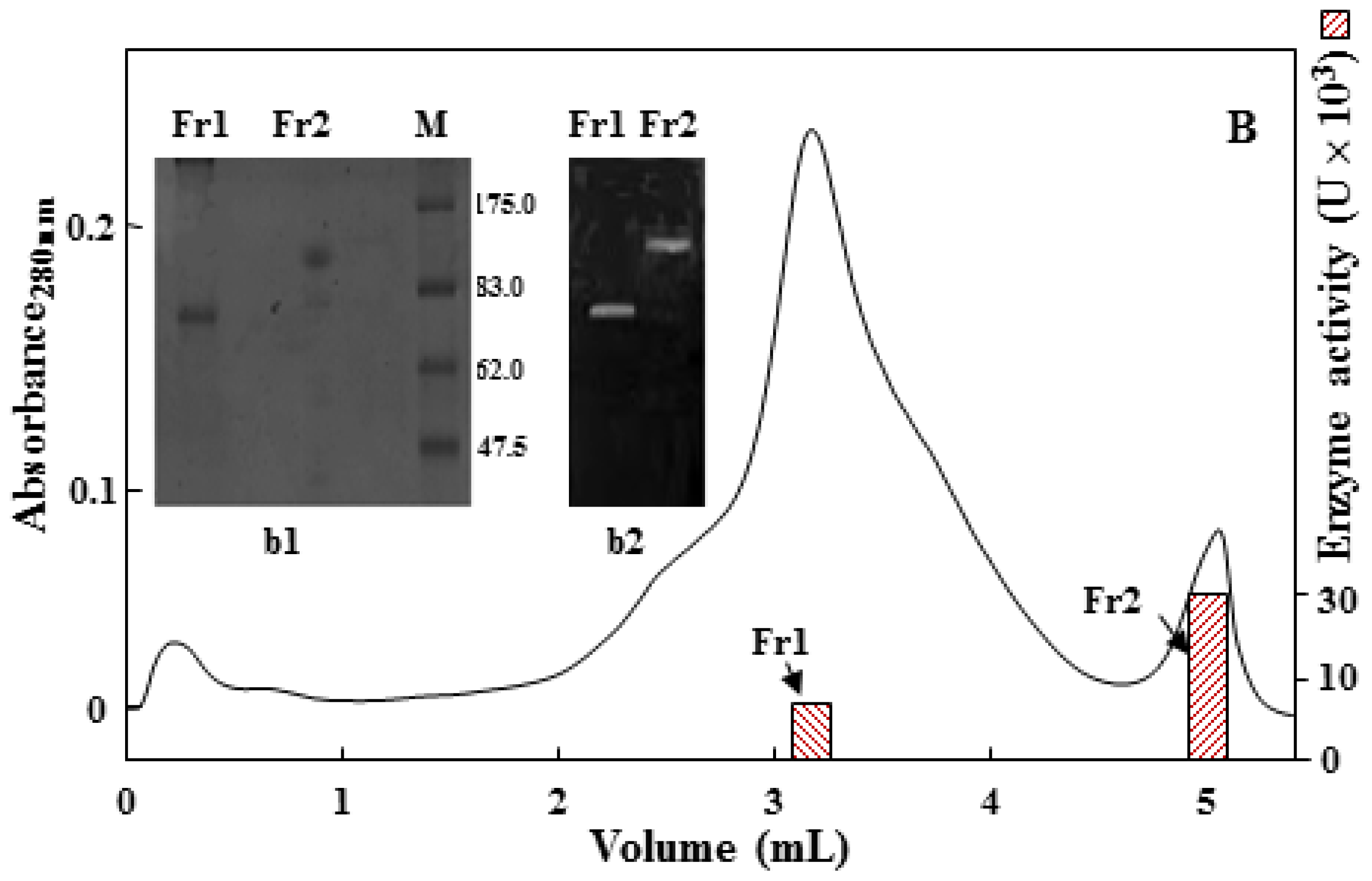
2.2. Purification of the 75 kDa Gelatinase from Tryptone, Yeast Extract and Sucrose (TYS) Culture
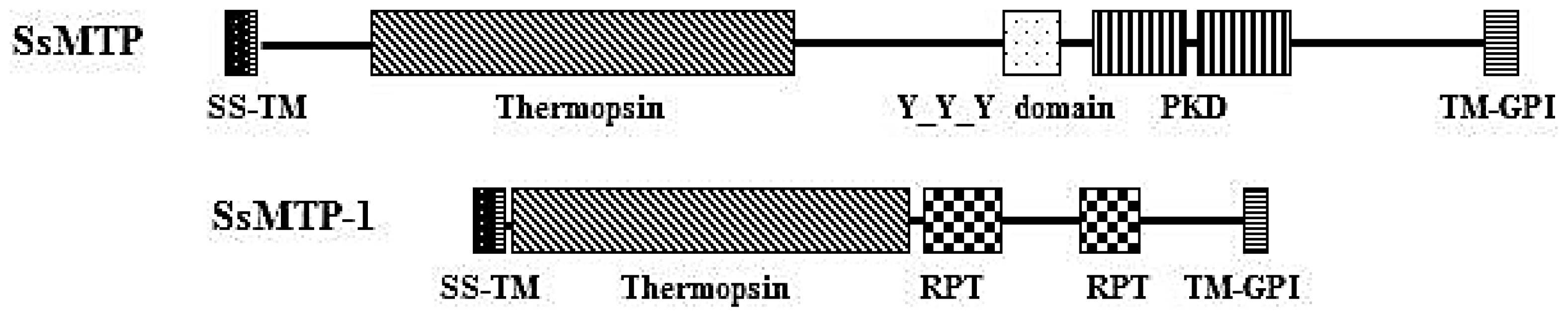
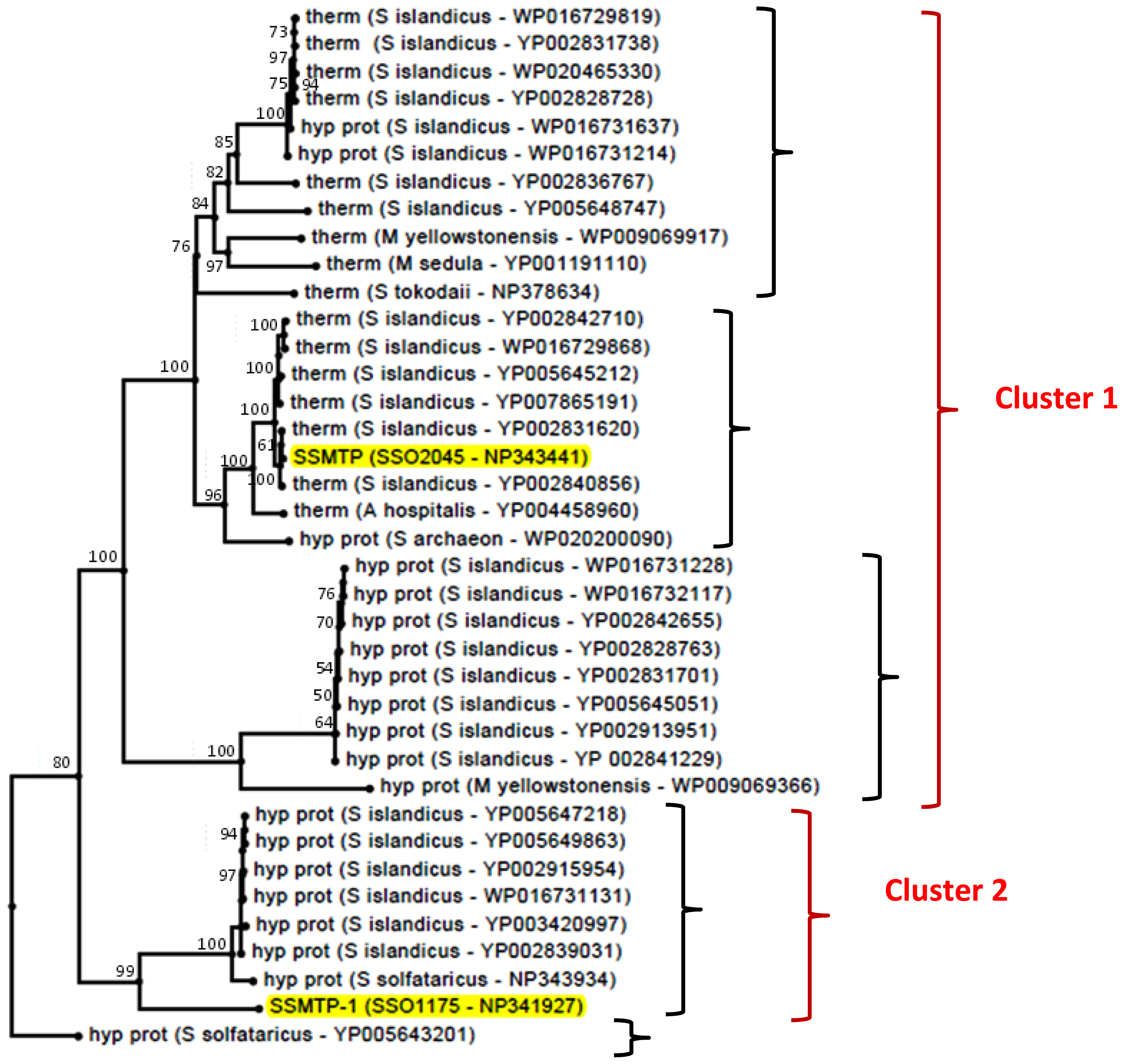
2.3. Structural and Phylogenetic Analyses
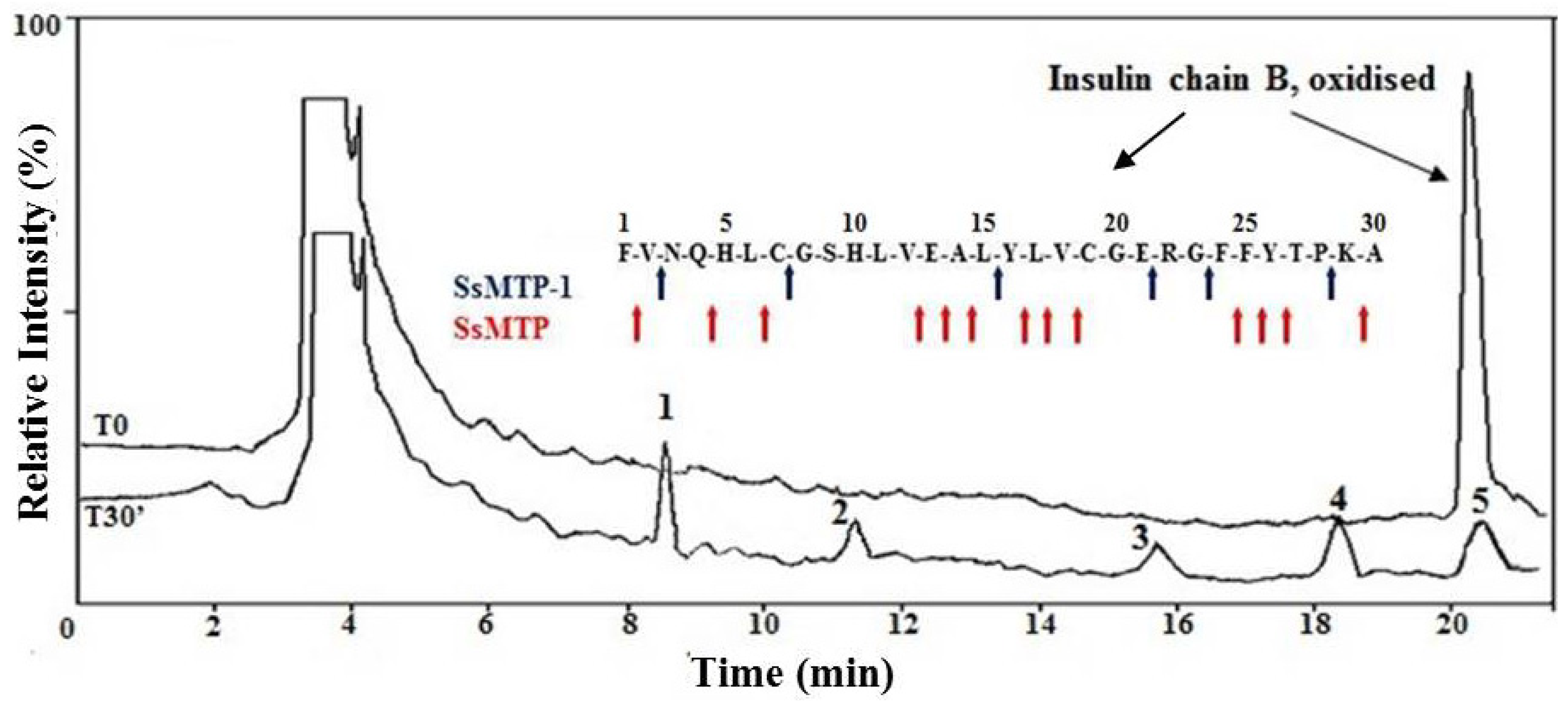
2.4. Enzymatic Properties of SsMTP-1 Protease
2.5. Computational Protein Function Predictions
3. Experimental Section
3.1. Enzymes and Reagents
3.2. Organism, Culture Conditions and Protein Production
3.3. Sequence and Structural Analysis
3.4. Purification of the SSO1175 Protein
3.5. Protease Activity Assays
3.6. Protease Analysis by Zymography
3.7. Substrate Specificity Assay on the Oxidized Insulin B Chain
3.8. Phylogenetic Analysis
4. Conclusions
Supplementary Information
ijms-15-03204-s001.pdfAcknowledgments
Conflicts of Interest
References
- Stetter, K.O. Hyperthermophylic prokaryotes. FEMS Microbial. Rev 1996, 18, 149–158. [Google Scholar]
- Schut, G.J.; Bridger, S.L.; Adams, M.W. Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: Characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J. Bacteriol 2007, 189, 4431–4441. [Google Scholar]
- Fusek, M.; Lin, X.L.; Tang, J. Enzymic properties of thermopsin. J. Biol.Chem 1990, 265, 1496–1501. [Google Scholar]
- Dib, R.; Chobert, J.M.; Dalgalarrondo, M.; Barbier, G.; Haertlé, T. Purification, molecular properties and specificity of a thermoactive and thermostable proteinase from Pyrococcus abyssi, strain st 549, hyperthermophilic archaea from deep-sea hydrothermal ecosystem. FEBS Lett 1998, 431, 279–284. [Google Scholar]
- Rasool, N.; Rashid, N.; Bashir, Q.; Siddiqui, M.A. Proteolytic inventory of Thermococcus kodakaraensis. Afr. J. Microbiol. Res 2013, 7, 3139–3150. [Google Scholar]
- Catara, G.; Ruggiero, G.; La Cara, F.; Digilio, A.; Capasso, A.; Rossi, M. A novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: Biochemical properties, cloning, and expression. Extremophiles 2003, 7, 391–399. [Google Scholar]
- Cannio, R.; Catara, G.; Fiume, I.; Balestrieri, M.; Rossi, M.; Palmieri, G. Identification of a cell-bound extracellular protease overproduced by Sulfolobus solfataricus in peptide-rich media. Protein Pept. Lett 2010, 17, 78–85. [Google Scholar]
- Ward, D.E.; Shockley, K.R.; Chang, L.S.; Levy, R.D.; Michel, J.K.; Conners, S.B.; Kelly, R.M. Proteolysis in hyperthermophilic microorganisms. Archaea 2002, 1, 63–74. [Google Scholar]
- De Castro, R.E.; Maupin-Furlow, J.A.; Giménez, M.I.; Herrera Seitz, M.K.; Sánchez, J.J. Haloarchaeal proteases and proteolytic systems. FEMS Microbiol. Rev 2006, 30, 17–35. [Google Scholar]
- Kelly, R.M.; Adams, M.W. Metabolism in hyperthermophilic microorganisms. Antonie van Leeuwenhoek 1994, 66, 247–270. [Google Scholar]
- Amend, J.P.; Shock, E.L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev 2001, 25, 175–243. [Google Scholar]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol 1972, 84, 54–68. [Google Scholar]
- Grogan, D.W. Phenotypic characterization of the archaebacterial genus Sulfolobus: Comparison of five wild-type strains. J. Bacteriol 1989, 171, 6710–6719. [Google Scholar]
- Lamble, H.J.; Theodossis, A.; Milburn, C.C.; Taylor, G.L.; Bull, S.D.; Hough, D.W.; Danson, M.J. Promiscuity in the part-phosphorylative Entner-Doudoroff pathway of the archaeon Sulfolobus solfataricus. FEBS Lett 2005, 579, 6865–6869. [Google Scholar]
- Kengen, S.W.M.; Stams, A.J.M.; de Vos, W.M. Sugar metabolism of hyperthermophiles. FEMS Microbiol. Rev 1996, 18, 119–137. [Google Scholar]
- Elferink, M.G.; Albers, S.V.; Konings, W.N.; Driessen, A.J. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol 2001, 39, 1494–1503. [Google Scholar]
- Albers, S.V.; Koning, S.M.; Konings, W.N.; Driessen, A.J. Insights into ABC transport in Archaea. J. Bioenerg. Biomembr 2004, 36, 5–15. [Google Scholar]
- KocabÂyÂk, S.; Özel, H. An extracellular—Pepstatin insensitive acid protease produced by Thermoplasma volcanium. Bioresour. Technol 2007, 98, 112–117. [Google Scholar]
- Bendtsen, J.D.; Nielsen, H.; von Heine, G.; Brunak, S. Improved prediction of signal peptides: Signal P 3.0. J. Mol. Biol 2004, 340, 783–795. [Google Scholar]
- Andrade, M.A.; Perez-Iratxeta, C.; Ponting, C.P. Protein repeats: Structures, functions, and evolution. J. Struct. Biol 2001, 134, 117–131. [Google Scholar]
- Eisenhaber, B.; Bork, P.; Eisenhaber, F. Post-translational GPI lipid anchor modification of proteins in kingdoms of life: Analysis of protein sequence data from complete genomes. Protein Eng 2001, 14, 17–25. [Google Scholar]
- Kobayashi, T.; Nishizaki, R.; Ikezawa, H. The presence of GPI-linked protein(s) in an archaeobacterium, Sulfolobus acidocaldarius, closely related to eukaryotes. Biochim. Biophys. Acta 1997, 1334, 1–4. [Google Scholar]
- Palmieri, G.; Casbarra, A.; Marino, G.; Catara, G.; Ruggiero, G.; Capasso, A.; Rossi, M. High cleavage specificity of a subtilisin-like protease from a hyperthermophilic archaeon under extreme conditions. Enzym. Microb. Technol 2005, 37, 745–749. [Google Scholar]
- Antelmann, H.; Tjalsma, H.; Voigt, B.; Ohlmeier, S.; Bron, S.; van Dijl, J.M.; Hecker, M. A proteomic view on genome-based signal peptide predictions. Genome Res 2001, 11, 1484–1502. [Google Scholar]
- Palmieri, G.; Balestrieri, M.; Peter-Katalinić, J.; Pohlentz, G.; Rossi, M.; Fiume, I.; Pocsfalvi, G. Surface-exposed glycoproteins of hyperthermophilic Sulfolobus solfataricus P2 show a common N-glycosylation profile. J. Proteome Res 2013, 12, 2779–2790. [Google Scholar]
- Reimann, J.; Esser, D.; Orell, A.; Amman, F.; Pham, T.K.; Noire, J.; Lindås, A.-C.; Bernander, R.; Wright, P.C.; Siebers, B.; Albers, S.-V. Archaeal signal transduction: Impact of protein phosphatase deletions on cell size, motility and energy metabolism in Sulfolobus acidocaldarius. Mol. Cell. Proteomics 2013, 12, 3908–3923. [Google Scholar]
- Bartolucci, S.; Rossi, M.; Cannio, R. Characterization and functional complementation of a nonlethal deletion in the chromosome of a β-glycosidase mutant of Sulfolobus solfataricus. J. Bacteriol 2003, 185, 3948–3957. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Dereeper, A.; Audic, S.; Claverie, J.M.; Blanc, G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol 2010, 10, 8. [Google Scholar]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res 2012, 40, D302–D305. [Google Scholar]
- Buchan, D.W.; Minneci, F.; Nugent, T.C.; Bryson, K.; Jones, D.T. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res 2013, 41, W349–W357. [Google Scholar]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 2008, 36, W465–W469. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004, 32, 1792–1797. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol 2007, 56, 564–577. [Google Scholar]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol 2010, 59, 307–321. [Google Scholar]
- Jordan, G.E.; Piel, W.H. PhyloWidget: Web-based visualizations for the tree of life. Bioinformatics 2008, 24, 1641–1642. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gogliettino, M.; Riccio, A.; Cocca, E.; Rossi, M.; Palmieri, G.; Balestrieri, M. A New Pepstatin-Insensitive Thermopsin-Like Protease Overproduced in Peptide-Rich Cultures of Sulfolobus solfataricus. Int. J. Mol. Sci. 2014, 15, 3204-3219. https://doi.org/10.3390/ijms15023204
Gogliettino M, Riccio A, Cocca E, Rossi M, Palmieri G, Balestrieri M. A New Pepstatin-Insensitive Thermopsin-Like Protease Overproduced in Peptide-Rich Cultures of Sulfolobus solfataricus. International Journal of Molecular Sciences. 2014; 15(2):3204-3219. https://doi.org/10.3390/ijms15023204
Chicago/Turabian StyleGogliettino, Marta, Alessia Riccio, Ennio Cocca, Mosè Rossi, Gianna Palmieri, and Marco Balestrieri. 2014. "A New Pepstatin-Insensitive Thermopsin-Like Protease Overproduced in Peptide-Rich Cultures of Sulfolobus solfataricus" International Journal of Molecular Sciences 15, no. 2: 3204-3219. https://doi.org/10.3390/ijms15023204




