Inhibition of Corneal Neovascularization with the Combination of Bevacizumab and Plasmid Pigment Epithelium-Derived Factor-Synthetic Amphiphile INTeraction-18 (p-PEDF-SAINT-18) Vector in a Rat Corneal Experimental Angiogenesis Model
Abstract
:1. Introduction
2. Results and Discussion
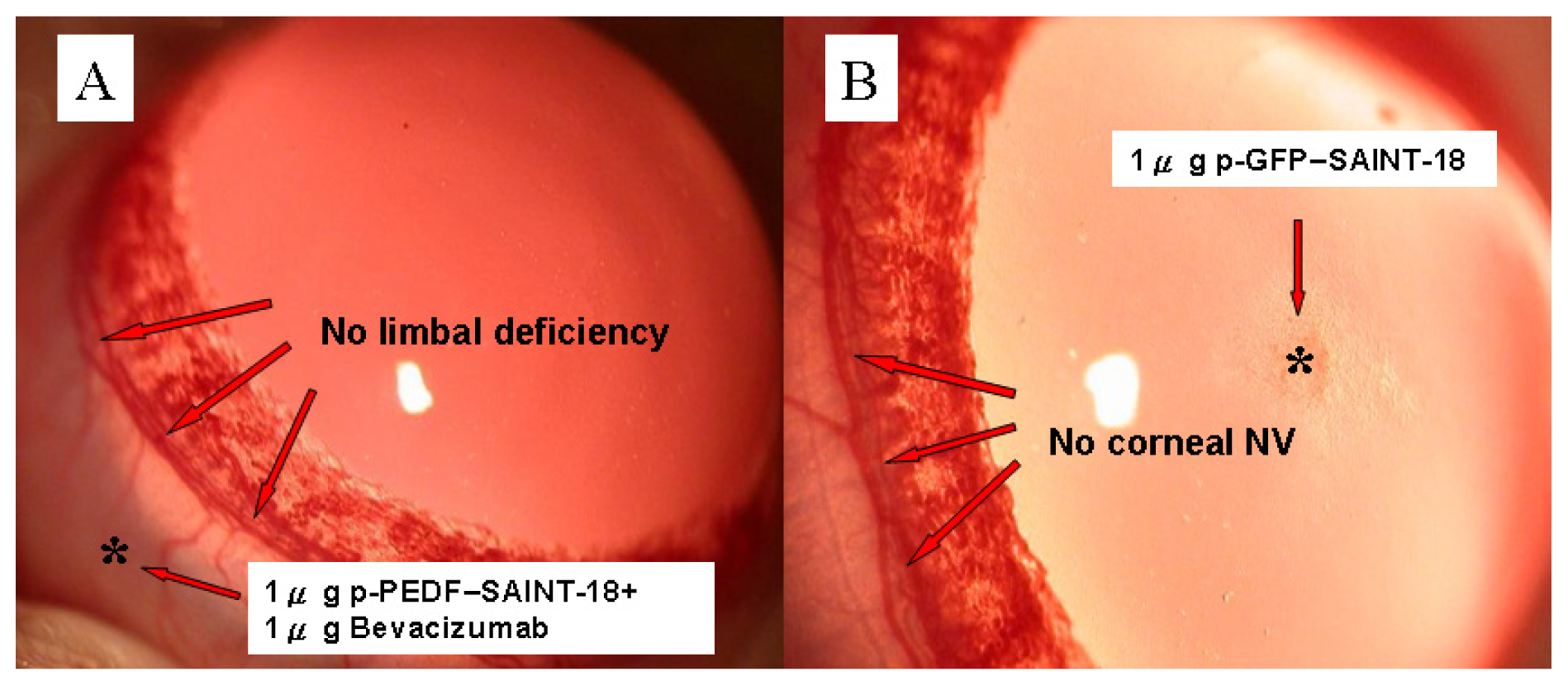
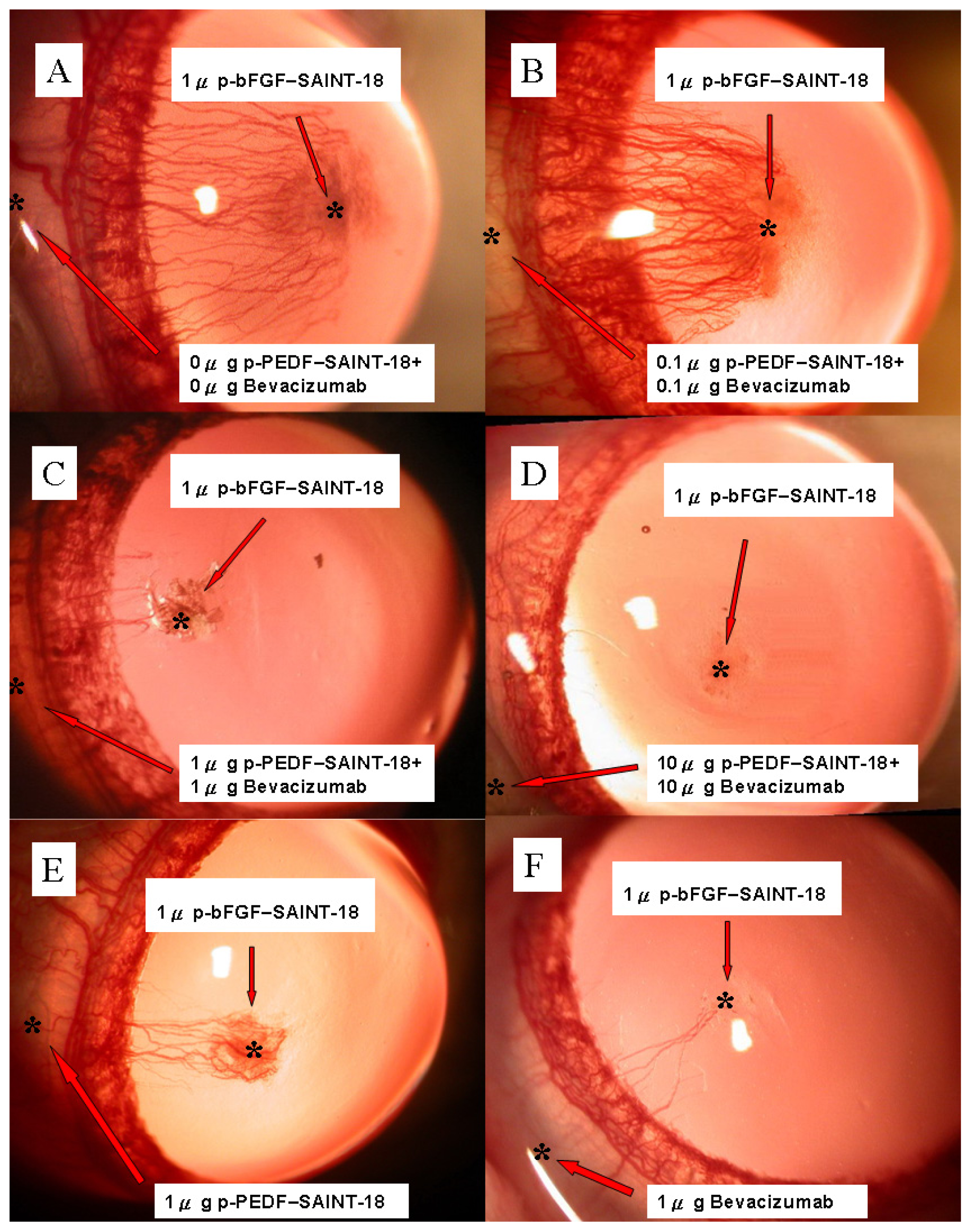
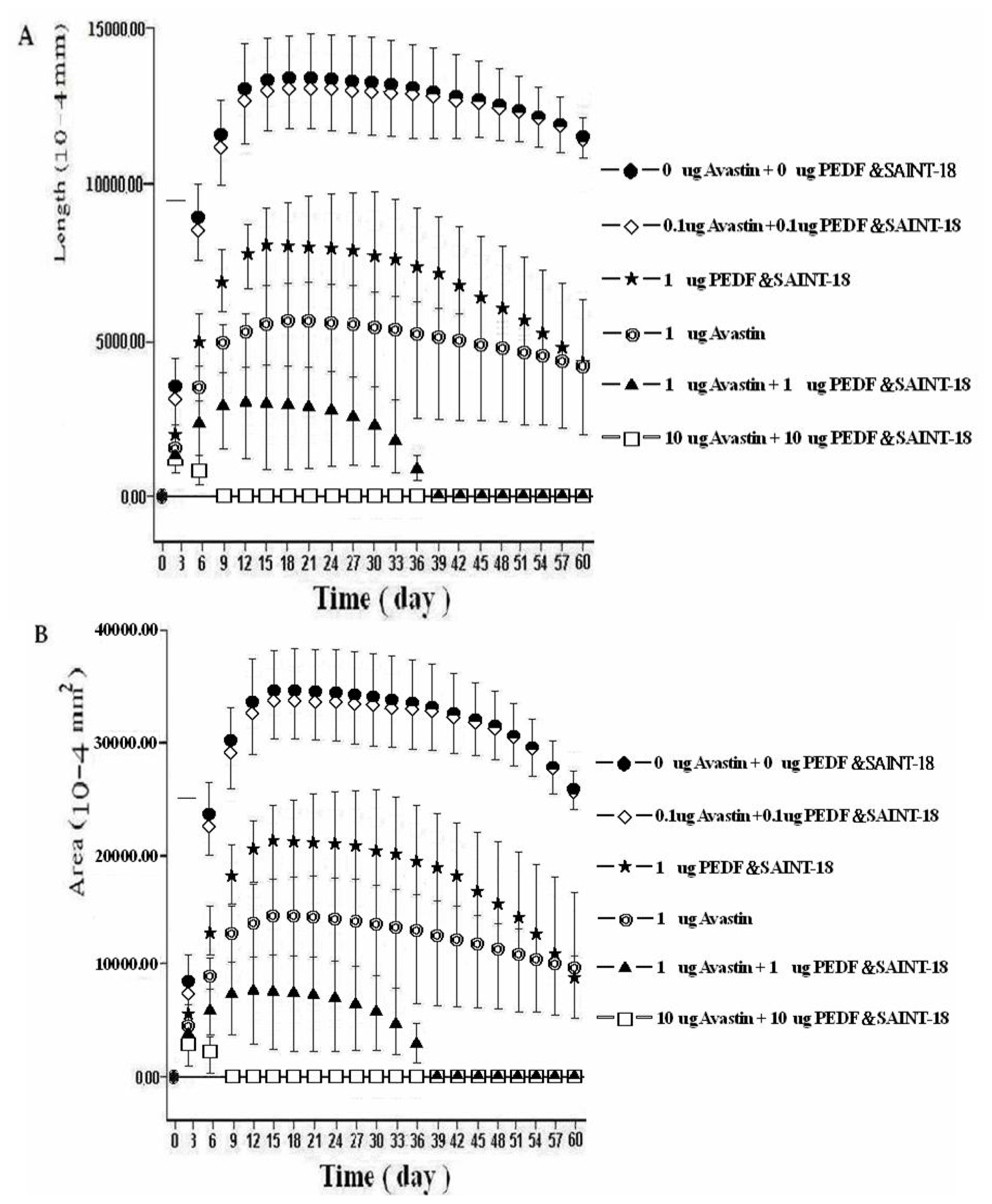
2.1. Biomicroscopic Examinations of Corneal NV
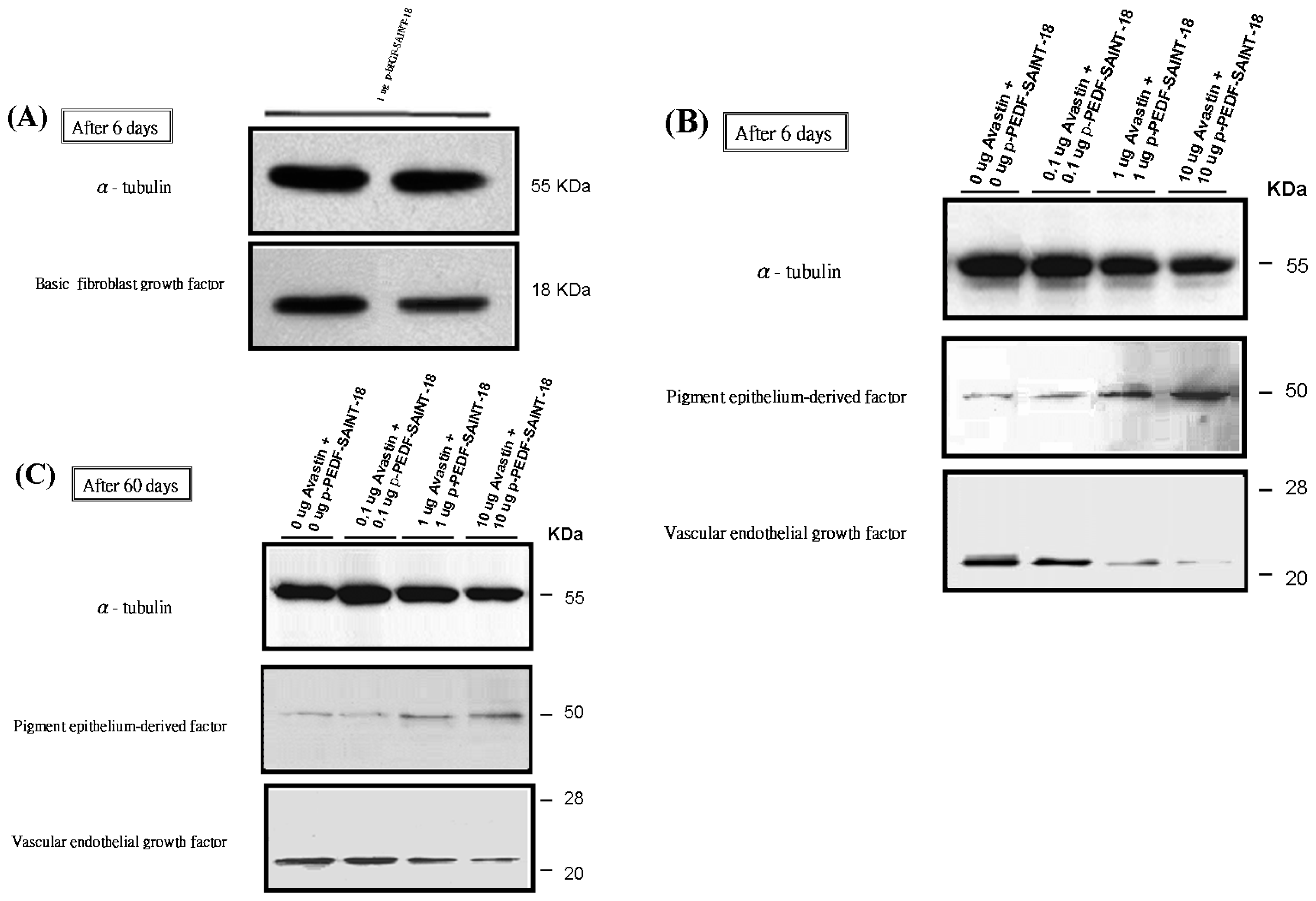
2.2. Western Blot Analysis
2.3. Histology
3. Experimental Section
3.1. Animals
3.2. Naked DNA Vector
3.3. p-DNA-SAINT-18 Complex Preparation
3.4. Corneal Pocket Assay
3.5. Visualization and Quantification of Corneal NV
3.6. Analysis of bFGF, VEGF and PEDF Protein Expression by Western Blot after Transfection
3.7. Histological Examination
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Abbreviations
| PEDF | pigment epithelium derived factor |
| BFGF | basic fibroblast growth factor |
| SAINT-18 | synthetic amphiphile interaction-18, 1-methyl-4-(cis-9-dioleyl) methylpyridinium-chloride |
| VEGF | vascular endothelial growth factor |
| GFP | green fluorescein protein |
| NV | neovascularization |
References
- Epstein, R.J.; Stulting, R.D.; Hendricks, R.L.; Harris, D.M. Corneal neovascularization pathogenesis and inhibition. Cornea 1987, 6, 250–257. [Google Scholar]
- Polverini, P.J. The pathophysiology of angiogenesis. Crit. Rev. Oral Biol. Med 1995, 6, 230–247. [Google Scholar]
- Benelli, U.; Bocci, G.; Danesi, R.; Lepri, A.; Bernardini, N.; Bianchi, F.; Lupetti, M.; Dolfi, A.; Campagni, A.; Agen, C.; et al. The heparin sulfate suleparoide inhibits rat corneal angiogenesis and in vitro neovascularization. Exp. Eye Res 1998, 67, 133–142. [Google Scholar]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar]
- Bocci, G.; Danesi, R.; Benelli, U.; Innocenti, F.; di Paolo, A.; Fogli, S.; del Tacca, M. Inhibitory effect of suramin in rat models of angiogenesis in vitro and in vivo. Cancer Chemother. Pharmacol 1999, 43, 205–212. [Google Scholar]
- Fotsis, T.; Pepper, M.; Adlercreutz, H.; Fleischmann, G.; Hase, T.; Montesano, R.; Schweigerer, L. Genistein, a dietary—Derived inhibitor of in vitro angiogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 2690–2694. [Google Scholar]
- Taniguchi, K.; Sasaki, K.; Watari, K.; Yasukawa, H.; Imaizumi, T.; Ayada, T.; Okamoto, F.; Ishizaki, T.; Kato, R.; Kohno, R.; et al. Suppression of Sproutys has a therapeutic effect for a mouse model of ischemia by enhancing angiogenesis. PLoS One 2009, 4, e5467. [Google Scholar]
- Liu, X.; Lin, Z.; Zhou, T.; Zong, R.; He, H.; Liu, Z.; Ma, J.X.; Liu, Z.; Zhou, Y. Anti-angiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS One 2011, 6, e16712. [Google Scholar]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; D’Amico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol 1994, 118, 445–450. [Google Scholar]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med 1994, 331, 1480–1487. [Google Scholar]
- Malecaze, F.; Clamens, S.; Simorre-Pinatel, V.; Mathis, A.; Chollet, P.; Favard, C.; Bayard, F.; Plouet, J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch. Ophthalmol 1994, 112, 1476–1482. [Google Scholar]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar]
- Adamis, A.P.; Shima, D.T.; Tolentino, M.J.; Gragoudas, E.S.; Ferrara, N.; Folkman, J.; D’Amore, P.A.; Miller, J.W. Inhibition of vascular endothelial growth factor prevents retinal ischemia associated iris neovascularization in a nonhuman primate. Arch. Ophthalmol 1996, 114, 66–71. [Google Scholar]
- Manzano, R.P.; Peyman, G.A.; Khan, P.; Carvounis, P.E.; Kivilcim, M.; Ren, M.; Lake, J.C.; Chévez-Barrios, P. Inhibition of experimental corneal neovascularization by bevacizumab (Avastin). Br. J. Ophthalmol 2007, 91, 804–807. [Google Scholar]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W. Bevacizumab plusirinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med 2004, 350, 2335–2342. [Google Scholar]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 363–372. [Google Scholar]
- Spaide, R.F.; Fisher, Y.L. Intravitreal Bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006, 26, 275–278. [Google Scholar]
- Papathanassiou, M.; Theodossiadis, P.G.; Liarakos, V.S.; Rouvas, A.; Giamarellos-Bourboulis, E.J.; Vergados, I.A. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am. J. Ophthalmol 2008, 145, 424–431. [Google Scholar]
- Yoeruek, E.; Ziemssen, F.; Henke-Fahle, S.; Tatar, O.; Tura, A.; Grisanti, S.; Bartz-Schmidt, K.U.; Szurman, P.; Tübingen. Bevacizumab Study xGroup. Safety, penetration and efficacy of topically applied bevacizumab: Evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol 2008, 86, 322–328. [Google Scholar]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar]
- Bouck, N. PEDF: Anti-angiogenic guardian of ocular function. Trends Mol. Med 2002, 8, 330–334. [Google Scholar]
- Becerra, S.P. Focus on molecules: Pigment epithelium-derived factor (PEDF). Exp. Eye Res 2006, 82, 739–740. [Google Scholar]
- Tombran-Tink, J.; Barnstable, C.J. Therapeutic prospects for PEDF: More than a promising angiogenesis inhibitor. Trends Mol. Med 2003, 9, 244–250. [Google Scholar]
- Duh, E.J.; Yang, H.S.; Suzuma, I.; Miyagi, M.; Youngman, E.; Mori, K.; Katai, M.; Yan, L.; Suzuma, K.; West, K.; et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF induced migration and growth. Invest. Ophthalmol. Vis. Sci 2002, 43, 821–829. [Google Scholar]
- Aplin, A.E.; Howe, A.; Alahari, S.K.; Juliano, R.L. Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev 1998, 50, 197–263. [Google Scholar]
- Alavi, A.; Hood, J.D.; Frausto, R.; Stupack, D.G.; Cheresh, D.A. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 2003, 301, 94–96. [Google Scholar]
- Conway, E.M.; Carmeliet, P. Cardiovascular biology: Signalling silenced. Nature 2003, 425, 139–141. [Google Scholar]
- Ren, J.G.; Jie, C.; Talbot, C. How PEDF prevents angiogenesis: A hypothesized pathway. Med. Hypotheses 2005, 64, 74–78. [Google Scholar]
- Kanda, S.; Mochizuki, Y.; Nakamura, T.; Miyata, Y.; Matsuyama, T.; Kanetake, H. Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J. Cell Sci 2005, 118, 961–970. [Google Scholar]
- Matsui, T.; Nishino, Y.; Maeda, S.; Yamagishi, S. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc Res 2012, 84, 105–108. [Google Scholar]
- Yu, C.Q.; Zhang, M.; Matis, K.I.; Kim, C.; Rosenblatt, M.I. Vascular endothelial growth factor mediates corneal nerve repair. Invest. Ophthalmol. Vis. Sci 2008, 49, 3870–3878. [Google Scholar]
- Kuo, C.N.; Yang, L.C.; Yang, C.T.; Chen, M.F.; Lai, C.H.; Chen, Y.H.; Chen, C.H.; Chen, C.H.; Wu, P.C.; Kou, H.K.; Tsai, J.C.; Hung, C.H. A novel vector system for gene transfer into the cornea using a partially dried plasmid expressing 18 basic fibroblast growth factor-synthetic amphiphile INTeraction-18 (SAINT-18) complex. Curr. Eye Res 2008, 33, 839–848. [Google Scholar]
- Kenyon, B.M.; Voest, E.E.; Chen, C.C.; Flynn, E.; Folkman, J.; D’Amato, R.J. A model of angiogenesis in the mouse cornea. Invest. Ophthalmol. Vis. Sci 1996, 37, 1625–1632. [Google Scholar]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol 2001, 12, 242–249. [Google Scholar]
- Mohan, R.R.; Tovey, J.C.; Sharma, A.; Schultz, G.S.; Cowden, J.W.; Tandon, A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One 2011, 6, e26432. [Google Scholar]
- Kuo, C.N.; Yang, L.C.; Yang, C.T.; Lai, C.H.; Chen, M.F.; Chen, C.Y.; Chen, C.H.; Wu, P.C.; Kou, H.K.; Chen, Y.J.; et al. Inhibition of corneal neovascularization with plasmid pigment epithelium-derived factor (p-PEDF) delivered by synthetic amphiphile INTeraction-18 (SAINT-18) vector in an experimental model of rat corneal angiogenesis. Exp. Eye Res 2009, 89, 678–85. [Google Scholar]
- Peters, S.; Heiduschka, P.; Julien, S.; Ziemssen, F.; Fietz, H.; Bartz-Schmidt, K.U.; Schraermeyer, U. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am. J. Ophthalmol 2007, 143, 995–1002. [Google Scholar]
- Kim, S.W.; Ha, B.J.; Kim, E.K.; Tchah, H.; Kim, T.I. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008, 115, e33–e38. [Google Scholar]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol 2009, 247, 1375–1382. [Google Scholar]
- Raviola, G. Conjunctival and episcleral blood vessels are permeable to blood-borne horseradish peroxidase. Invest. Ophthalmol. Vis. Sci 1983, 24, 725–736. [Google Scholar]
- Ranta, V.P.; Urtti, A. Transscleral drug delivery to the posterior eye: Prospects of pharmacokinetic modeling. Adv. Drug Deliv. Rev 2006, 58, 1164–1181. [Google Scholar]
- Robinson, M.R.; Lee, S.S.; Kim, H.; Kim, S.; Lutz, R.J.; Galban, C.; Bungay, P.M.; Yuan, P.; Wang, N.S.; Kim, J.; Csaky, K.G. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res 2006, 82, 479–487. [Google Scholar]
- Amrite, A.C.; Edelhauser, H.F.; Kompella, U.B. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest. Ophthalmol. Vis. Sci 2008, 49, 320–332. [Google Scholar]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci 2007, 48, 2545–2552. [Google Scholar]
- Kim, T.I.; Kim, S.W.; Kim, S.; Kim, T.; Kim, E.K. Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea 2008, 27, 349–352. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-N.; Chen, C.-Y.; Chen, S.-N.; Yang, L.-C.; Lai, L.-J.; Lai, C.-H.; Chen, M.-F.; Hung, C.-H.; Chen, C.-H. Inhibition of Corneal Neovascularization with the Combination of Bevacizumab and Plasmid Pigment Epithelium-Derived Factor-Synthetic Amphiphile INTeraction-18 (p-PEDF-SAINT-18) Vector in a Rat Corneal Experimental Angiogenesis Model. Int. J. Mol. Sci. 2013, 14, 8291-8305. https://doi.org/10.3390/ijms14048291
Kuo C-N, Chen C-Y, Chen S-N, Yang L-C, Lai L-J, Lai C-H, Chen M-F, Hung C-H, Chen C-H. Inhibition of Corneal Neovascularization with the Combination of Bevacizumab and Plasmid Pigment Epithelium-Derived Factor-Synthetic Amphiphile INTeraction-18 (p-PEDF-SAINT-18) Vector in a Rat Corneal Experimental Angiogenesis Model. International Journal of Molecular Sciences. 2013; 14(4):8291-8305. https://doi.org/10.3390/ijms14048291
Chicago/Turabian StyleKuo, Chien-Neng, Chung-Yi Chen, San-Ni Chen, Lin-Cheng Yang, Li-Ju Lai, Chien-Hsiung Lai, Miao-Fen Chen, Chia-Hui Hung, and Ching-Hsein Chen. 2013. "Inhibition of Corneal Neovascularization with the Combination of Bevacizumab and Plasmid Pigment Epithelium-Derived Factor-Synthetic Amphiphile INTeraction-18 (p-PEDF-SAINT-18) Vector in a Rat Corneal Experimental Angiogenesis Model" International Journal of Molecular Sciences 14, no. 4: 8291-8305. https://doi.org/10.3390/ijms14048291
APA StyleKuo, C.-N., Chen, C.-Y., Chen, S.-N., Yang, L.-C., Lai, L.-J., Lai, C.-H., Chen, M.-F., Hung, C.-H., & Chen, C.-H. (2013). Inhibition of Corneal Neovascularization with the Combination of Bevacizumab and Plasmid Pigment Epithelium-Derived Factor-Synthetic Amphiphile INTeraction-18 (p-PEDF-SAINT-18) Vector in a Rat Corneal Experimental Angiogenesis Model. International Journal of Molecular Sciences, 14(4), 8291-8305. https://doi.org/10.3390/ijms14048291






