Targeted Silencing of MART-1 Gene Expression by RNA Interference Enhances the Migration Ability of Uveal Melanoma Cells
Abstract
:1. Introduction
2. Results and Discussion
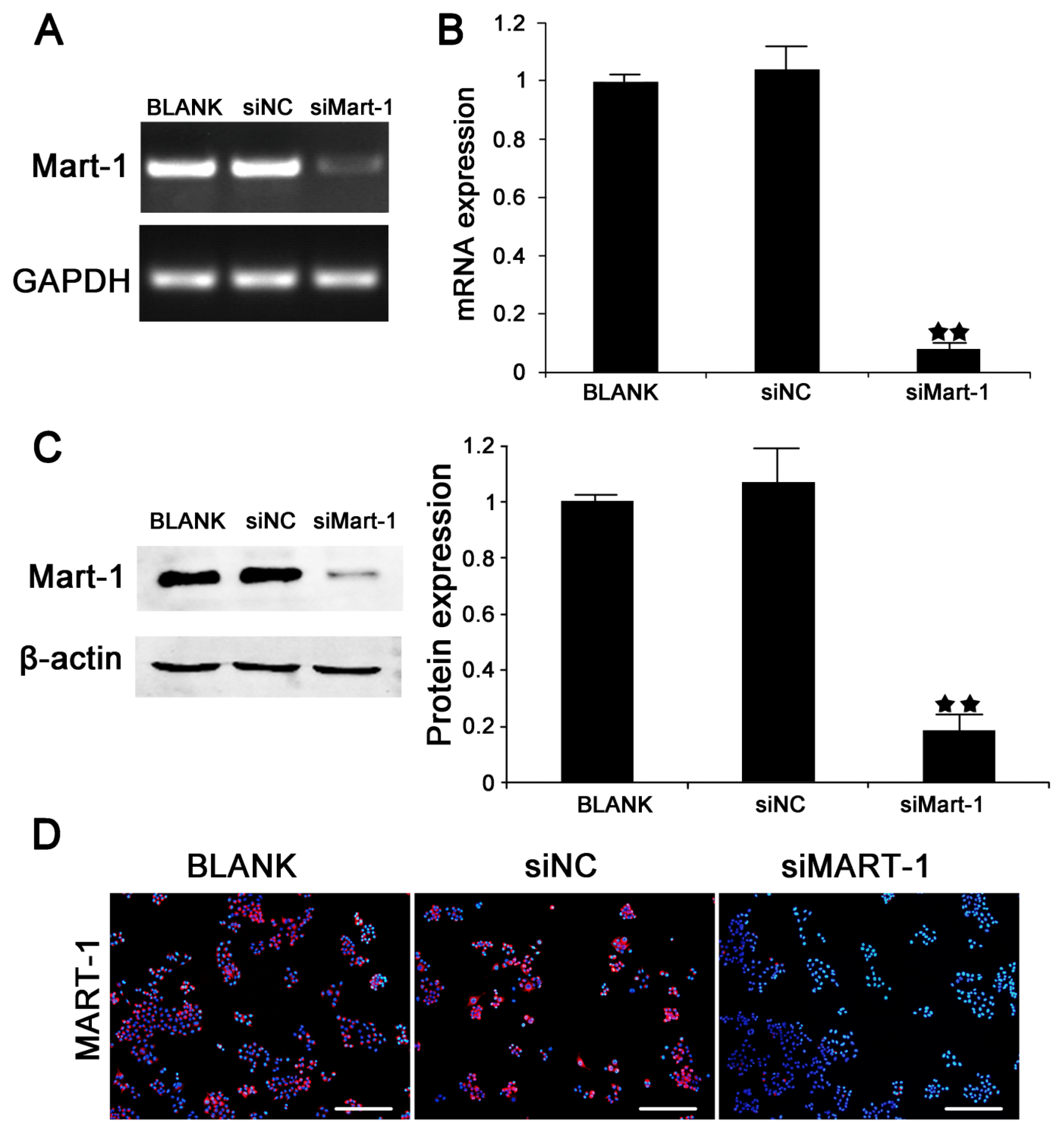
2.1. Silencing of MART-1 in SP6.5 Cells
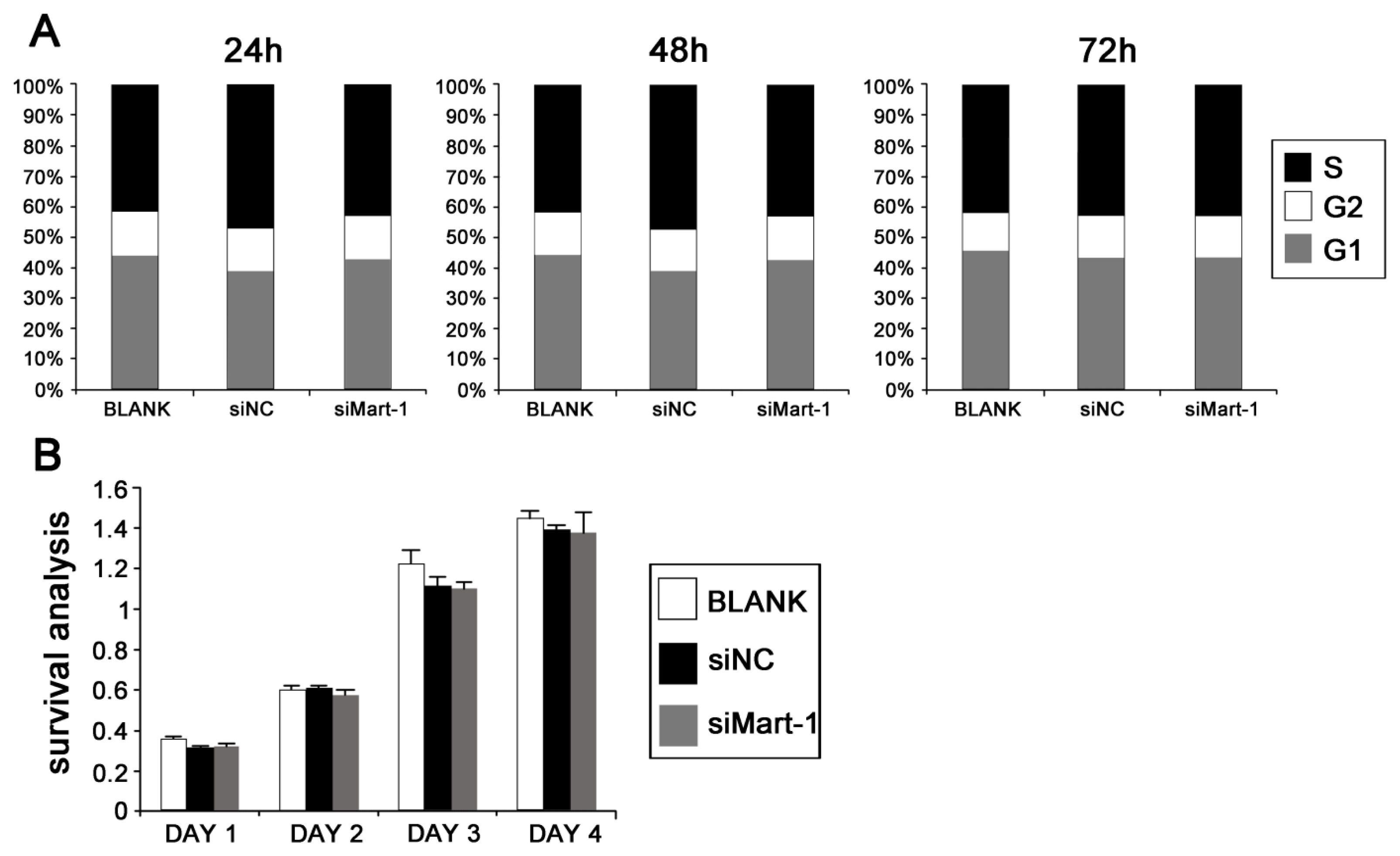
2.2. Cell Cycle and Cell Proliferation in SP6.5 Cells with MART-1 Targeted Gene Silencing
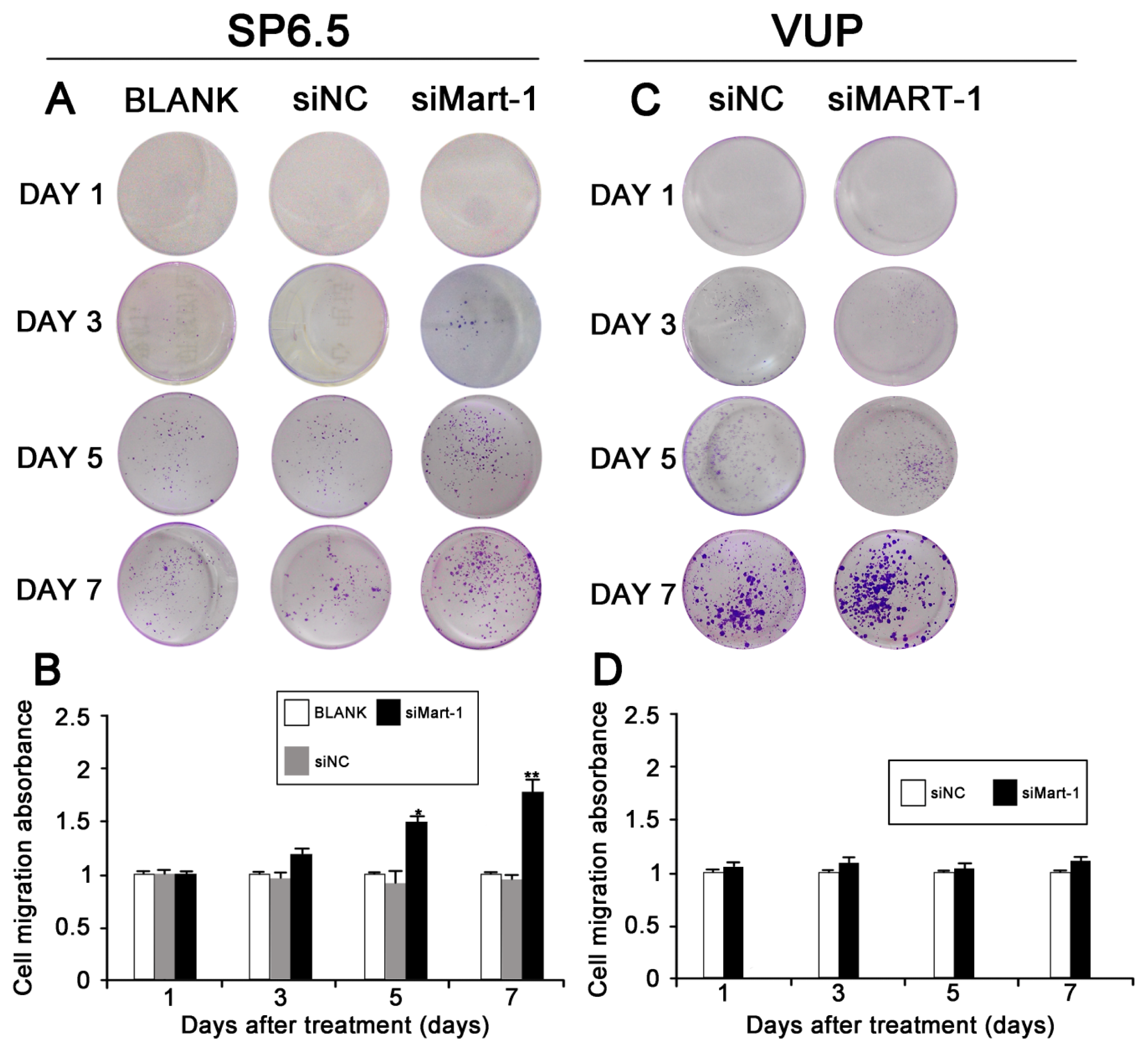
2.3. Effect of MART-1 Silencing on Cell Migration
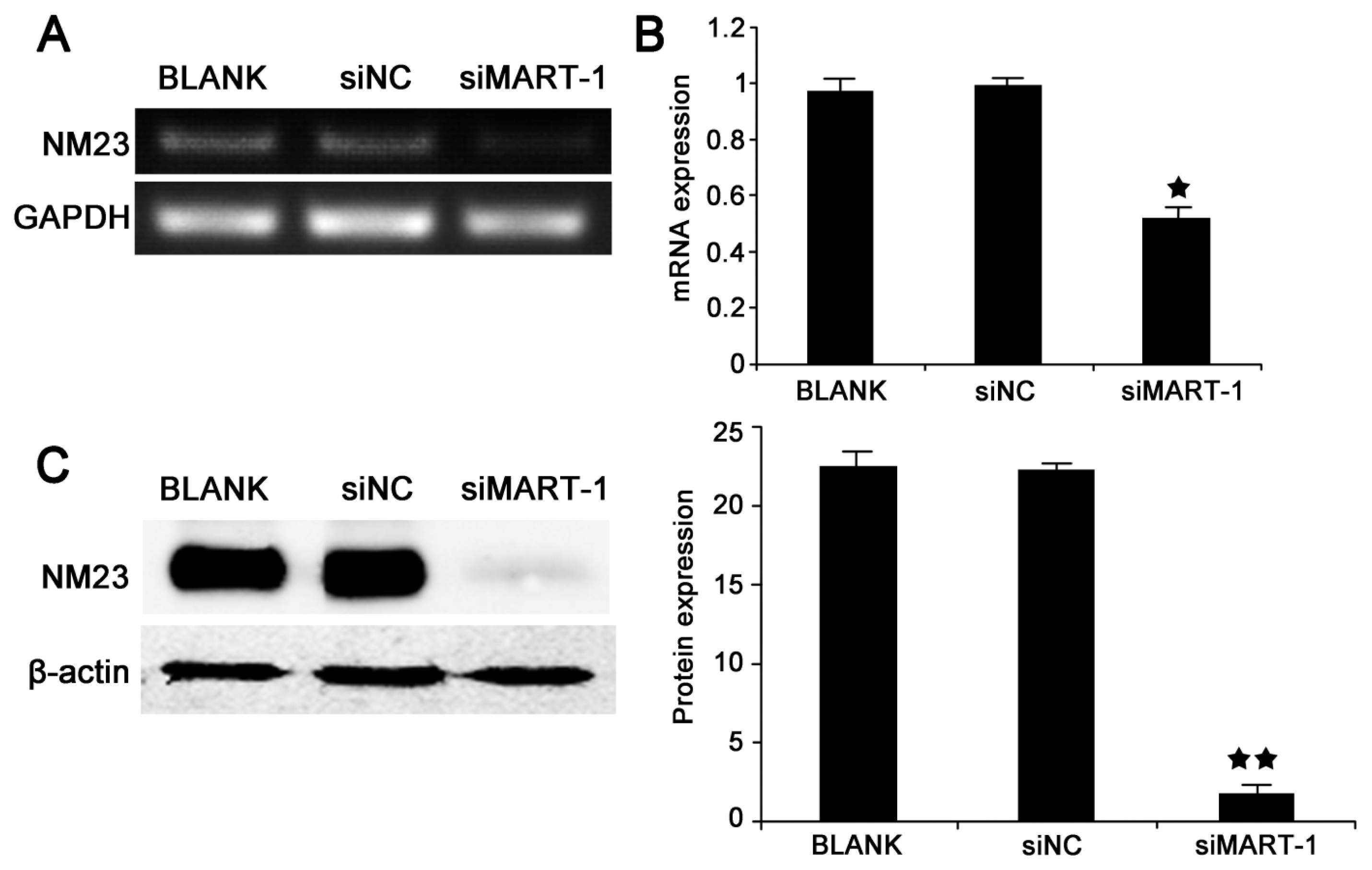
2.4. MART-1 Silencing Decreases NM23 mRNA and Protein Levels
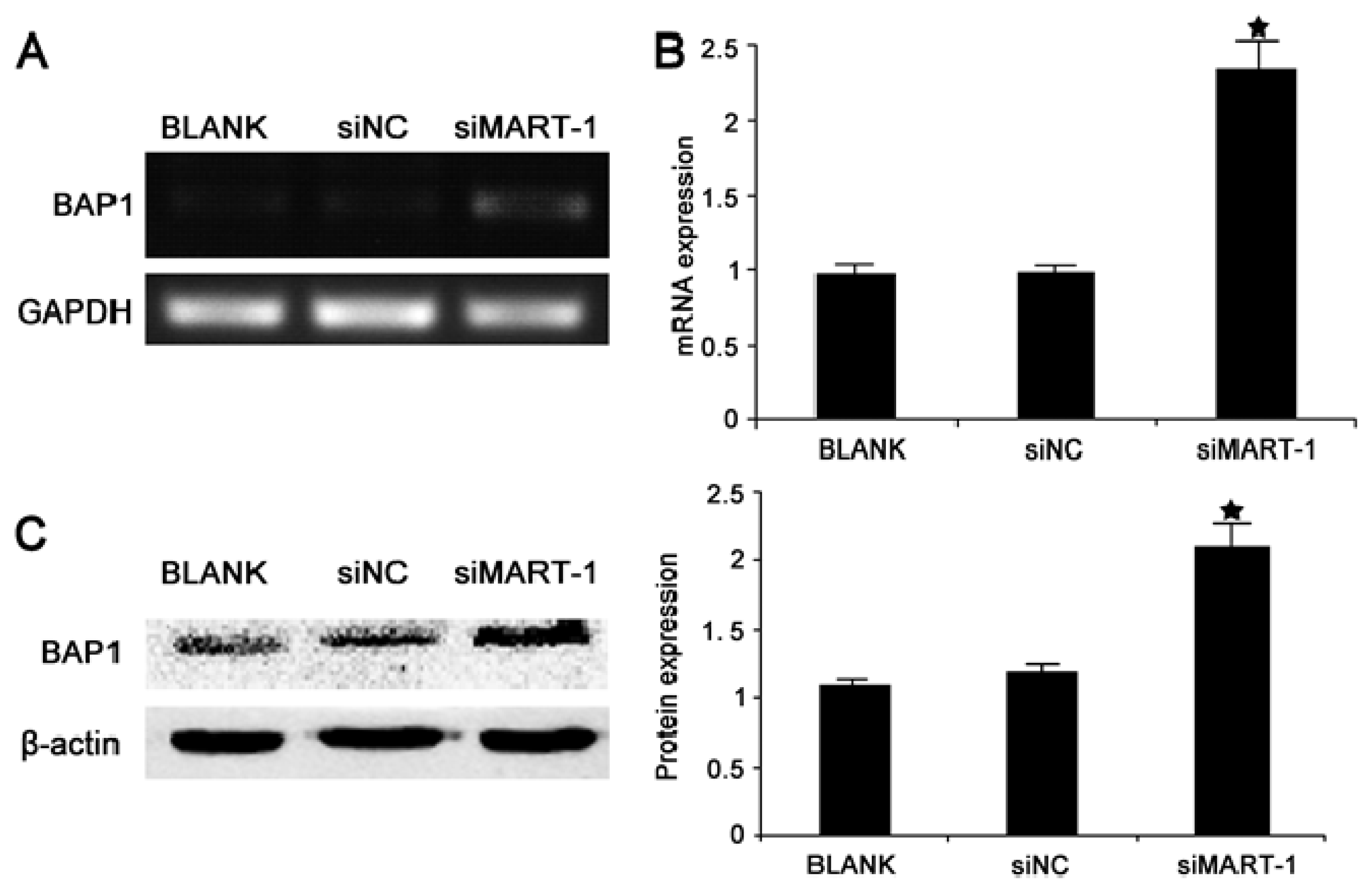
2.5. Effect of MART-1 Silencing on BAP1 mRNA and Protein Levels
2.6. Discussion
3. Experimental Section
3.1. Cell Lines and Cell Culture
3.2. MART-1 siRNA Oligonucleotides
3.3. SiRNA Transfection
3.4. RNA Extraction and Reverse Transcription-PCR Analysis
3.5. Quantitative Real-Time PCR
3.6. Western Blot Analysis
3.7. Immunocytochemistry
3.8. Cell Cycle
3.9. MTT Assay
3.10. Migration Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Scotto, J.; Fraumeni, J.J. Melanomas of the eye and other noncutaneous sites: Epidemiologic aspects. J. Natl. Cancer Inst 1976, 56, 489–491. [Google Scholar]
- Bergman, L.; Seregard, S. Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest. Ophthalmol. Vis. Sci 2002, 43, 2579–2583. [Google Scholar]
- Coupland, S.E.; Anastassiou, G. The prognostic value of cyclin D1, p53, and MDM2 protein expression in uveal melanoma. J. Pathol 2000, 191, 120–126. [Google Scholar]
- Kawakami, Y.; Battles, J.K. Production of recombinant MART-1 proteins and specific antiMART-1 polyclonal and monoclonal antibodies: Use in the characterization of the human melanoma antigen MART-1. J. Immunol. Methods 1997, 202, 13–25. [Google Scholar]
- Hoashi, T.; Watabe, H. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem 2005, 280, 14006–14016. [Google Scholar]
- Wang, J.; Jia, R. Differential expression of Mart-1 in human uveal melanoma cells. Mol. Med. Rep 2011, 4, 799–803. [Google Scholar]
- Diener-West, M.; Hawkins, B.S. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch. Ophthalmol 1992, 110, 245–250. [Google Scholar]
- Hannon, G.J.; Rossi, J.J. Unlocking the potential of the human genome with RNA interference. Nature 2004, 431, 371–378. [Google Scholar]
- Aris, M.; Zubieta, M.R. MART-1- and gp100-expressing and -non-expressing melanoma cells are equally proliferative in tumors and clonogenic in vitro. J. Investig. Dermatol 2012, 132, 365–374. [Google Scholar]
- Hu, S.; Luo, Q. The pharmacological NF-kappaB inhibitor BAY11–7082 induces cell apoptosis and inhibits the migration of human uveal melanoma cells. Int. J. Mol. Sci 2012, 13, 15653–15667. [Google Scholar]
- Laurent, C.; Valet, F. High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res 2011, 71, 666–674. [Google Scholar]
- Ye, M.; Hu, D. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest. Ophthalmol. Vis. Sci 2008, 49, 497–504. [Google Scholar]
- Florenes, V.A.; Aamdal, S. Levels of NM23 messenger RNA in metastatic malignant melanomas: Inverse correlation to disease progression. Cancer Res 1992, 52, 6088–6091. [Google Scholar]
- Greco, I.M.; Calvisi, G. An immunohistochemical analysis of NM23 gene product expression in uveal melanoma. Melanoma Res 1997, 7, 231–236. [Google Scholar]
- Zhang, Q.; McCorkle, J.R. Metastasis suppressor function of NM23-H1 requires its 3′-5′ exonuclease activity. Int. J. Cancer 2011, 128, 40–50. [Google Scholar]
- Buxton, I.L.; Yokdang, N. Extracellular NM23 signaling in breast cancer: Incommodus verum. Cancers 2011, 3, 2844–2857. [Google Scholar]
- Luo, M.; Zhu, D. Lentivirus-mediated stable silencing of NM23-H1 gene in lung cancer cells and the influence on biological behavior (in Chinese). Zhongguo Fei Ai Za Zhi 2012, 15, 139–145. [Google Scholar]
- Jensen, D.E.; Proctor, M. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar]
- Abdel-Rahman, M.H.; Pilarski, R. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J. Med. Genet 2011, 48, 856–859. [Google Scholar]
- Jensen, D.E.; Rauscher, F.R. BAP1, a candidate tumor suppressor protein that interacts with BRCA1. Ann. N. Y. Acad. Sci 1999, 886, 191–194. [Google Scholar]
- Ventii, K.H.; Devi, N.S. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008, 68, 6953–6962. [Google Scholar]
- Harbour, J.W.; Onken, M.D. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar]
- Jia, R.; Jiao, Z. Functional significance of B7-H1 expressed by human uveal melanoma cells. Mol. Med. Rep 2011, 4, 163–167. [Google Scholar]
- Albert, D.M.; Ruzzo, M.A. Establishment of cell lines of uveal melanoma. Methodology and characteristics. Invest. Ophthalmol. Vis. Sci 1984, 25, 1284–1299. [Google Scholar]
- Song, X.; Wang, H. Combined treatment with an oncolytic adenovirus and antitumor activity of vincristine against retinoblastoma cells. Int. J. Mol. Sci 2012, 13, 10736–10749. [Google Scholar]
- Giordano, F.; Bonetti, C. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum. Mol. Genet 2009, 18, 4530–4545. [Google Scholar]
- Song, X.; Zhou, Y. Inhibition of retinoblastoma in vitro and in vivo with conditionally replicating oncolytic adenovirus H101. Invest. Ophthalmol. Vis. Sci 2010, 51, 2626–2635. [Google Scholar]
- Yao, Y.; Li, L. Enhanced therapeutic efficacy of vitamin K2 by silencing BCL-2 expression in SMMC-7721 hepatocellular carcinoma cells. Oncol. Lett 2012, 4, 163–167. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Jia, R.; Wang, J.; Xu, X.; Yao, Y.; Ge, S.; Fan, X. Targeted Silencing of MART-1 Gene Expression by RNA Interference Enhances the Migration Ability of Uveal Melanoma Cells. Int. J. Mol. Sci. 2013, 14, 15092-15104. https://doi.org/10.3390/ijms140715092
Zhang Y, Jia R, Wang J, Xu X, Yao Y, Ge S, Fan X. Targeted Silencing of MART-1 Gene Expression by RNA Interference Enhances the Migration Ability of Uveal Melanoma Cells. International Journal of Molecular Sciences. 2013; 14(7):15092-15104. https://doi.org/10.3390/ijms140715092
Chicago/Turabian StyleZhang, Yidan, Renbing Jia, Jing Wang, Xiaofang Xu, Yuting Yao, Shengfan Ge, and Xianqun Fan. 2013. "Targeted Silencing of MART-1 Gene Expression by RNA Interference Enhances the Migration Ability of Uveal Melanoma Cells" International Journal of Molecular Sciences 14, no. 7: 15092-15104. https://doi.org/10.3390/ijms140715092



