2.1. Equilibration of the Membrane System, Cross-Sectional Area per Lipid and Membrane Thickness
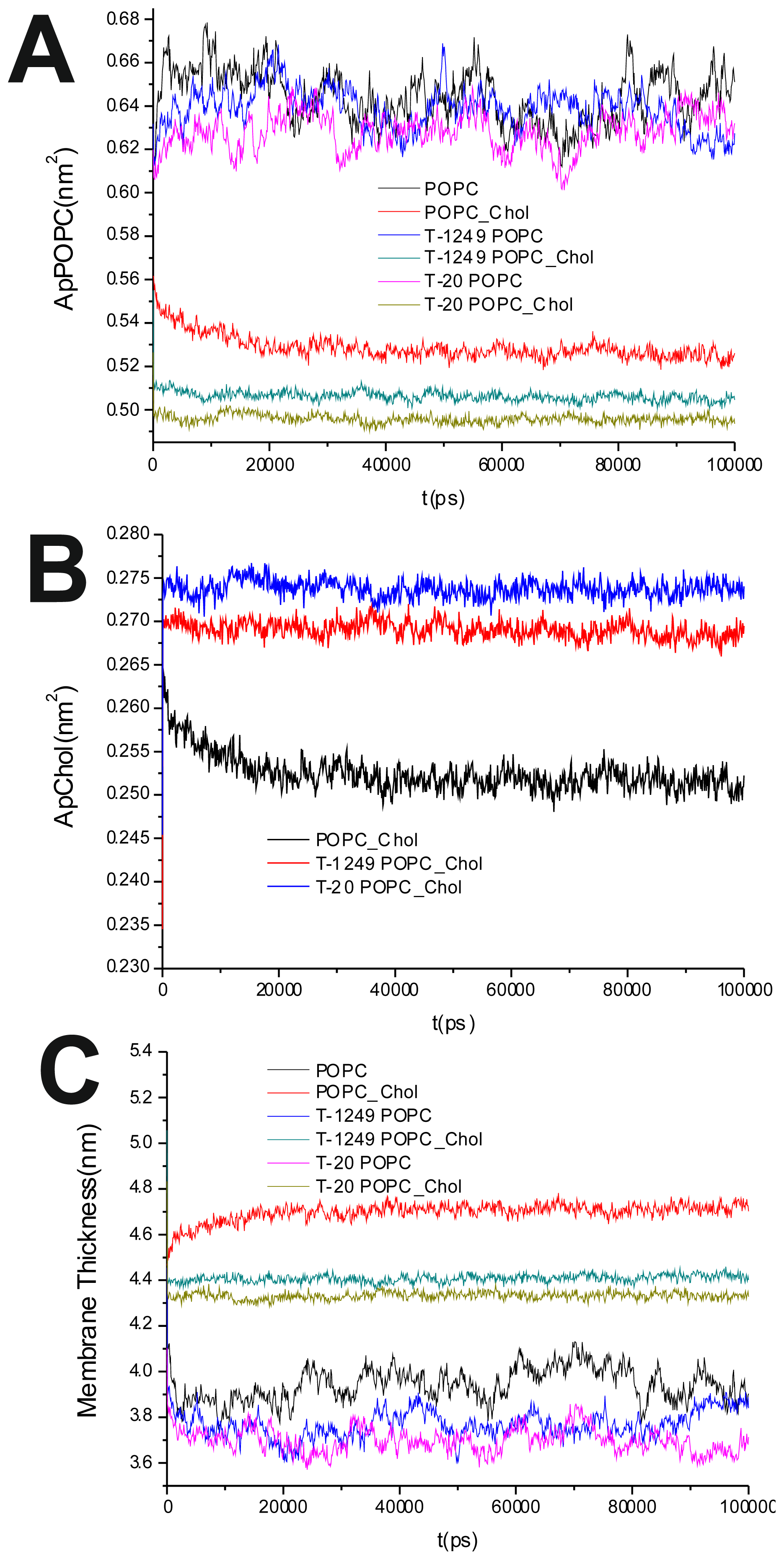
To evaluate the process of the systems’ equilibration, time profiles of the surface area/POPC (
Figure 1A), surface area/Chol (
Figure 1B) and membrane thickness were calculated as in [
22] [
Equations (1) and
(2)] and recorded for the production simulation (100 ns):
In these equations,
ApPOPC is the cross-sectional area per POPC molecule,
ApCHOL is the cross-sectional area per Chol molecule,
Abox is the area of the
xy plane of the simulation box,
Vbox is the simulation box total volume,
Nw is the number of water molecules,
Vw is the volume of the water molecule,
x = 0.00 or 0.50 is the Chol mole fraction,
Nlipid is the number of lipid molecules,
VChol is the volume of the Chol molecule [
22] and
Vpeptide is the volume of the peptide molecule, determined from the peptide simulation in water by averaging
Vpeptide =
Vbox −
Nw ×
Vw for the last 25 ns of the simulation.
The average of the surface area per lipid was stable over the final 80 ns of the simulation, which led us to the conclusion that the simulated systems had reached a steady state after 20 ns of simulation (
Figure 1A,B). This was also verified by observation of the membrane thickness parameter. Membrane thickness was determined by the ensemble average of the P–P distance between phosphate P atoms of opposite leaflets.
Figure 1C shows the time variation of the latter, and as can be observed, stabilization is achieved and maintained over the last 80 ns of the simulation.
It has been shown that the cross-sectional area per POPC decreases, whereas the cross-sectional area per Chol increases, in all systems upon peptide adsorption (
Table 1). This suggests that peptide adsorption would have a condensation-like effect on the bilayers, but also that Chol molecules in the POPC/Chol bilayers are more exposed to solvent molecules in membranes interacting with either T-20 or T-1249. Peptide adsorption has little or no effect in the membrane thickness (
Table 1) of POPC bilayers (the effect appears to be always a decrease, larger for T-20) and induces a decrease in the membrane thickness of POPC/Chol bilayers (here, too, the effect is greater for T-20). In these membranes, the free volume is low, so if the peptides are inducing decreases in both the cross-sectional area per POPC and the membrane thickness, this could account for the exposure of the Chol molecules to water, reducing the umbrella effect that protects and stabilizes Chol molecules in these bilayers [
23].
2.2. H Bonds between POPC, Chol and Sol Molecules
Formation of H-bonds between POPC, Chol and water molecules was investigated. For this analysis, an H-bond for a given donor—the H-acceptor triad was registered each time—the donor acceptor distance was <0.35 nm and the H-donor-acceptor angle was <30°.
Table 2 shows the average number of H-bonds formed between the three groups under scrutiny (POPC, Chol and Sol—solvent,
i.e., water). Both peptides when interacting with the model bilayers cause a decrease in the number of H bonds formed between POPC and solvent molecules. This effect is generally more pronounced in the T-1249 systems, namely in the T-1249/POPC system, where the decrease is greater. Behind this effect appears to be the fact that T-1249 is able to interact more strongly via H bonds with the bilayer lipids than T-20 [
19,
20], involving more lipid molecules that would, otherwise, be available for interaction with the solvent molecules. This observation is also supported by the fact that the decrease in POPC-Sol H bonds is always greater in the top bilayer, suggesting the peptide adsorption as a direct cause to this effect. Peptide interaction also causes a decrease in the Chol-water H bond number, but this decrease is so small, that it appears to be due mainly to a reduction in the number of Chol molecules accessible to the water molecules (because they are covered by the adsorbing peptide) and, thus, capable of linking via H bonds. Finally, the interaction of both peptides with POPC/Chol bilayers induces a small increase in the number of H bonds between POPC and Chol molecules, apparently caused by the tighter packing of the bilayer lipids, due to the peptide adsorption to the top leaflet that pushes [
19,
20], as reported earlier, the top leaflet bilayer lipids (located closer to the peptide) towards the hydrophobic core.
In order to evaluate H bond dynamics, the H bond’s lifetime was calculated as described in the GROMACS manual [
24]. In short, the lifetime of the H-bonds, shown on
Table 3, is calculated from the average over all autocorrelation functions of the existence functions (either zero or one) of all H-bonds:
with Si(t) ɛ {0; 1} for an H-bond, i, at time, t. The integral of C(τ) then gives an estimate of the average H-bond lifetime, τHB:
Upon peptide adsorption, τ
HB for the POPC-Sol H bonds increases in the top leaflet of the POPC bilayers under scrutiny (
Table 3). In the bottom leaflet, τ
HB for POPC-Sol H bonds remains mostly unaltered. Peptide adsorption creates a burrow-like crater in the POPC bilayers [
19,
20], further exposing the POPC molecules of the crater’s rim. This exposure to the Sol molecules may be responsible for the increase in POPC-Sol H bond lifetime; the effect is local, and hence, the average is only slightly increased. In POPC/Chol bilayers, which are significantly more rigid and, therefore, do not allow for an exposure in the same order as in the POPC bilayers [
19,
20], the effect is virtually insignificant.
Peptide adsorption also evokes an increase in the Chol-Sol and POPC-Chol H bond lifetimes, especially in the latter. In the Chol-Sol H bonds, the increase is small (and smaller in the T-1249/POPC/Chol system), and it may be caused by the increased exposure of Chol molecules to Sol: cross-sectional area per Chol increases upon peptide adsorption (also less in the T-1249/POPC/Chol system). This further exposure to Sol molecules would facilitate H bond formation and persistence. As T-1249 interacts with Chol via H bonds [
20], this effect is lessened.
POPC-Chol H bond lifetimes are significantly increased upon peptide adsorption. The effect has a greater magnitude in the top bilayer of both peptide containing systems. Peptide adsorption induces pressure on the bilayer surface. This pressure pushes one leaflet against each other (in the z direction), but also, the molecules against each other in the xy plane. This increased proximity facilitates H bond formation and persistence. POPC/Chol membranes have very little free volume and slow dynamics (much slower that POPC membranes), and as such, effects as these should propagate easily inside the bilayer, thus causing the POPC-Chol H bonds to have longer lifetimes. Nonetheless this effect appears to be dependent on the distance to the peptide, since it is, in both cases, weaker in the bottom monolayer.
2.4. Lateral Diffusion of POPC and Chol
The lateral diffusion coefficients, Dlat, of the lipids were calculated from the two-dimensional mean square displacement (MSD) using the Einstein relation:
in which MSD (t) can be defined as:
Where
is the (
x,
y) position of the center of mass of the molecule,
i, of a given species, and the averaging is carried out over all molecules of this kind and time origins. To eliminate noise due to fluctuations in the center of mass of each leaflet, all lipid
MSD analyses were carried out using trajectories with a fixed center of mass of one of the leaflets [
25,
26]. MSD and respective
Dlat were calculated for POPC and Chol as the average of both leaflets and for the leaflets separately.
Table 5 shows
Dlat for the molecules under scrutiny, POPC and Chol, in all systems under study. In the POPC bilayer systems, peptide adsorption induces a decrease in POPC’s
Dlat more pronounced in the top bilayer and for the T-1249 peptide’s adsorption. Adsorption may be causing a slowing of the membrane diffusion dynamics. In the T-20/POPC/Chol system, peptide interaction has the opposite effect; an increase in
Dlat. T-20, for most of the simulation run, only interacts with the POPC/Chol bilayer via the
C-terminus amino acids, leaving the rest of the helix with a high rotational and translational freedom [
19] and, also, a high
Dlat[
19]. This mobility may be thus influencing POPC’s diffusion dynamics, increasing its
Dlat. In the T-1249/POPC/Chol system, the POPC’s
Dlat in the top leaflet of this system decreases. T-1249 interacts more strongly with the bilayers than T-20 [
18–
20], and so, the effect observed for the POPC bilayers appears to be maintained in this system. Chol molecules show an increase in
Dlat in all peptide-containing systems. This increase may be a result of the disordering caused by the peptides’ adsorption (see Section 2.6.,
Tables 6 and
7). The smaller increase is in the top leaflet’s Chol of the system containing T-1249, and that may be caused by the fact that Chol interacts more strongly with T-1249 (via H bonds) than with T-20 (which showed no such interaction) [
19].
2.5. Rotational Dynamics of Selected Axis of Membrane Lipids
The rotational dynamics of several axes (POPC P–N axis (P8→N4),
sn-1 long axis (C2→C15),
sn-2 long axis (C2→C17) and Chol long axis (C2→C20)) was studied. In this way, motions both in the headgroup and in the hydrophobic regions were addressed. To this end, rotational auto correlation functions C(
t), defined in
Equation 7, were calculated.
Here, θ(ζ), for the sake of commodity, is the angle between a vector defined in the molecular framework at times, ζ and t + ζ, and P
2(x) = (3x
2 − 1)/2 is the second order Legendre polynomial. Averaging is performed over ζ, which, assuming a sufficiently ergodic trajectory, is an approximation of the ensemble average. All calculated C(t) functions decay to a residual value, C
∞ > 0, probably denoting a hindered rotation [
26,
27].
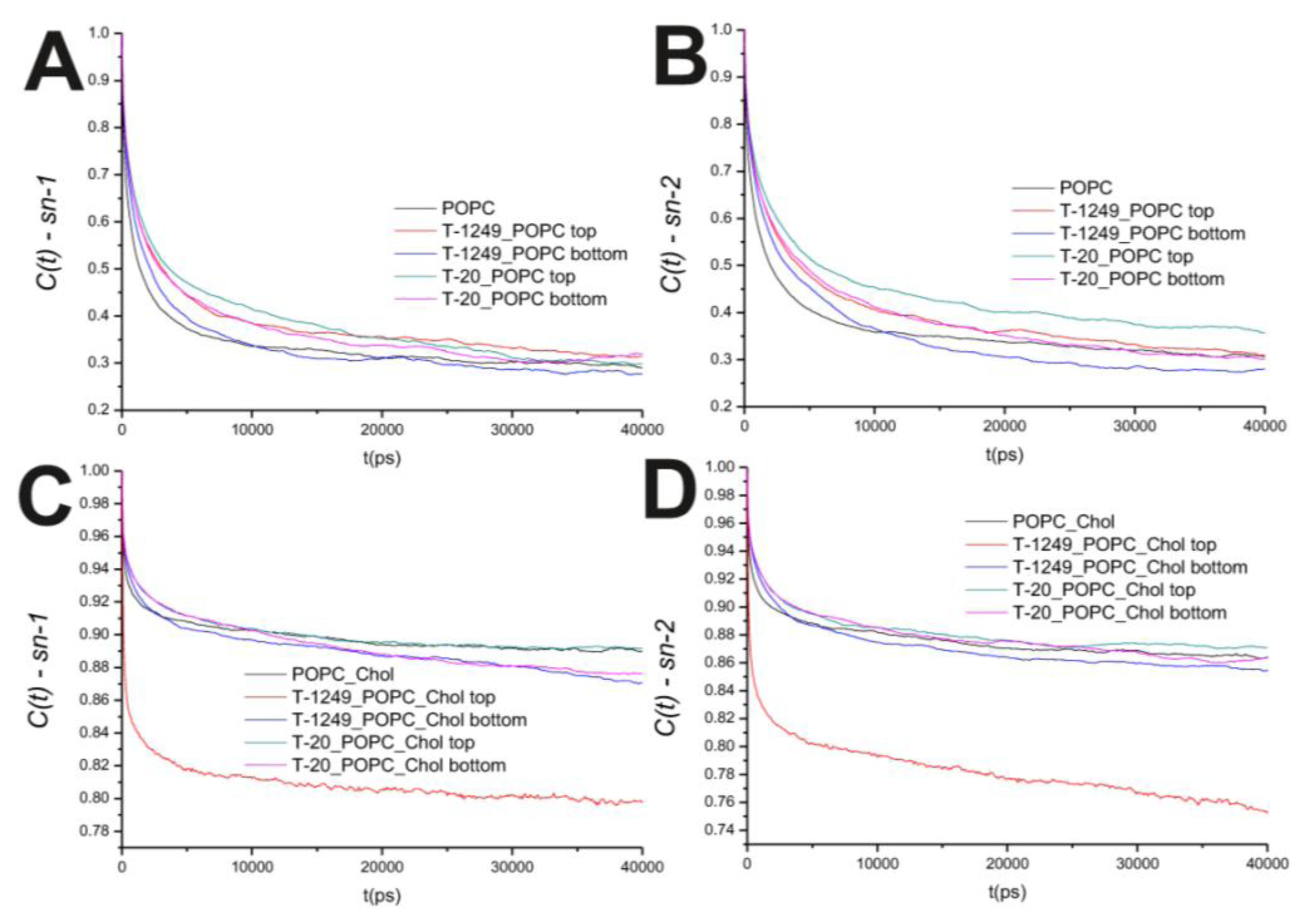
In the POPC bilayer systems, peptide interaction with the model membranes causes an increased hindrance to rotational movements in the P–N axis (
Figure 2B). This is mostly restricted to the top leaflet, which suggests that peptide adsorption and interaction with POPC molecules slows P–N axis rotational movement at the interface. However, this is not restricted to the interface, and a similar effect is verified in both the
sn-1 and
sn-2 axis located in the hydrophobic core (
Figure 3). In the POPC/Chol systems, peptide adsorption has almost no effect in the dynamics of the
sn-1 and
sn-2 axes in both leaflets, with the exception of the top leaflet interacting with T-1249, in which both axes show less restricted rotation,
i.e., tumbling of the rotational autocorrelation function to lower values (
Figure 3C,D). In these systems, the dynamics of the P–N axis is slowed for the top leaflets and faster in the bottom leaflets; this effect is of greater magnitude in the T-1249 adsorption, which implies direct correlation to the degree of interaction of the peptide with the membrane (
Figure 3C). Chol dynamics is generally slowed upon peptide adsorption in both leaflets in the T-20 system and in the bottom leaflet in the T-1249 system (
Figure 3A). Chol shows a faster dynamics in the top leaflet of the T-1249/POPC/Chol system, and this appears to be a similar phenomenon, as in the acyl chain axis. Since Chol interacts strongly with T-1249, namely via H bonds [
20], and because T-1249 has a diffusion dynamics faster than the bilayer lipids [
20], it induces Chol to assume a faster dynamics also, interfering, thus, with the bilayer core dynamics.
2.6. Order Parameters
The order parameter tensor, S, is defined as:
where θ
a (or α
b) is the angle made by a
th (or b
th) molecular axis with the bilayer normal and δ
ab is the Kronecker delta (< > denotes both ensemble and time averaging). In our simulations, using a united atom force field, the order parameter for saturated and unsaturated carbons, −
SCD, can be determined using the following relations [
28]:
−
SCD may vary between 0.5 (full order along the bilayer normal) and −0.25 (full order along the bilayer plane), whereas −
SCD = 0 denotes isotropic orientation. Due to the slow convergence of this parameter [
27], analysis was restricted to the last 50 ns of the simulations. For an immediate comparison, −
SCD ensemble averages for the last 50 ns of each simulation, along the
sn-1 chains, are shown in
Table 6 for the systems under study.
In the 50 ns time scale, T-1249 has a larger effect in the −
SCD, on both POPC and POPC/Chol bilayers. T-1249 was shown to be able to interact in a stronger manner with the model membranes [
19,
20]; both its Lennard-Jones and Coulomb interaction energies (T-1249-POPC) are higher than T-20’s [
20]. Additionally, it forms more H bonds with the bilayer’s POPC than T-20, and the bonds are distributed more evenly throughout the length of the peptide helix [
20], apparently stabilizing this interaction in a more effective way. T-20 and T-1249 have opposite overall average effects in the −
SCD of the
sn-1 acyl chain of the POPC bilayer, while T-1249 decreases the average of −
SCD, T-20 increases this parameter, albeit by a small amount. This stronger interaction may account for the influence it has on
sn-1. T-1249 has a fast (faster than POPC’s) diffusion dynamics [
19] and is able to, nonetheless, form H bonds with POPC, thus dragging POPC molecules in its movement. This enhanced mobility should reflect in a disordering of the acyl chains and account for the lowering of −
SCD. The same phenomenon appears to be responsible for the lowering of −
SCD in the POPC/Chol bilayers. These bilayers are in the lo phase, highly ordered and with a slow diffusional dynamics. Upon T-20 and T-1249 interaction with these bilayers, −
SCD decreases (
Table 6). The peptides have faster dynamics than the bilayer molecules and are able to form H bonds with POPC (and Chol in the T-1249’s case) [
18–
20], thus “dragging” POPC or Chol molecules faster than the surrounding ones that are not linked to the peptide and, thus, interfering with the highly ordered structure of the bilayer. This effect is more pronounced in the T-1249 adsorption. The fact that its interaction with membrane lipids is stronger (and the only peptide that forms H bonds with Chol) [
20] appears to be a significant influence in the lowering of −
SCD.
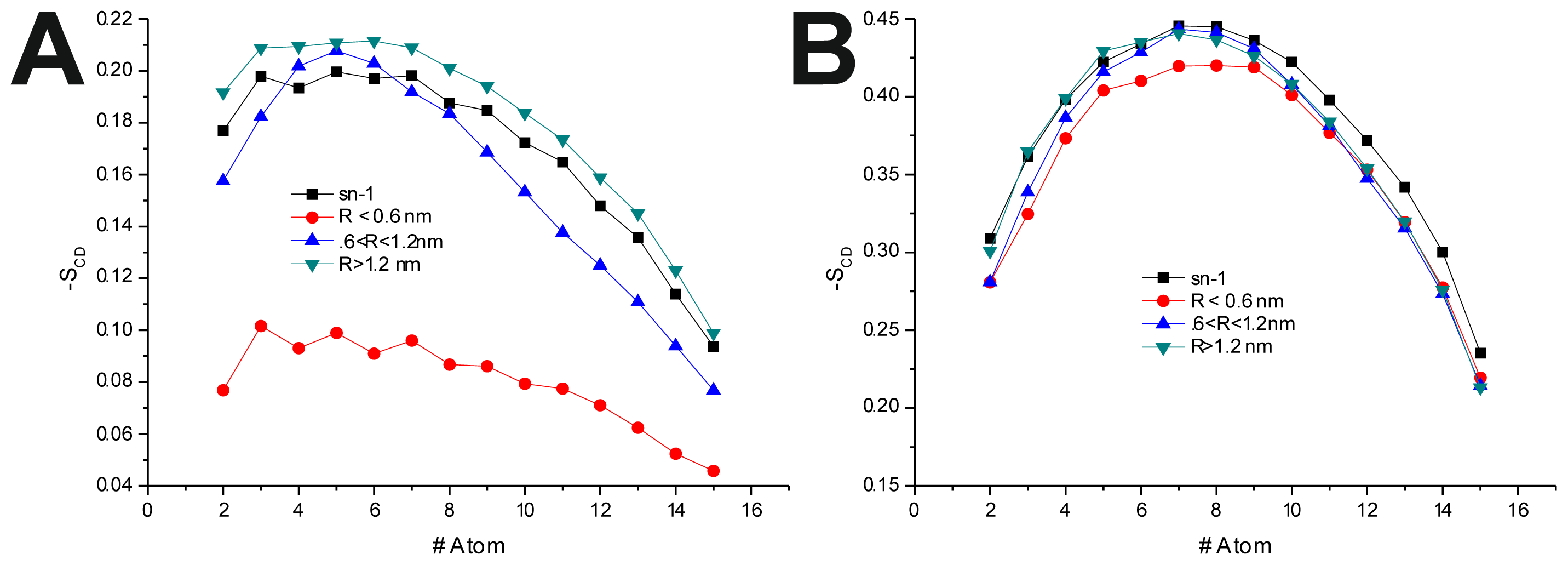
To further investigate the effect of the peptide adsorption to the membrane surface on the −
SCD, these parameters were calculated as a function of the minimal atomic distance (for three different distance bins:
R < 0.6 nm, 0.6 nm <
R < 1.2 nm and
R > 1.2 nm, also, in the last 50 ns of each production run) between the peptide’s TRD and PBD domains and each individual POPC molecule (
Figure 4 and
Table 6). In the disordered POPC systems, proximity to the TRD (
R < 1.2 nm) causes a decrease in −
SCD in the
sn-1 of the POPC molecules closer to the TRD domain (
Figure 4A,C and
Table 7), this effect being stronger in the T-20-containing system. In the T-1249 + POPC system, PBD domain proximity (
R < 1.2 nm) causes an increase in −
SCD of the
sn-1 acyl chain (
Figure 4E). In this system, for
R > 1.2 nm, the effects of interaction with both TRD and PBD domains, in both cases, are reversed from those described above, which appears to be due to the interaction of the POPC molecules with the opposite domain of the peptide (
Figure 4 and
Table 7). T-20, having no PBD domain, suffers no such effect and, as such, imposes a stronger decrease in −
SCD of the
sn-1 acyl chain. The localized compression of T-20 induces around its TRD, resulting from the way it burrows itself in the membrane interface and compresses the POPC molecules, both in
z direction and the
xy plane, and the latter may be responsible for the increase in −
SCD of the
sn-1 acyl chain observed for
R > 1.2 nm.
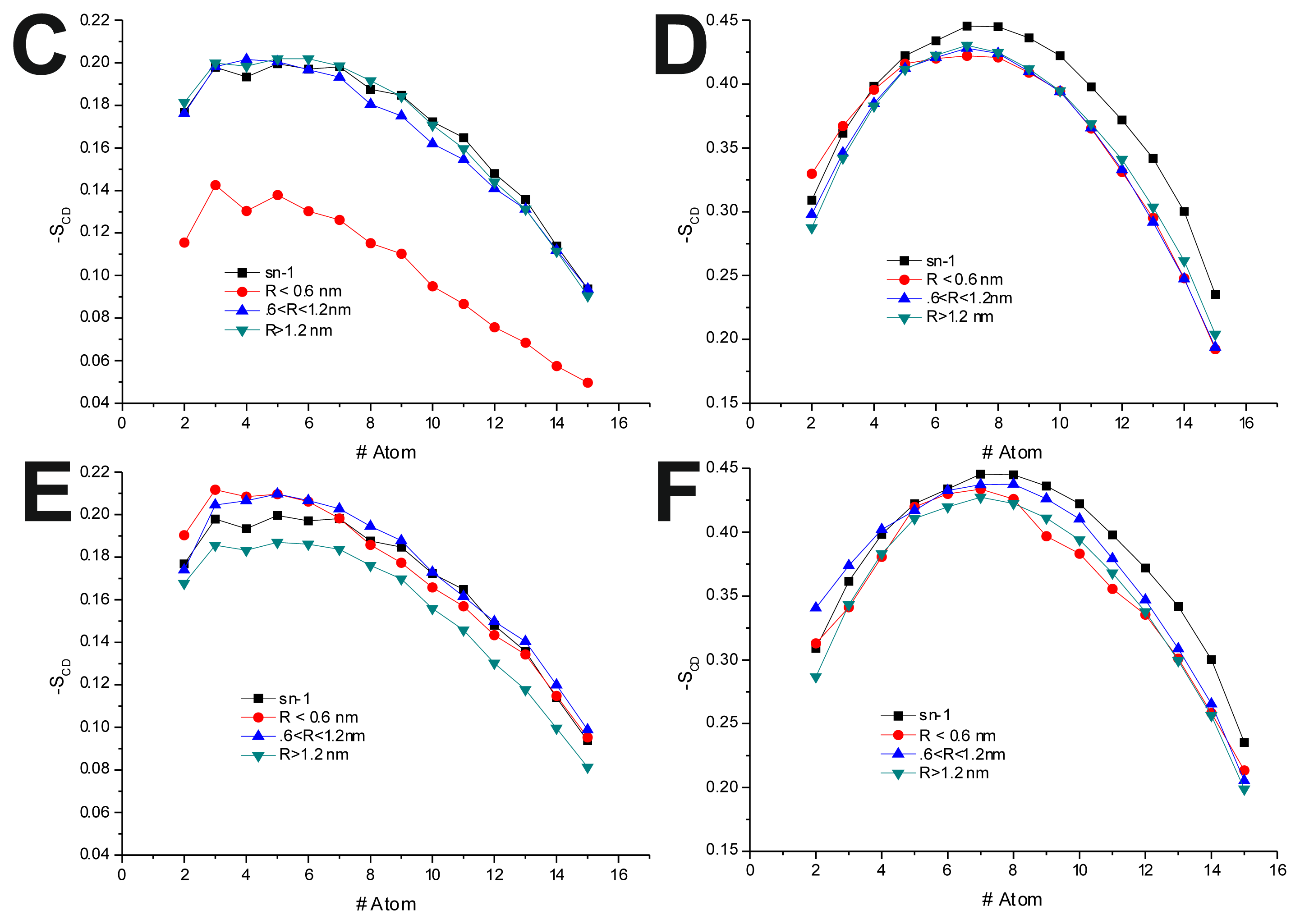
In the POPC/Chol, lo, systems, interaction with the TRD and PBD domains causes an overall decrease in −
SCD on all bins (
Figure 4B,D,F and
Table 7). In the T-20 + POPC/Chol system, this effect decreases with the distance from the TRD domain, whereas in the T-1249 + POPC/Chol system, there is almost no variation from bin to bin (
Figure 4B,D and
Table 7). A similar observation can be made for the PBD effect on −
SCD, in which the decrease in −
SCD is similar in the closer and outer bin, being smaller in the middle bin (
Figure 4F and
Table 7). These membranes are in a highly ordered state, and the interaction of these peptides that diffuse more rapidly than the lipids with which they interact, as described earlier, causes a disordering effect in the bilayer. This effect is stronger in the T-1249-containing systems, as it interacts more strongly with POPC/Chol bilayers.